Abstract
Cytomegalovirus (CMV) enzyme-linked immunosorbent spot (ELISPOT) and CMV QuantiFERON assays were examined as potential biomarkers predictive of congenital CMV (cCMV) transmission. Fifty-seven pregnant women with primary CMV infection and 23 with nonprimary CMV infection were recruited in the study. Maternal age, CMV IgG avidity, viremia, and viruria were also included among the potential predictors. Spearman's statistical correlation analysis revealed a positive correlation between the CMV ELISPOT and CMV QuantiFERON assay results (P < 0.001), but only the CMV ELISPOT assay correlated with cCMV (P < 0.001). cCMV was positively correlated with maternal viremia and viruria (P < 0.05) and negatively correlated with CMV IgG avidity (P < 0.01). Maternal age and CMV QuantiFERON assay results were not statistically associated with cCMV. CMV-specific cell-mediated immunity detected by the CMV ELISPOT assay plays a critical role in cCMV.
INTRODUCTION
Congenital cytomegalovirus (cCMV) infection affects about 0.7% of newborns worldwide (1–3). The clinical CMV-related sequelae at birth are highly variable and related to maternal serostatus and the time of onset of congenital infection during pregnancy (4–9). Whenever clinically evident, CMV-induced damages include sensorineural hearing loss (SNHL), visual impairment, delayed psychomotorial development, and retardation (10–13). Understanding the risk factors and biomarkers associated with the maternal transmission of CMV infection represents a leading priority for both diagnosis and clinical management of cCMV. Recently, it was shown that maternal CMV cell-mediated immunity (CMI) plays a critical role in determining cCMV (14–16). Several assays are available to assess CMV-specific CMI, and the large majority of these assays are based on interferon gamma (IFN-γ) release assays (IGRAs) (17–20). In this study, two IGRAs that detect CMV-specific CMI, the CMV enzyme-linked immunosorbent spot (ELISPOT) and CMV QuantiFERON assays, were compared for their prediction of cCMV. Both the CMV ELISPOT and CMV-QuantiFERON assays detect IFN-γ produced by antigen-stimulated peripheral blood mononuclear cells (PBMCs). The main differences between the assays include the antigen stimulus composition, with stimulation of CD8+ T-cell responses in the CMV QuantiFERON assay (21) and stimulation of both CD4+ and CD8+ T-cell responses in the CMV ELISPOT assay (22, 23). Moreover, the CMV QuantiFERON assay detects IFN-γ in a volume of ∼1 ml of whole blood, while the CMV ELISPOT assay detects IFN-γ secreted by ∼2 × 105 PBMCs (22, 23). Recent studies suggest that the CMV ELISPOT and CMV QuantiFERON assays may display large variability on an individual basis (24, 25).
In order to have a more comprehensive view of the maternal factors associated with cCMV, this study also investigated maternal parameters such as maternal age, viremia, viruria, and CMV immunoglobulin G (IgG) avidity.
(The data in this study were partly presented at the Congenital CMV Conference, Brisbane, Australia, 2015.)
MATERIALS AND METHODS
Patients.
Eighty pregnant Caucasian women were referred from January 2012 to January 2013 to the Padua Reference Center for Gestational and Congenital Infections for suspected infection and potential risk for the fetus. Patients and patient specimens were previously described in other studies (15, 25). The Padua Reference Center represents the main referral hub for congenital infections, serving about 950,000 women ranging in age from 15 to 45 years in the Veneto region (National Statistic Institute [ISTAT] data [see http://www.istat.it/en/veneto]). Patient exclusion criteria were (i) women with preexisting or acquired immunodeficiency or (ii) women exhibiting primary CMV infection after the 20th week of gestation. The median age of the pregnant women was 31 years (range, 17 to 42 years). These cases were classified as primary CMV infection (57 women) and nonprimary CMV infection (23 women). Primary maternal CMV infection was defined by (i) seroconversion in previously seronegative mothers or (ii) detection of maternal CMV immunoglobulin M (IgM) and concomitant low maternal CMV IgG avidity (<25%). Nonprimary CMV infection was defined by the presence of CMV viruria in already CMV IgG-positive pregnant women and detection of CMV IgG avidity of >45% within the 14th week of gestation. All serologic and molecular tests were performed at the Padua General Hospital Microbiology and Virology Diagnostic Laboratory. In primarily infected pregnant women, the estimated timing of CMV infection occurred within a median of 6 weeks of gestation (range, 0 to 20 weeks), and CMV ELISPOT and CMV QuantiFERON assays were performed within a median of 8 weeks (range, 2 to 17 weeks) after CMV infection. Of the women experiencing primary CMV infection, 16/57 (28%) transmitted the infection to the fetus, 19/57 (33%) had episodes of viremia, and 43/57 (75%) had viruria. Of the 23 nonprimary infections, no cases of CMV viremia were reported, all women experienced CMV viruria, and no cases of congenital transmission occurred. Fetal or newborn CMV infection was assessed by CMV DNA detection in amniotic fluid at 20 to 21 gestational weeks of age or in urine at birth (26, 27). The Padua General Hospital Ethical Committee approved the study. The participants provided written informed consent.
Detection of maternal CMV DNA in blood and urine, CMV IgM and IgG, CMV IgG avidity, CMV QuantiFERON, and CMV ELISPOT tests.
CMV IgM and CMV IgG (Siemens Immulite) and CMV IgG avidity (Tecnogenetics) tests were performed according to the manufacturers' instructions. CMV DNA detection in blood, amniotic fluid, and urine was performed by using real-time quantitative PCR (28). The lowest limit of CMV DNA detection is <1,000 copies/ml of whole blood. In primary and nonprimary CMV infections, CMV ELISPOT and QuantiFERON assays were performed after confirmation of infection. CMV ELISPOT and QuantiFERON tests were performed at the same time.
Blood was drawn into 10-ml tubes containing sodium citrate (CMV ELISPOT assay), and ∼3 ml was collected into 3 CMV QuantiFERON tubes (positive control, negative control, and CMV, with ∼1 ml/tube), according to the manufacturers' instructions. CMV QuantiFERON tubes (Qiagen) were kept overnight at 37°C, and samples were then processed according to the manufacturer's instructions. PBMCs were isolated from whole blood by Ficoll density gradient centrifugation and used for the CMV ELISPOT assay (Autoimmune Diagnostika) as previously described (29). Freshly isolated PBMCs were stimulated with commercially available peptides spanning the highly immunogenic CMV pp65 (ppUL83) protein (Autoimmune Diagnostika). In order to avoid false-negative responses due to peptide misrecognition, the whole CMV lysate was included as an internal control, as described previously (29).
Statistical analysis.
Data including maternal age, maternal CMV ELISPOT assay result, maternal CMV QuantiFERON assay result, maternal IgG avidity, maternal CMV detection in blood and urine, and CMV transmission to the newborn were analyzed by using Spearman's pairwise correlation analysis. For statistical analysis, viremia, viruria, and CMV transmission were considered qualitative categorical binary variables (0 [yes] or 1 [no]). Maternal age, CMV ELISPOT assay result, CMV QuantiFERON assay result, and CMV IgG avidity were quantitative continuous variables. The Spearman rank method was employed to analyze the correlation of CMV ELISPOT and QuantiFERON assay results with the time of CMI assay determination to assess if the time of assay determination (2 to 17 weeks) introduced any bias for the CMI assays and thus for determination of cCMV. P values of <0.05 were considered statistically significant. To determine the diagnostic performance of the CMV ELISPOT and CMV QuantiFERON assays, a receiver operating characteristic (ROC) analysis was performed. The area under the curve (AUC) indicates the performance of the diagnostic test. Youden's index was employed to determine the optimum diagnostic cutoff point (30). Statistical analysis was performed by using STATA 14 software. R software (open-source software; R Foundation [see http://www.r-project.org/]) was used for translating the numerical data reported in Table 1 and Fig. 1. The distance between the nodes is decreased when the nodes are highly correlated.
TABLE 1.
Spearman pairwise correlation analysisa
| Variable | Correlation coefficient |
||||||
|---|---|---|---|---|---|---|---|
| Maternal age | Maternal CMV ELISPOT assay result | Maternal CMV QuantiFERON assay result | Maternal CMV IgG avidity | CMV detection in maternal blood | CMV detection in maternal urine | CMV transmission to newborn | |
| Maternal age | 1 | ||||||
| Maternal CMV ELISPOT assay result | 0.04 | 1 | |||||
| Maternal CMV QuantiFERON assay result | −0.1053 | 0.3629*** | 1 | ||||
| Maternal CMV IgG avidity | −0.0196 | −0.3101** | −0.0958 | 1 | |||
| CMV detection in maternal blood | −0.0996 | 0.3195** | 0.1429 | −0.6042*** | 1 | ||
| CMV detection in maternal urine | −0.2228* | 0.0912 | −0.0399 | 0.0755 | 0.1699 | 1 | |
| CMV transmission to newborn | −0.0177 | 0.4357*** | −0.1131 | −0.3075** | 0.2565* | 0.2537* | 1 |
Negative values indicate negative correlations. Maternal age, CMV ELISPOT assay result, CMV QuantiFERON assay result, and CMV IgG avidity were quantitative variables, while CMV viremia and viruria and CMV transmission to the newborn were expressed as binary variables (0 or 1). Significance is indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
FIG 1.
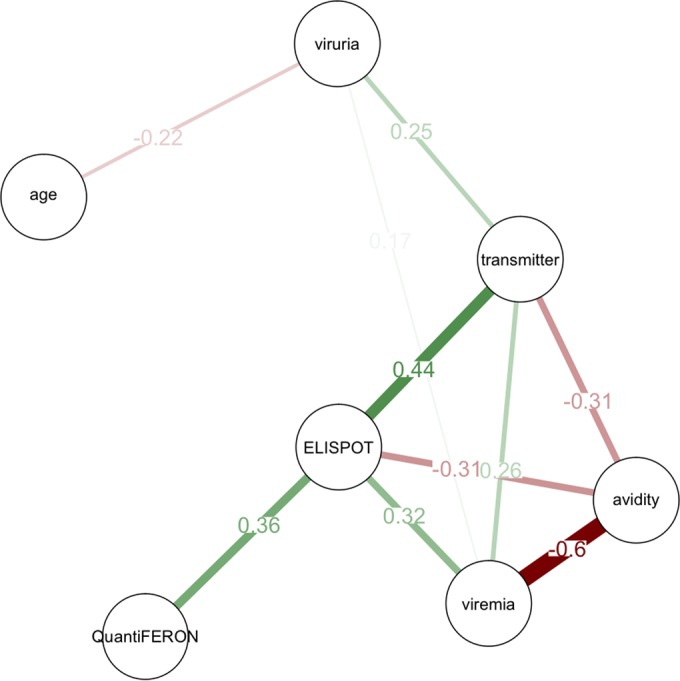
Graphical representation of the factors associated with congenital CMV transmission. Green lines indicate a positive correlation, while red lines indicate a negative correlation. The thickness of the lines and intensity of the color are directly proportional to the significance. The numbers indicated within the connecting lines represent the correlation coefficients. The distance between nodes is proportional to the degree of correlation.
RESULTS
In order to examine the factors associated with cCMV, a pairwise Spearman statistical correlation analysis was performed, and congenital transmission, maternal age, CMV ELISPOT assay result, CMV QuantiFERON assay result, CMV IgG avidity, and CMV DNA detection in maternal blood and urine were considered (Table 1). CMV transmission to the newborn (Fig. 1) was positively associated with the maternal CMV ELISPOT assay result (correlation coefficient, 0.4357; P < 0.001) and detection of CMV DNA in maternal blood and urine (correlation coefficients, 0.2565 and 0.2537, respectively; P < 0.05 for both) and negatively associated with maternal IgG avidity (correlation coefficient, −0.3075; P < 0.01). Remarkably, the CMV ELISPOT assay result was positively correlated with the CMV QuantiFERON assay result and viremia (correlation coefficients, 0.3629 and 0.3195, respectively; P < 0.001 and P < 0.01, respectively) and negatively correlated with CMV IgG avidity (correlation coefficient, −0.3101; P < 0.01). CMV IgG avidity was negatively correlated with CMV viremia (correlation coefficient, −0.6042; P < 0.001). The factors not statistically correlated with cCMV were maternal age (correlation coefficient, −0.0177) and CMV QuantiFERON assay result (correlation coefficient, −0.1131).
The different patterns of CMV ELISPOT and CMV QuantiFERON assay results for transmitting and nontransmitting mothers with primary infection and for mothers with nonprimary infection are shown in Fig. 2. As previously described (25), primary infections display statistically significantly higher CMI than nonprimary infections. To estimate the diagnostic performance and determine the diagnostic thresholds for cCMV, a ROC analysis was performed for the CMV ELISPOT assay, the CMV QuantiFERON assay, and the combination of both the CMV ELISPOT and CMV QuantiFERON assays (Fig. 3).
FIG 2.
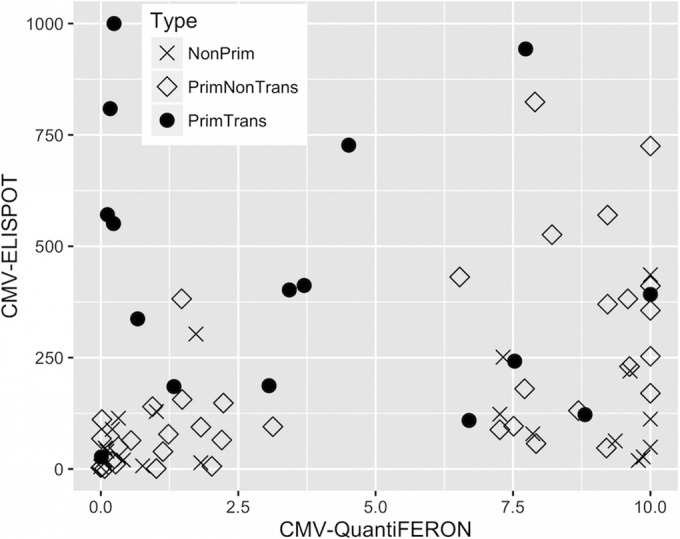
Scatterplot distributions of CMV ELISPOT and CMV QuantiFERON assay results for transmitting (PrimTrans) and nontransmitting (PrimNonTrans) pregnant women with primary CMV infection and pregnant women with nonprimary CMV infection (NonPrim). Detection by the CMV ELISPOT assay was limited to 1,000 spots/2 × 105 PBMCs, while detection by the CMV QuantiFERON assay was limited to 10 IU/ml.
FIG 3.
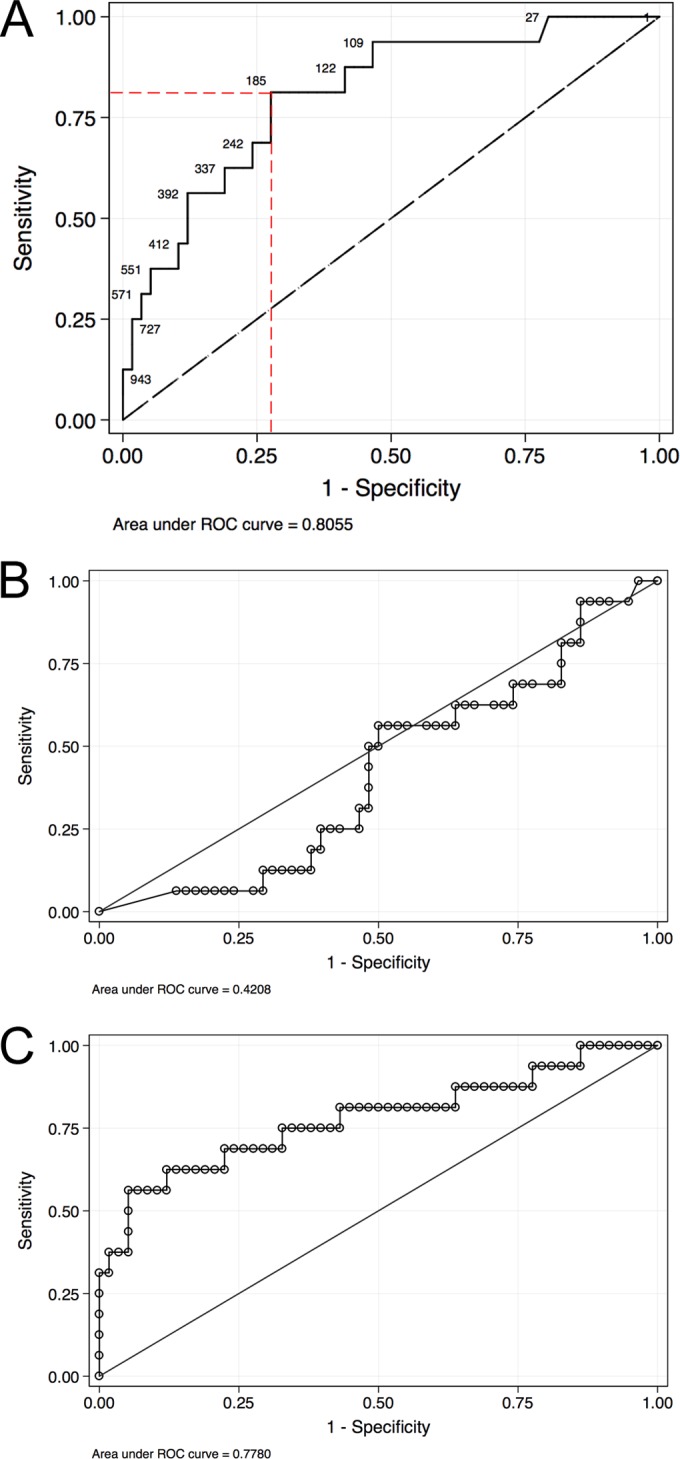
Receiver operating characteristic (ROC) analysis of the CMV ELISPOT assay alone (A), the CMV QuantiFERON assay alone (B), and the combination of both the CMV ELISPOT and CMV QuantiFERON assays (C). Panel A also shows Youden's index with the highest specificity and sensitivity.
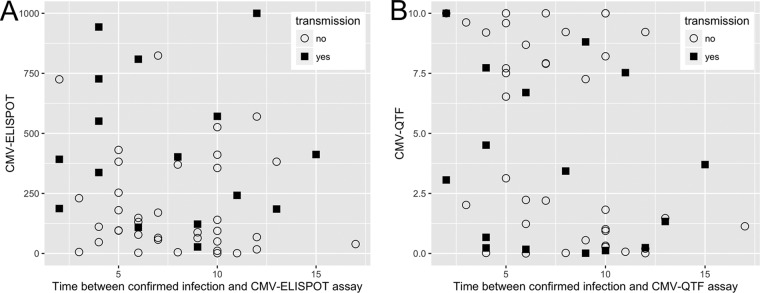
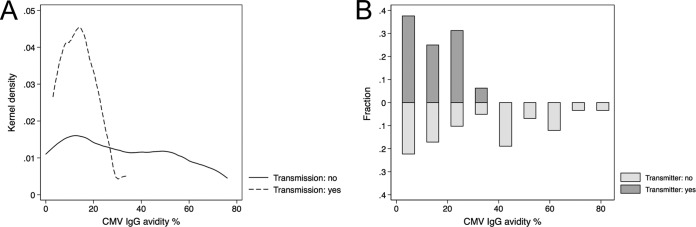
The CMV ELISPOT assay had an AUC value of 0.805, and Youden's index was calculated to be 185 spots/2 × 105 PBMCs for the CMV ELISPOT assay (Fig. 3A). Youden's index defines the point with the highest sensitivity and specificity, generally used for cutoff determination. This result was previously described (15). The CMV QuantiFERON assay had an AUC of 0.42, below the reference (AUC = 0.5) and thus a null value (Fig. 3B). For this reason, a CMV QuantiFERON assay cutoff was not determined. The combination of both the CMV ELISPOT and CMV QuantiFERON assays had an AUC value of 0.778 (Fig. 3C) and thus did not improve the overall diagnostic performance. Since the CMV ELISPOT and CMV QuantiFERON assays were executed within 8 weeks (range, 2 to 17 weeks) after confirmation of infection, we investigated whether the time of assay execution may have influenced the robustness of the immune response, introducing a bias into the analysis of cCMV. Figure 4 shows the plots of CMV ELISPOT and CMV QuantiFERON assay results for transmitting and nontransmitting mothers and the time of assay execution. The correlation of both assays was investigated by using the Spearman rank method regression. The test P values were 0.2277 (Spearman's rho = −0.1638) for the CMV ELISPOT assay and 0.0653 (Spearman's rho = −0.2481) for the CMV QuantiFERON assay. For both assays, the P values were not significant, indicating that both the CMV ELISPOT and CMV QuantiFERON assay results were independent of the time of the assay. The effect of CMV IgG avidity on cCMV was also investigated: Fig. 5 shows the kernel density and fraction of CMV IgG avidity patterns in transmitting versus nontransmitting mothers, showing that CMV IgG avidities of between 2 and 20% were associated with cCMV.
FIG 4.
Scatterplot distributions of the CMV ELISPOT (A) and CMV QuantiFERON (B) assay results over time (weeks) after infection.
FIG 5.
CMV IgG avidity in transmitting and nontransmitting CMV-infected pregnant women. (A) Kernel density distribution; (B) fraction.
DISCUSSION
This study, conducted on 80 pregnant women with primary and nonprimary CMV infections, evaluated the CMV ELISPOT and CMV QuantiFERON assays as potential biomarkers associated with cCMV. Other known biomarkers, such as maternal viremia, viruria, CMV IgG avidity, and age, were also included in this study. This study showed that the CMV ELISPOT assay result was strongly associated with an increased risk of cCMV. Other factors associated with cCMV were low IgG avidity and virus detection in blood and urine. Maternal age does not influence the risk of cCMV. The CMV QuantiFERON assay result correlated with the CMV ELISPOT assay result but not with cCMV. If both the CMV ELISPOT and CMV QuantiFERON assays are used to determine the risk of cCMV, the combined diagnostic performance is lower than that of the CMV ELISPOT assay alone. The optimal cutoff for cCMV in the CMV ELISPOT assay was 185 spots/2 × 105 PBMCs. This study and a previous one (15) showed an increased rate of cCMV in the presence of low CMV IgG avidity and a robust maternal T-cell response to CMV; thus, cCMV may be a consequence of an imbalanced Th1/Th2 response to CMV. In particular, we speculate that an altered CMV-specific CD4+ T-cell response, detected by the CMV ELISPOT assay but not by the CMV QuantiFERON assay, may promote cCMV. A second hypothesis is that cCMV may be a consequence of a proinflammatory state at the placental level caused by a large number of circulating CMV-specific activated T cells secreting IFN-γ. Under proinflammatory conditions, the placenta may express atypical receptors and molecules, facilitating CMV transplacental passage (31–34). The third hypothesis is that the strong T-cell response observed for cCMV may be directly related to a preceding protracted or high-level viremia: evidence from the transplant field suggests that high viral loads in blood promote higher-level T-cell responses (35). The latter hypothesis would be technically difficult to assess for pregnancy since T-cell assays are performed after the occurrence of CMV infection.
ACKNOWLEDGMENTS
We thank the Padua General Hospital-Azienda Ospedaliera di Padova for providing us with the IFN-γ ELISPOT plates and Cellestis and ADA, Italy, for providing the CMV QuantiFERON assay, materials, and reagents. We are deeply indebted to Gelinda Chies and Alessandra degli Agostini for their help with sample collection and Daniel Tinto and Federica Franceschi for technical aid. We thank Edward S. Mocarski, Emory Vaccine Center, Emory University, USA, for the critical discussion of the manuscript.
D.A., A.S., G.F., and C.M. analyzed the data and wrote the manuscript. A.S. and G.F. collected and analyzed the CMV ELISPOT assay and CMV QuantiFERON assay data. G.F. and N.G. collected the clinical and diagnostic data. C.M. performed the statistical analysis. G.P. and N.G. supervised the study.
We declare no conflicts of interest.
The University of Padua provided 60% funding in 2014 and 2015 (60A07-8071/14 and 60A07-4239/15).
REFERENCES
- 1.Dollard SC, Grosse SD, Ross DS. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Boppana SB, Ross SA, Fowler KB. 2013. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57(Suppl 4):S178–S181. doi: 10.1093/cid/cit629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler KB, Stagno S, Pass RF. 2003. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 7.Nigro G, Adler SP. 2011. Cytomegalovirus infections during pregnancy. Curr Opin Obstet Gynecol 23:123–128. doi: 10.1097/GCO.0b013e328342f1f6. [DOI] [PubMed] [Google Scholar]
- 8.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. 2006. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 35:216–220. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Schleiss MR. 2013. Cytomegalovirus in the neonate: immune correlates of infection and protection. Clin Dev Immunol 2013:501801. doi: 10.1155/2013/501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosse SD, Ross DS, Dollard SC. 2008. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 41:57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 2002. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 110:762–767. doi: 10.1542/peds.110.4.762. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal LS, Fowler KB, Boppana SB, Britt WJ, Pass RF, Schmid SD, Stagno S, Cannon MJ. 2009. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J 28:515–520. doi: 10.1097/INF.0b013e318198c724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson EC, Schleiss MR. 2013. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 60:335–349. doi: 10.1016/j.pcl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialas KM, Tanaka T, Tran D, Varner V, Cisneros De La Rosa E, Chiuppesi F, Wussow F, Kattenhorn L, Macri S, Kunz EL, Estroff JA, Kirchherr J, Yue Y, Fan Q, Lauck M, O'Connor DH, Hall AH, Xavier A, Diamond DJ, Barry PA, Kaur A, Permar SR. 2015. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci U S A 112:13645–13650. doi: 10.1073/pnas.1511526112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saldan A, Forner G, Mengoli C, Gussetti N, Palu G, Abate D. 2015. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 61:1228–1234. doi: 10.1093/cid/civ561. [DOI] [PubMed] [Google Scholar]
- 16.Schleiss MR. 2016. Preventing congenital cytomegalovirus infection: protection to a ‘T’. Trends Microbiol 24:170–172. doi: 10.1016/j.tim.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanekar SA, Nomura LE, Suni MA, Picker LJ, Maecker HT, Maino VC. 2001. Gamma interferon expression in CD8(+) T cells is a marker for circulating cytotoxic T lymphocytes that recognize an HLA A2-restricted epitope of human cytomegalovirus phosphoprotein pp65. Clin Diagn Lab Immunol 8:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giulieri S, Manuel O. 2011. QuantiFERON-CMV assay for the assessment of cytomegalovirus cell-mediated immunity. Expert Rev Mol Diagn 11:17–25. doi: 10.1586/erm.10.109. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt T, Sester M. 2013. Detection of antigen-specific T cells based on intracellular cytokine staining using flow-cytometry. Methods Mol Biol 1064:267–274. doi: 10.1007/978-1-62703-601-6_19. [DOI] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, Bell S, Gailbraith A, McNeil K, Jones S, Khanna R. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis 9:165–170. doi: 10.1111/j.1399-3062.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, Varma A, Deeks SG, McCune JM, Nixon DF, Sinclair E. 2003. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods 283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Maecker HT, Maino VC, Picker LJ. 2000. Immunofluorescence analysis of T-cell responses in health and disease. J Clin Immunol 20:391–399. doi: 10.1023/A:1026403724413. [DOI] [PubMed] [Google Scholar]
- 24.Abate D, Saldan A, Mengoli C, Fiscon M, Silvestre C, Fallico L, Peracchi M, Furian L, Cusinato R, Bonfante L, Rossi B, Marchini F, Sgarabotto D, Rigotti P, Palu G. 2013. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV QuantiFERON gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol 51:2501–2507. doi: 10.1128/JCM.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saldan A, Forner G, Mengoli C, Tinto D, Fallico L, Peracchi M, Gussetti N, Palu G, Abate D. 2016. Comparison of the cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV QuantiFERON cell-mediated immune assays in CMV-seropositive and -seronegative pregnant and nonpregnant women. J Clin Microbiol 54:1352–1356. doi: 10.1128/JCM.03128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries JJ, van der Eijk AA, Wolthers KC, Rusman LG, Pas SD, Molenkamp R, Claas EC, Kroes AC, Vossen AC. 2012. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 53:167–170. doi: 10.1016/j.jcv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev 15:680–715. doi: 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengoli C, Cusinato R, Biasolo MA, Cesaro S, Parolin C, Palu G. 2004. Assessment of CMV load in solid organ transplant recipients by pp65 antigenemia and real-time quantitative DNA PCR assay: correlation with pp67 RNA detection. J Med Virol 74:78–84. doi: 10.1002/jmv.20149. [DOI] [PubMed] [Google Scholar]
- 29.Abate D, Saldan A, Forner G, Tinto D, Bianchin A, Palu G. 2014. Optimization of interferon gamma ELISPOT assay to detect human cytomegalovirus specific T-cell responses in solid organ transplants. J Virol Methods 196:157–162. doi: 10.1016/j.jviromet.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Youden WJ. 1950. Index for rating diagnostic tests. Cancer 3:32–35. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Fisher S, Genbacev O, Maidji E, Pereira L. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 74:6808–6820. doi: 10.1128/JVI.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, Craig ME, Rawlinson WD. 2012. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 7:e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleiss MR, Aronow BJ, Handwerger S. 2007. Cytomegalovirus infection of human syncytiotrophoblast cells strongly interferes with expression of genes involved in placental differentiation and tissue integrity. Pediatr Res 61:565–571. doi: 10.1203/pdr.0b013e318045be6d. [DOI] [PubMed] [Google Scholar]
- 34.Scott GM, Chow SS, Craig ME, Pang CN, Hall B, Wilkins MR, Jones CA, Lloyd AR, Rawlinson WD. 2012. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J Infect Dis 205:1305–1310. doi: 10.1093/infdis/jis186. [DOI] [PubMed] [Google Scholar]
- 35.Gerna G, Lilleri D, Chiesa A, Zelini P, Furione M, Comolli G, Pellegrini C, Sarchi E, Migotto C, Bonora MR, Meloni F, Arbustini E. 2011. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant 11:2463–2471. doi: 10.1111/j.1600-6143.2011.03636.x. [DOI] [PubMed] [Google Scholar]