Abstract
We analyzed the diagnostic value of microorganisms cultured from negative-pressure-wound-therapy (NPWT) foam samples compared to that of microorganisms cultured from deep tissue samples from patients with vascular graft infections. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 58%, 86%, 81%, and 66%, respectively. The diagnostic value of microbiological cultures from NPWT foams was poor.
TEXT
To date, little is known about the interpretation of microbiological results from negative-pressure-wound-therapy (NPWT) foams despite the fact that NPWT is increasingly being used in wound care and the treatment of prosthetic vascular graft infections (PVGI) (1–15). Specimens are often taken not only from the site of infection but also from these foams with the notion that microbes on the foam might represent the bacterial flora of the adjacent wound tissue (16). We aimed to analyze the diagnostic value of NPWT foam microbiology in patients with a PVGI.
We included patients from the Vascular Graft Cohort Study (VASGRA; ClinicalTrials registration no. NCT01821664), an ongoing observational research project at the University Hospital Zurich recruiting adult patients with a PVGI since May 2013 (6, 17). Data entries until December 2015 were considered. A few retrospective patients with a PVGI and NPWT therapy were added to the analysis. The study was approved by the IEC Zürich (KEK_2012-0583). At our institution, PVGI are generally operated on in a graft-preserving manner. During revisions, patients undergo surgical debridement of infected tissue and recurring NPWT dressing changes (7).
The samples were analyzed at the Institute for Medical Microbiology, Zurich. The foam samples were inoculated onto aerobic sheep blood agar plates without antibiotics (COS; bioMérieux, Marcy l'Etoile, France) and with colistin-nalidixic acid (CNA) for Gram-positive bacteria (bioMérieux) and onto MacConkey (MCK) plates (bioMérieux) for the detection of Gram-negative bacteria. All plates were incubated for 3 days at 37°C; in addition, Sabouraud (Sab) plates for yeasts (BD, Basel, Switzerland) were inoculated and incubated for 7 days. After inoculation, the plates were put into thioglycolate broth without indicator (BD) for growth enrichment. If growth occurred within 3 days, samples were subcultured onto aerobic and anaerobic plates.
Deep wound and superficial wound swabs were inoculated onto the same battery of plates with incubation in thioglycolate broth and also on aerobic selective chocolate agar plates (HAE2; bioMérieux), anaerobic sheep blood agar plates with hemin and vitamin K1 (Brucella agar; BD), laked sheep blood Brucella agar plates with kanamycin and vancomycin (BD), and phenylethylalcohol agar plates with vitamin K1 (BD). The plates were incubated for 1 week, the thioglycolate broth was inoculated for 10 days, and the Sabouraud agar plates were incubated for 3 weeks (18). Sequence identification was performed according to standard methods, including matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) and 16S RNA gene sequencing (19, 20). Susceptibility testing was performed according to the EUCAST guidelines (19, 20).
We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) (21) of the deep wound tissue cultures compared with those for NWPT foam cultures. The results from the deep wound tissue cultures were considered the gold standard. Statistical analysis was performed with Stata SE 14 (StataCorp., TX, USA).
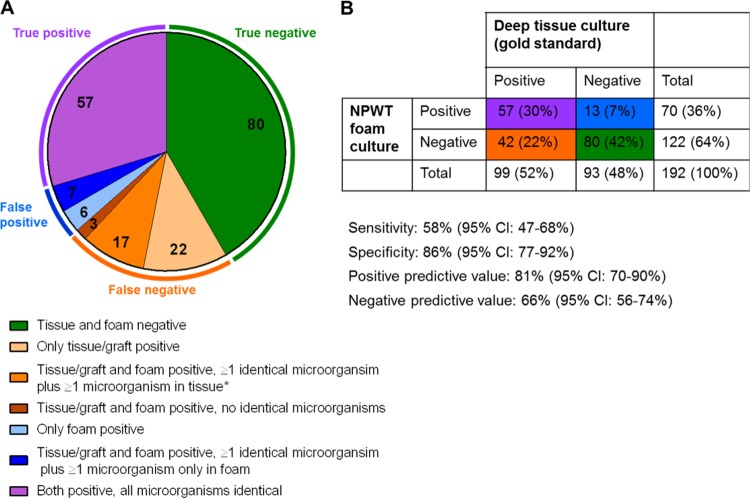
We included a total of 192 samples from 54 patients, all of whom had received NPWT (154 VASGRA samples; 38 retrospective samples). The patient characteristics and detailed information on the samples are listed in Appendix S1 in the supplemental material. Ninety of 192 (47%) foam and 107 of 192 (56%) tissue samples were positive (Table 1). The numbers of different types of microorganisms were comparable between the foam and tissue samples with a median (range) of 1 (1 to 3) and 1 (1 to 4) (P = 0.869 [t test]), respectively. In 20% and 18% wound and foam samples, respectively, multiple microorganisms were found (Pexact = 0.603). The anaerobe species tended to be poorly identified in NPWT foam samples. We obtained an overall sensitivity, specificity, PPV, and NPV of 58% (95% confidence interval [CI], 47 to 68%), 86% (77 to 92%), 81% (70 to 90%), and 66% (56 to 74%), respectively (Fig. 1).
TABLE 1.
Diagnostic value of the microbiological examination of NPWT foams compared to that of deep wound tissue samples adjacent to an infected vascular graft (gold standard)
| Organism | NPWT foams (n [%])a | Deep tissue (n [%])b | NPWT foams and deep tissue (n [%]) | None detected (n [%]) | Sensitivity (% [95% CIc]) | Specificity (% [95% CI]) | Positive predictive value (% [95% CI]) | Negative predictive value (% [95% CI]) |
|---|---|---|---|---|---|---|---|---|
| Gram-positive | ||||||||
| Coagulase-negative staphylococci | 11 (5.7) | 14 (7.3) | 33 (17.2) | 134 (69.8) | 70 (55–83) | 92 (87–96) | 75 (60–87) | 91 (85–95) |
| Corynebacteria | 0 (0.0) | 1 (0.5) | 0 (0.0) | 191 (99.5) | NAe | NA | NA | NA |
| Enterococcus spp. | 5 (2.6) | 6 (3.1) | 16 (8.3) | 165 (85.9) | 73 (50–89) | 97 (93–99) | 76 (53–92) | 96 (93–99) |
| Staphylococcus aureus | 0 (0.0) | 1 (0.5) | 2 (1.0) | 189 (98.4) | 67 (9–99) | 100 (98–100) | 100 (16–100) | 99 (97–100) |
| Streptococcus spp. | 0 (0.0) | 0 (0.0) | 1 (0.5) | 191 (99.5) | 100 (3–100) | 100 (98–100) | 100 (3–100) | 100 (98–100) |
| Gram-negative | ||||||||
| Acinetobacter baumannii | 0 (0.0) | 0 (0.0) | 1 (0.5) | 191 (99.5) | 100 (3–100) | 100 (98–100) | 100 (3–100) | 100 (98–100) |
| Citrobacter freundii | 1 (0.5) | 2 (1.0) | 4 (2.1) | 185 (96.4) | 67 (22–96) | 99 (97–100) | 80 (28–99) | 99 (96–100) |
| Enterobacter cloacae | 1 (0.5) | 1 (0.5) | 1 (0.5) | 189 (98.4) | 50 (1–99) | 99 (97–100) | 50 (1–99) | 99 (97–100) |
| Escherichia coli | 1 (0.5) | 3 (1.6) | 6 (3.1) | 182 (94.8) | 67 (30–93) | 99 (97–100) | 86 (42–100) | 98 (95–100) |
| Klebsiella spp. | 0 (0.0) | 2 (1.0) | 6 (3.1) | 184 (95.8) | 75 (35–97) | 100 (98–100) | 100 (54–100) | 99 (96–100) |
| Proteus mirabilis | 0 (0.0) | 2 (1.0) | 0 (0.0) | 190 (99.0) | NA | NA | NA | NA |
| Pseudomonas aeruginosa | 2 (1.0) | 2 (1.0) | 3 (1.6) | 185 (96.4) | 60 (15–95) | 99 (96–100) | 60 (15–95) | 99 (96–100) |
| Serratia marcescens | 0 (0.0) | 0 (0.0) | 1 (0.5) | 191 (99.5) | 100 (3–100) | 100 (98–100) | 100 (3–100) | 100 (98–100) |
| Stenotrophomonas maltophilia | 0 (0.0) | 0 (0.0) | 1 (0.5) | 191 (99.5) | 100 (3–100) | 100 (98–100) | 100 (3–100) | 100 (98–100) |
| Anaerobes | ||||||||
| Peptostreptococcus | 0 (0.0) | 2 (1.0) | 1 (0.5) | 189 (98.4) | 33 (1–91) | 100 (98–100) | 100 (3–100) | 99 (96–100) |
| Other anaerobesd | 0 (0.0) | 5 (2.6) | 0 (0.0) | 187 (97.4) | NA | NA | NA | NA |
| Fungi | ||||||||
| Candida spp. | 2 (1.0) | 7 (3.6) | 29 (15.1) | 154 (80.2) | 81 (64–92) | 99 (95–100) | 94 (79–99) | 96 (91–98) |
In addition, 2 Granulicatella adjacens and 1 Raoultella species were found in NPWT foam/graft samples (n = 3).
In addition, the following other organisms were found in deep tissue samples: 1 Aspergillus fumigatus, 3 Granulicatella adiacens, 1 Mycobacterium chimaera, and 1 Dermabacter hominis.
CI, confidence interval.
In 5 deep tissue samples the following anaerobes found: 1 Bacteroides thetaiotaomicron, 1 Finegoldia adiacens, 3 Finegoldia magna, and 3 Prevotella bivia.
NA, not applicable.
FIG 1.
(A) Number of positive and negative samples in tissue/graft culture (gold standard) and NPWT (negative pressure wound therapy) foams (total number of samples = 192). Samples were categorized based on the concordance of the detected species. If at least one microorganism species was detected only in one type of material, the sample was considered false positive or false negative, respectively. A sample was classified as false negative, if all found species were discordant. *Additionally, in 3 of 17 NPWT foam samples a nonidentical organism was found which was not detected in the tissue culture. B. Diagnostic results of NPWT foams compared to deep tissue/graft cultures.
In 19 NPWT foam samples, 26 different microorganisms which were not found in the deep tissue samples were detected. Fourteen (58%) microorganisms had been previously found in tissue/graft or blood culture samples from the respective patient, and an additional 3 (13%) have been found at a later time point (<31 days). A chart review noted that one of these three microorganisms was judged as clinically relevant (Pseudomonas aeruginosa), meaning that the detection did lead to a treatment adaption. Seven microorganisms were not detected in the adjacent wound tissue sample (see Appendix S2 in the supplemental material). We did not find any evidence for a different diagnostic agreement between the NPWT foams and deep tissue examination either for the different pressures applied to the vascular wound or for differences in the time intervals between foam changes or for the time since infection (see Appendix S3 in the supplemental material).
We found that the diagnostic value of NPWT foam culture was poor. Our study did not confirm the hypothesis that high negative pressure leads to an enrichment of microorganisms in the foam samples compared to that in wound tissue samples. Most microorganisms which were isolated from the NPWT foam samples but not from the corresponding tissue samples were identified in previous or subsequent samples.
To our knowledge, this is the first study to look at the diagnostic value of the microbiological cultures of NPWT foam samples in a patient population with PVGI. A smaller study examined 41 NPWT foam and tissue samples from an orthopedic patient population (2). For 17 of 22 negative foam samples, the tissue samples were also negative, whereas the remaining 5 tissue samples contained organisms. The authors concluded that tissue samples and parts of the foam should be examined. Another study found high bacterial loads in NPWT foam samples (1). The number of foam samples in which microorganisms were found was higher than in our study, which might be explained by the higher number of patients receiving antibiotic treatment in our study (80% versus 12%). Neither higher negative pressure nor longer intervals between foam changes or the time since infection affected the diagnostic concordance. Negative pressure levels and intervals of foam changes might be adapted to the clinical needs without compromising the diagnostic results (22).
The strength of our study is the large sample size. No published study has reported information as detailed as ours regarding sensitivity, specificity, PPV, and NPV. The small number of patients taken off antibiotic treatment (19%) might limit the generalization of the results.
The microbiological examination of NPWT foam samples had limited diagnostic value in a patient population with PVGI. Antimicrobial therapy should primarily be based on deep wound cultures. Since few other studies have addressed this issue, additional studies are needed to draw general conclusions.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to our patients for their commitment and thank B. Ruehe, C. Rüegg, A. Wolfensberger, P. Paioni, and U. Matt for excellent patient care. We also thank C. Müller and S. Bürgin as our study nurses and C. Laich and A. Thoma for administrative assistance.
The members of the VASGRA Cohort Study are G. Bloemberg, B. Hasse, L. Husmann, M. Lachat, D. Mayer, Z. Rancic, A. U. Scherrer, R. Weber, and A. S. Zinkernagel.
B.H. and D.M. designed the study. C.F. and S.F. collected patient data. A.U.S. analyzed the data. A.U.S. and B.H. wrote the first draft, and A.U.S., B.H., G.B., R.Z., A.S.Z., Z.R., and D.M. wrote the final version of the manuscript. All investigators contributed to data collection and interpretation of the data, reviewed drafts of the manuscript, and approved the final manuscript.
This study was financed within the framework of the Vascular Graft Cohort Study (VASGRA) supported by the Swiss National Science Foundation grants 320030_144277/1 and 32473B_163132/1 (to B.H.), the Vontobel Foundation (to B.H.), and the Rozalia Foundation (to B.H.).
We declare no conflicts of interest.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01102-16.
REFERENCES
- 1.Yusuf E, Jordan X, Clauss M, Borens O, Mader M, Trampuz A. 2013. High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen 21:677–681. doi: 10.1111/wrr.12088. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostakos K, Mosser P. 2012. Bacteria identification on NPWT foams: clinical relevance or contamination? J Wound Care 21:333–334, 336–339. doi: 10.12968/jowc.2012.21.7.333. [DOI] [PubMed] [Google Scholar]
- 3.Nordmyr J, Svensson S, Bjorck M, Acosta S. 2009. Vacuum assisted wound closure in patients with lower extremity arterial disease. The experience from two tertiary referral-centres. Int Angiol 28:26–31. [PubMed] [Google Scholar]
- 4.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. 1997. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Pinocy J, Albes JM, Wicke C, Ruck P, Ziemer G. 2003. Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of a polyvinyl alcohol-vacuum sponge system. Wound Repair Regen 11:104–109. doi: 10.1046/j.1524-475X.2003.11205.x. [DOI] [PubMed] [Google Scholar]
- 6.Szilagyi DE, Smith RF, Elliott JP, Vrandecic MP. 1972. Infection in arterial reconstruction with synthetic grafts. Ann Surg 176:321–333. doi: 10.1097/00000658-197209000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer D, Hasse B, Koelliker J, Enzler M, Veith FJ, Rancic Z, Lachat M. 2011. Long-term results of vascular graft and artery preserving treatment with negative pressure wound therapy in Szilagyi grade III infections justify a paradigm shift. Ann Surg 254:754–759; discussion 760. doi: 10.1097/SLA.0b013e3182365864. [DOI] [PubMed] [Google Scholar]
- 8.Berger P, de Bie D, Moll FL, de Borst GJ. 2012. Negative pressure wound therapy on exposed prosthetic vascular grafts in the groin. J Vasc Surg 56:714–720. doi: 10.1016/j.jvs.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Dosluoglu HH, Schimpf DK, Schultz R, Cherr GS. 2005. Preservation of infected and exposed vascular grafts using vacuum assisted closure without muscle flap coverage. J Vasc Surg 42:989–992. doi: 10.1016/j.jvs.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Saziye K, Afksendiyos K. 2015. The vacuum-assisted closure (V.A.C.) system for surgical site infection with involved vascular grafts. Vascular 23:144–150. doi: 10.1177/1708538114537488. [DOI] [PubMed] [Google Scholar]
- 11.Monsen C, Acosta S, Mani K, Wann-Hansson C. 2015. A randomised study of NPWT closure versus alginate dressings in peri-vascular groin infections: quality of life, pain and cost. J Wound Care 24:252, 254-256, 258–260. doi: 10.12968/jowc.2015.24.6.252. [DOI] [PubMed] [Google Scholar]
- 12.Monsen C, Wann-Hansson C, Wictorsson C, Acosta S. 2014. Vacuum-assisted wound closure versus alginate for the treatment of deep perivascular wound infections in the groin after vascular surgery. J Vasc Surg 59:145–151. doi: 10.1016/j.jvs.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 13.Borgquist O, Gustafsson L, Ingemansson R, Malmsjo M. 2010. Micro- and macromechanical effects on the wound bed of negative pressure wound therapy using gauze and foam. Ann Plast Surg 64:789–793. doi: 10.1097/SAP.0b013e3181ba578a. [DOI] [PubMed] [Google Scholar]
- 14.Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, Orgill DP. 2006. Microdeformational wound therapy: effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg 56:418–422. doi: 10.1097/01.sap.0000202831.43294.02. [DOI] [PubMed] [Google Scholar]
- 15.Chen SZ, Li J, Li XY, Xu LS. 2005. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg 28:211–217. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 16.Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. 2004. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 17.FitzGerald SF, Kelly C, Humphreys H. 2005. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother 56:996–999. doi: 10.1093/jac/dki382. [DOI] [PubMed] [Google Scholar]
- 18.Washington JA. (ed). 2012. Laboratory procedures in clinical microbiology. Springer, New York, NY. [Google Scholar]
- 19.Bosshard PP, Zbinden R, Abels S, Boddinghaus B, Altwegg M, Bottger EC. 2006. 16S rRNA gene sequencing versus the API 20 NE system and the VITEK 2 ID-GNB card for identification of nonfermenting Gram-negative bacteria in the clinical laboratory. J Clin Microbiol 44:1359–1366. doi: 10.1128/JCM.44.4.1359-1366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulthess B, Bloemberg GV, Zbinden R, Bottger EC, Hombach M. 2014. Evaluation of the Bruker MALDI Biotyper for identification of Gram-positive rods: development of a diagnostic algorithm for the clinical laboratory. J Clin Microbiol 52:1089–1097. doi: 10.1128/JCM.02399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.TDR Diagnostics Evaluation Expert Panel, Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. 2010. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 8:S17–S29. [PubMed] [Google Scholar]
- 22.Seabrook GR, Schmitt DD, Bandyk DF, Edmiston CE, Krepel CJ, Towne JB. 1990. Anastomotic femoral pseudoaneurysm: an investigation of occult infection as an etiologic factor. J Vasc Surg 11:629–634. doi: 10.1016/0741-5214(90)90207-Q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.