Abstract
Strain typing of Treponema pallidum, using the three-target enhanced classification scheme, was performed with 191 samples obtained between 2004 and 2011 in Sydney, Australia. The most common strain type was 14d/g (92/191 samples [48%]). Two new TP0548 gene types were detected (m and n). Strain type was associated with macrolide resistance and possible acquisition outside Australia.
TEXT
Over the past 15 years, many developed countries have witnessed a resurgence in syphilis notifications. In Sydney, Australia, just 16 cases of infectious syphilis were reported in 1999, compared to 745 cases in 2014, mainly among men who have sex with men (MSM) (1). Despite numerous public health and testing campaigns, this epidemic shows no signs of abating, and increasing reports of macrolide resistance create further challenges (2). An enhanced understanding of the dynamics of this epidemic is required to better inform public health responses. Molecular typing is a powerful and well-established tool for this purpose. The aims of this study were to determine, for the first time, the range of syphilis strain types circulating in Sydney, Australia, using an enhanced three-target molecular classification system for Treponema pallidum (3), and to explore the relationships between strain type, macrolide resistance, and clinical and epidemiological features.
Samples that had been collected between 2004 and 2011 and were analyzed previously for the macrolide resistance mutation A2058G (n = 353) (2) were subjected to strain typing targeting the arp, tpr, and TP0548 genes. Strain types are assigned based on the number of repeats within arp, followed by the restriction fragment length polymorphism (RFLP) pattern of a region of tpr and the sequence type of the TP0548 gene. For example, strain type 14d/f indicates 14 arp repeats, tpr pattern d, and TP0548 type f.
Amplification of arp was performed using touchdown PCR, with 1× HotStarTaq master mix (Qiagen), 200 nM 6-carboxyfluorescein (FAM)-labeled forward (ARP N1, 5′-ATCTTTGCCGTCCCGTGTGC-3′) and reverse (ARP N2, 5′-CCGAGTGGGATGGCTGCTTC-3′) primers, and 10 μl of DNA, in a total reaction volume of 50 μl. Cycling conditions were 95°C for 15 min; 13 cycles of 95°C for 30 s, 74°C for 45 s, and 72°C for 60 s; 25 cycles of 95°C for 30 s, 64°C for 45 s, and 72°C for 60 s; and a final extension at 72°C for 60 s. Amplicons were analyzed at the Flinders Genomics Facility (Flinders University, Adelaide, Australia) on a 3130xl Genetic Analyzer (Thermo Fisher Scientific), and the number of repeats within arp was determined according to peak size, as described previously (4).
The target region of tpr was amplified using nested PCR (4). First, a 2,186-bp region was amplified using 1× HotStarTaq master mix (Qiagen), 400 nM forward (B1, 5′-ACTGGCTCTGCCACACTTGA-3′) and reverse (A2, 5′-CTACCAGGAGAGGGTGACGC-3′) primers, 1 mM MgCl2, and 10 μl of DNA, in a total reaction volume of 25 μl. PCR conditions were 95°C for 15 min; 40 cycles of 94°C for 60 s, 60°C for 60 s, and 72°C for 2 min; and a final extension at 72°C for 10 min. Secondary amplification included 1× MyTaq Red Mix (Bioline), 500 nM published primers IP6 (5′-CAGGTTTTGCCGTTAAGC-3′) and IP7 (5′-AATCAAGGGAGAATACCGTC-3′), 1 mM MgCl2, and 1 μl (diluted 10−2) of the 2,186-bp product, in a total reaction volume of 25 μl. PCR conditions were 95°C for 60 s and 40 cycles of 95°C for 30 s, 60°C for 60 s, and 72°C for 2 min, resulting in a 1,836-bp amplicon. RFLP analysis was performed with 10 μl of the secondary product, after overnight digestion with MseI at 37°C and separation on a 1.5% agarose gel at 150 V for 2 h. RFLP patterns were manually reviewed and compared to previously published patterns designated a to p (5).
The TP0548 gene was amplified as described previously (3), using the primers sense 1 (5′-GCGTGGTGGTGAGTTCTTCT-3′) and antisense 1 (5′-ACGGCAGGCTAGTTGAGAAT-3′). Sequencing was performed at the Australian Genome Research Facility; sequences were analyzed using UGENE (Unipro) and compared to typing groups identified previously (a to l) (3, 6, 7).
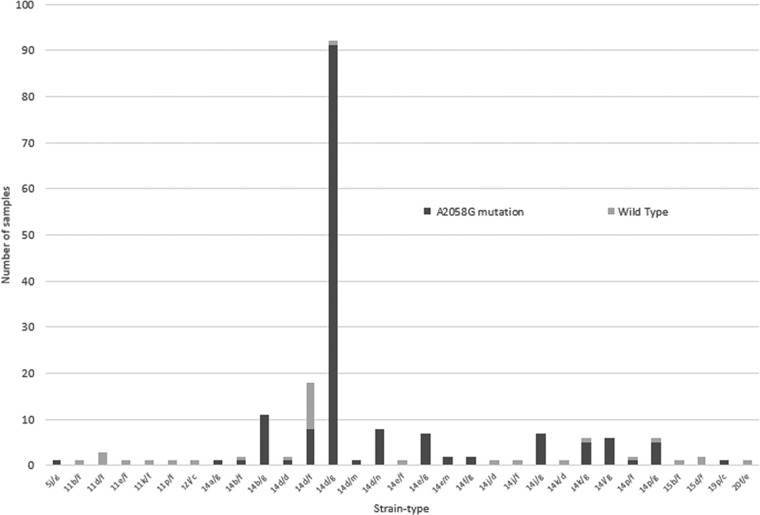
Complete strain-typing profiles were obtained for 191/353 samples (54%), representing a number of years (2004, n = 5; 2005, n = 14; 2006, n = 2; 2007, n = 24; 2008, n = 34; 2009, n = 34; 2010, n = 38; 2011, n = 40). Thirty-one individual strain types were identified, with 14d/g representing the most common in each of the study years, accounting for 92/191 samples (48%) (Fig. 1). Strain type 14d/g was also reported as the dominant strain type in London (80%) (8), Paris (69%) (6), and Denmark (53%) (9) and has been identified in Seattle (10) and San Francisco (11), all settings in which MSM represent the majority of syphilis diagnoses. Interestingly, a similar study from Melbourne, Australia (12), using the original two-target subtyping scheme, reported 14e as the most common subtype (31%), with 14d (of which strain type 14d/g is a member) representing only 16.7% of samples. This suggests different epidemics of syphilis occurring among MSM in these two Australian cities, with limited evidence of sexual mixing. Two previously unreported TP0548 gene sequences were confirmed by sequencing in both the forward and reverse directions and were designated TP0548 gene types m and n (Fig. 2).
FIG 1.
Strain types of Treponema pallidum and association with A2058G mutation.
FIG 2.
TP0548 gene sequence patterns a to l and two new patterns, m and n, identified in this study. The region of the TP0548 gene shown here spans position 131 to position 216.
Overall, 159/191 samples (83%) harbored the A2058G macrolide resistance mutation. Significantly, 91/92 samples (99%) of the dominant strain type 14d/g were macrolide resistant, compared with 68/99 non-14d/g samples (67%) (odds ratio [OR], 41.5 [95% confidence interval [CI], 5.5 to 311.5]; P < 0.001). Of strain types represented by more than 5 samples (n = 161), 148 (92%) had the A2058G mutation, compared with 11/30 strain types (37%) with less than 5 samples (OR, 19.7 [95% CI, 7.7 to 50.0]; P < 0.001). This suggests an association between the success of a strain type and the acquisition of macrolide resistance (Fig. 1).
Clinical data (gender, age at diagnosis, history of syphilis, gender of sexual partners, stage of syphilis, aboriginality, HIV status, and sex during overseas travel within the past year) were available for 91/191 samples (48%), originating from four clinics (Taylor Square Private Clinic, East Sydney Doctors, Holdsworth House Medical Practice, and Sydney Sexual Health Centre). Ninety of these samples were from men, 78 (87%) of whom reported sex with other men. No patients reported being of aboriginal or Torres Strait Islander background. The median age at diagnosis was 39 years (range, 21 to 63 years), and 64 samples (70%) were from primary syphilis lesions, with the remainder being from secondary lesions.
There was no association of HIV status with strain type, i.e., 20/41 strains of type 14d/g (49%) were from HIV-positive patients, compared with 23/50 non-14d/g strains (46%) (P = 0.79). This lack of association with HIV status was also apparent in Melbourne (12), suggesting that, for men acquiring syphilis in Australia, there is significant sexual mixing between HIV-positive and HIV-negative men. There was, however, an association of strain types with probable overseas acquisition. Just 2/41 patients (5%) with 14d/g strains reported sex overseas, compared with 11/50 patients (22%) with non-14d/g strains (OR, 5.5 [95% CI, 1.1 to 26.5]; P = 0.033).
Two cases of secondary syphilis had a presumptive diagnosis of neurosyphilis (positive cerebrospinal fluid Venereal Disease Research Laboratory [VDRL] test result and severe headache), and both were assigned strain type 14d/f. Two of 6 patients with strain type 14d/f developed neurosyphilis, compared with 0/85 with non-14d/f strains (P = 0.005). This strain type was associated previously with a higher probability of neurosyphilis (3) and in our analysis the association reached statistical significance, but the very small number of cases requires cautious interpretation.
The limitations of this study include those associated with retrospective analyses, such as degradation of stored samples and inconsistent referral of patient samples to the diagnostic laboratory. However, the study sample is reflective of the broader syphilis epidemic among MSM in Sydney. Another limitation was the reliance on self-reported sexual activity and travel experience, although this is common to similar studies.
This study reports the first three-target strain-typing data for T. pallidum samples collected from an Australian population and demonstrates a wide diversity of strain types. The local epidemiology appears to be dominated by MSM with a few strains associated with macrolide resistance mutations, with sporadic importation of macrolide-sensitive strains. Further investigation is required to determine how the Sydney epidemic relates to other parts of Australia and the Asia Pacific region.
ACKNOWLEDGMENTS
We thank Allan Pillay for technical advice on the strain-typing assay, Hossinur Rahman for technical assistance, and the staff members at Holdsworth House Medical Practice, Taylor Square Private Clinic, East Sydney Doctors, and Sydney Sexual Health Centre for help accessing clinical information.
Laboratory costs in this study were partly funded by a RACP Novartis research fellowship grant. Neither the RACP nor Novartis hand any role in study design, conduct, analysis, or reporting.
None of the authors has any potential conflicts of interest to declare.
REFERENCES
- 1.The Kirby Institute. 2015. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2015. The Kirby Institute, UNSW Australia, Sydney, Australia. [Google Scholar]
- 2.Read P, Jeoffreys N, Tagg K, Guy R, Gilbert G, Donovan B. 2014. Azithromycin-resistant syphilis-causing strains in Sydney: prevalence and risk factors. J Clin Microbiol 52:2776–2781. doi: 10.1128/JCM.00301-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra C, Sahi S, Tantalo L, Godornes C, Reid T, Behets F, Rompalo A, Klausner JD, Yin Y, Mulcahy F, Golden MR, Centurion-Lara A, Lukehart SA. 2010. Enhanced molecular typing of Treponema pallidum: geographical distribution of strain types and association with neurosyphilis. J Infect Dis 202:1380–1388. doi: 10.1086/656533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillay A, Liu H, Chen C, Holloway B, Sturm W, Steiner B, Morse SA. 1998. Molecular subtyping of Treponema pallidum subspecies pallidum. Sex Transm Dis 25:408–414. doi: 10.1097/00007435-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sena A, Pillay A, Cox DL, Radolf JD. 2015. Treponema and Brachyspira, human host-associated spirochetes, p 1055–1081. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 6.Grange P, Allix-Beguec C, Chanal J, Benhaddou N, Gerhardt P, Morini JP, Deleuze J, Lassau F, Janier M, Dupin N. 2013. Molecular subtyping of Treponema pallidum in Paris, France. Sex Transm Dis 40:641–644. doi: 10.1097/OLQ.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 7.Grillova L, Strouhal M, Mikalova L, Smajs D. 2015. The 2 simultaneously published “k” sequence variants of tp0548 locus of Treponema pallidum ssp. pallidum isolates differ: the one published later has to be renamed as “l”. Sex Transm Dis 42:53. doi: 10.1097/OLQ.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 8.Tipple C, McClure M, Taylor GP. 2011. High prevalence of macrolide resistant Treponema pallidum strains in a London centre. Sex Transm Infect 87:486–488. doi: 10.1136/sextrans-2011-050082. [DOI] [PubMed] [Google Scholar]
- 9.Salado-Rasmussen K, Cowan S, Gerstoft J, Larsen HK, Hoffmann S, Knudsen TB, Katzenstein TL, Jensen JS. 2016. Molecular typing of Treponema pallidum in Denmark: a nationwide study of syphilis. Acta Derm Venereol 96:202–206. doi: 10.2340/00015555-2190. [DOI] [PubMed] [Google Scholar]
- 10.Grimes M, Sahi SK, Godornes BC, Tantalo LC, Roberts N, Bostick D, Marra CM, Lukehart SA. 2012. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex Transm Dis 39:954–958. doi: 10.1097/OLQ.0b013e31826ae7a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillay A, Chen C, Morshed M, Philip S, Ballard R. 2011. P4-S3.02: subtyping of Treponema pallidum strains by sequence analysis of tp0279 and tp0548. Sex Transm Infect 87(Suppl 1):A312. doi: 10.1136/sextrans-2011-050108.517. [DOI] [Google Scholar]
- 12.Azzato F, Ryan N, Fyfe J, Leslie D. 2012. Molecular subtyping of Treponema pallidum during a local syphilis epidemic in men who have sex with men in Melbourne, Australia. J Clin Microbiol 50:1895–1899. doi: 10.1128/JCM.00083-12. [DOI] [PMC free article] [PubMed] [Google Scholar]