Abstract
GP5+/6+-based PCR followed by reverse line blot hybridization (GP5+/6+RLB) and multiplex type-specific PCR (E7-MPG) are two human papillomavirus (HPV) genotyping methodologies widely applied in epidemiological research. We investigated their relative analytical performance in 4,662 samples derived from five studies in Bhutan, Rwanda, and Mongolia coordinated by the International Agency for Research on Cancer (IARC). A total of 630 samples were positive by E7-MPG only (13.5%), 24 were positive by GP5+/6+RLB only (0.5%), and 1,014 were positive (21.8%) by both methods. Ratios of HPV type-specific positivity of the two tests (E7-MPG:GP5+/6+RLB ratio) were calculated among 1,668 samples that were HPV positive by one or both tests. E7-MPG:GP5+/6+RLB ratios were >1 for all types and highly reproducible across populations and sample types. E7-MPG:GP5+/6+RLB ratios were highest for HPV53 (7.5) and HPV68 (7.1). HPV16 (1.6) and HPV18 (1.7) had lower than average E7-MPG:GP5+/6+RLB ratios. Among E7-MPG positive infections, median mean fluorescence intensity (MFI; a semiquantitative measure of viral load) tended to be higher among samples positive for the same virus type by GP5+/6+RLB than for those negative for the same type by GP5+/6+RLB. Exceptions, however, included HPV53, -59, and -82, for which the chances of being undetected by GP5+/6+RLB appeared to be MFI independent. Furthermore, the probability of detecting an additional type by E7-MPG was higher when another type was already detected by GP5+/6+RLB, suggesting the existence of masking effects due to competition for GP5+/6+ PCR primers. In conclusion, this analysis is not an evaluation of clinical performance but may inform choices for HPV genotyping methods in epidemiological studies, when the relative merits and dangers of sensitivity versus specificity for individual types should be considered, as well as the potential to unmask nonvaccine types following HPV vaccination.
INTRODUCTION
Human papillomavirus (HPV) DNA detection and genotyping are central to molecular epidemiological studies of HPV natural history and carcinogenicity, as well as to evaluations of HPV vaccine efficacy and effectiveness. There are various PCR-based methods for HPV DNA detection and genotyping, of which one of the most commonly used in epidemiological studies is the GP5+/6+ primer set targeting conserved sequences within the L1 gene, thus allowing detection of a broad range of mucosal HPV types in a single reaction. The HPV genotype can subsequently be identified by various methods, of which the most commonly used is hybridization with type-specific probes using a reverse line blot hybridization assay (henceforth referred to as GP5+/6+RLB) (1). The GP5/6 primers were initially developed to maximize detection of HPV6, -11, -16, -18, -31, and -33 (2) and were subsequently elongated at their 3′ ends to generate the primers GP5+ and GP6+ to improve the detection of a broader range of HPV types (3). This methodology has been widely validated against clinical outcomes in large population-based screening programs and shows favorable clinical performance in comparison to cervical cytology (4, 5).
More recently, other assays have been developed to increase analytical sensitivity for detection of HPV DNA, including a Luminex bead-based assay for the genotyping of products from the multiplex type-specific E7 PCR (henceforth referred to as E7-MPG). E7-MPG detects DNA from 21 HPV types, has been validated for analytical sensitivity in a global proficiency study (6), and has been shown to be more sensitive than most other broad-spectrum primer-based PCR methods in detecting low viral copy numbers and multiple infections (7). Increased detection of multiple infections has been partly attributed to overcoming the masking effects of competition for consensus primers by the use of HPV type-specific primers (7). E7-MPG was neither designed nor validated for cervical cancer screening. However, the balance of analytical sensitivity versus specificity remains an important question for epidemiological studies, most notably those monitoring HPV vaccination efficacy (8, 9).
MATERIALS AND METHODS
Populations.
Samples reported here arise from five different studies coordinated by the International Agency for Research on Cancer (IARC) (Table 1).
TABLE 1.
Description of study populations and concordance of positivity for 21 HPV types by E7-MPG and GP5+/6+RLB testing
| Country | Sample type | Median subject age (5%–95% range [yr]) | Na | No. (%) of samples by E7-MPG and GP5+/6+RLB resultsb |
|||
|---|---|---|---|---|---|---|---|
| +/+ | +/− | −/+ | −/− | ||||
| Bhutan | Cells | 22 (19–24) | 884 | 207 (23.4) | 107 (12.1) | 6 (0.7) | 564 (63.8) |
| Rwanda | Cells | 22 (18–24) | 1,169 | 380 (32.5) | 189 (16.2) | 7 (0.6) | 593 (50.7) |
| Mongolia | Cells | 34 (21–54) | 635 | 264 (41.6) | 177 (27.9) | 5 (0.8) | 189 (29.8) |
| Bhutan | Urine | 19 (18–38) | 1,062 | 87 (8.2) | 77 (7.3) | 5 (0.5) | 893 (84.1) |
| Rwanda | Urine | 19 (17–20) | 912 | 76 (8.3) | 80 (8.8) | 1 (0.1) | 755 (82.8) |
| Total | 4,662 | 1,014 (21.8) | 630 (13.5) | 24 (0.5) | 2,994 (64.2) | ||
N, number of samples.
+/+, positive by both tests; +/−, positive only by E7-MPG; −/+, positive only by GP5+/6+RLB; −/−, negative by both tests.
(i) Exfoliated cell samples from 884 women from Bhutan.
During a population-based survey in 2011 to 2012, 2,505 women aged 18 to 69 years were invited for the collection of exfoliated cervical cells in PreservCyt medium for HPV testing with GP5+/6+RLB (10). Women aged 18 to 24 years were systematically (884 of 885) tested with E7-MPG. Women were sexually active, and 97.2% were unvaccinated for HPV.
(ii) Exfoliated cell samples from 1,169 women from Rwanda.
During a population-based survey in 2013 to 2014, 2,508 women aged 18 to 69 years were invited for the collection of exfoliated cervical cells in PreservCyt medium for HPV testing with GP5+/6+RLB (11). Women aged 18 to 24 years were systematically (1,169 of 1,179) tested with E7-MPG. Women were sexually active, and 97.2% were unvaccinated for HPV.
(iii) Exfoliated cell samples from 635 women from Mongolia.
During a population-based survey in 2005, 969 women aged 18 to 69 years were invited for the collection of exfoliated cervical cells in PreservCyt medium for HPV testing with GP5+/6+RLB (12). Almost all GP5+/6+RLB-positive women (321 of 339) and a random selection of GP5+/6+RLB-negative women (314 of 630) were also tested with E7-MPG (7). All women were sexually active and unvaccinated.
(iv) Urine samples from 1,062 women from Bhutan.
In 2013, 973 high school students self-collected a urine sample using a device designed to collect first-void urine immediately into conservation medium (13). A total of 871 students (90%) reported to be virgins, and 896 (92%) reported having been vaccinated against HPV6/-11/-16/-18 (8). A further 89 urine samples were obtained from Bhutanese women aged 30 years or more in the Bhutan cervical cell survey described above approximately 18 months after an initial diagnosis of GP5+/6+RLB-positive normal cytology. All samples were tested with both GP5+/6+RLB and E7-MPG.
(v) Urine samples from 912 women from Rwanda.
In 2013 to 2014, 912 high school students self-collected a urine sample as described above. A total of 720 students (79%) reported to be virgins, and 393 (43%) reported having been vaccinated against HPV6/-11/-16/-18 (8). All samples were tested with both GP5+/6+RLB and E7-MPG.
All included studies had approval from both the local Research Ethical Boards (in Bhutan, Rwanda, and Mongolia) and the IARC Ethics Committee.
DNA extraction.
For exfoliated cell samples, DNA was extracted from PreservCyt samples at the Department of Pathology, VU University Medical Center, Amsterdam, using magnetic beads (Macherey-Nagel) on a robotic system (Hamilton Robotics) according to the manufacturer's instructions.
For urine samples, DNA was extracted at the Centre for the Evaluation of Vaccination, University of Antwerp, Belgium, using a protocol that first concentrated DNA by centrifugation in an Amicon Ultra-4 50K (50,000 nominal molecular weight limit) filter device (Merck Millipore, Belgium). Subsequently DNA was lysed with NucliSENS lysis buffer (bioMérieux, Benelux) and extracted using a generic easyMAG off-board lysis protocol (reported in detail in reference 8).
HPV DNA detection and genotyping.
HPV detection and genotyping of all DNA samples were first performed by GP5+/6+RLB at the Department of Pathology, VU University Medical Center, Amsterdam. Beta-globin PCR analysis was conducted to confirm human DNA in all specimens (14). Presence of HPV DNA was determined by conducting a general primer GP5+/6+-based PCR, which permits the detection of a broad spectrum of genital HPV types (14, 15). HPV positivity was assessed by hybridization of PCR products in an enzyme immunoassay with two oligonucleotide probe cocktails that, together, detect 44 mucosal HPV types: 6, 11, 16, 18, 26, 30, 31, 32, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 61, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 85, 86, 89, and 90. Subsequent HPV genotyping was conducted by reverse line blot hybridization of GP5+/6+ PCR products (1).
E7-MPG was performed using a Luminex bead-based platform (7), either at the Group of Infections and Cancer Biology, International Agency for Research on Cancer, in Lyon, France (for Bhutan and Rwandan samples), or at the German Cancer Research Center (DKFZ) in Heidelberg, Germany (for Mongolian samples). The assay detected DNA from 21 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, and 82), except for Mongolia, where types 6 and 11 were not included. PCRs were performed with a Qiagen multiplex PCR kit according to the instructions of the manufacturer (16), and beta-globin primers were included to control DNA quality. Results are expressed as the median of the mean fluorescence intensity (MFI) of at least 100 beads per set. MFI values reflect a semiquantitative measure of the number of copies of target DNA in the sample. For each probe, the cutoff for positivity was computed as described previously (7, 17).
Statistical analyses.
Type-specific positivity for E7-MPG and GP5+/6+RLB were compared graphically, and type-specific positivity ratios were calculated (E7-MPG:GP5+/6+RLB ratio) only for women who were HPV positive according to either or both tests; women who were HPV negative by both tests (doubly negative) were excluded, but their inclusion would not have changed E7-MPG:GP5+/6+RLB ratios. In addition, HPV positivity refers only to positivity for the 21 HPV types in common to both HPV genotyping methods (HPVs 6, 11, 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, and 82), whereas other HPV types detected by GP5+/6+RLB were ignored in this analysis. Ninety-five percent confidence intervals (CIs) of ratios were computed assuming a binomial distribution. Median MFI values were compared for each HPV by a Wilcoxon-Mann-Whitney test, adjusted for study.
To aid comparisons between tests and between populations, graphs of type-specific positivity include two lines. On the figures, a solid line represents a theoretical scenario where both tests detect types equally, which corresponds to an E7-MPG:GP5+/6+RLB ratio of 1. A dotted line represents the slope of a linear regression passing through the origin and the 21 type-specific points, hence representing the average E7-MPG:GP5+/6+RLB positivity ratio. The strength of the association of this linear regression was assessed by using the coefficient of determination, R2, or explained variation.
Log-binomial regression was used to model positivity for an additional HPV type by E7-MPG given the GP5+/6+RLB positivity status, adjusted for study-only or for lifetime number of sexual partners (0, 1, 2, or 3+). In this analysis (which concerns the issue of the potential masking of multiple infections), the definition of GP5+/6+RLB positive inevitably includes also positivity for other HPV types detected by GP5+/6+RLB.
RESULTS
A total of 4,662 adequate (i.e., beta-globin-positive) samples were tested with both GP5+/6+RLB and E7-MPG and included in the following analyses (Table 1). With respect to the 21 HPV types detected by both tests, 1,668 samples were HPV positive (35.8%), including 630 positive by E7-MPG only (13.5%), 24 positive by GP5+/6+RLB only (0.5%), and 1,014 (21.8%) positive by both tests (Table 1). HPV positivity was hence 35.3% by E7-MPG (of which 49.3% were multiple infections) and 22.3% by GP5+/6+RLB (of which 32.9% were multiple infections). Type-specific prevalence stratified by single versus multiple infections is shown separately by both tests in Fig. S1 in the supplemental material.
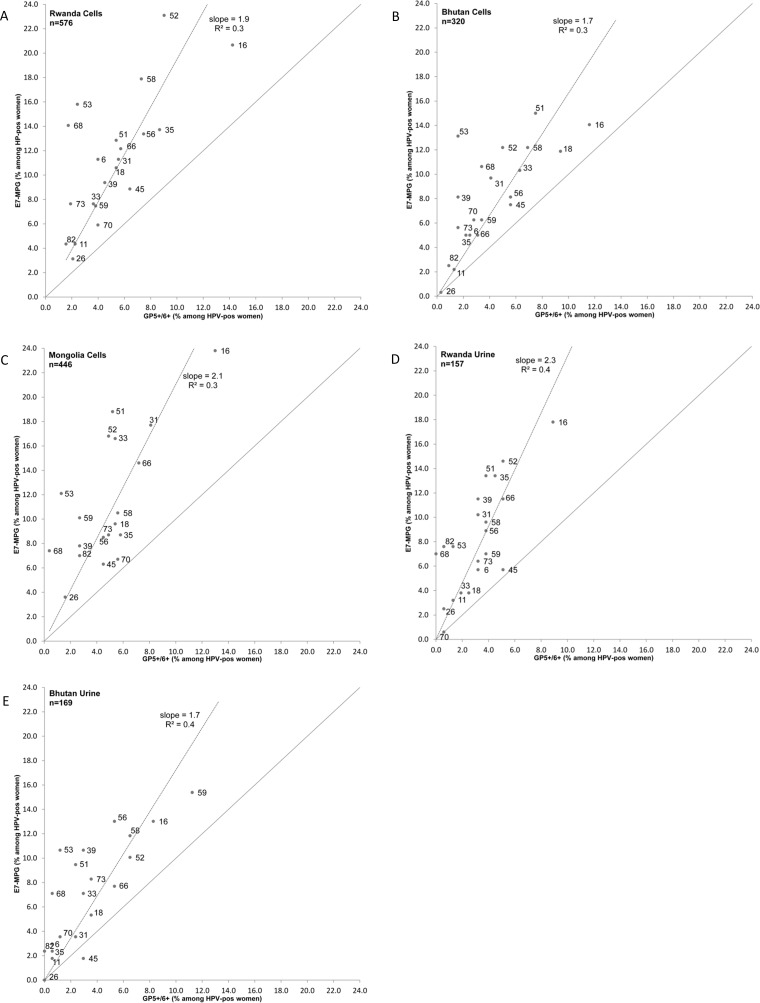
Figure 1A to E compare type-specific positivity by E7-MPG and GP5+/6+RLB, separately for each of the five studies, for women who were HPV positive for any of the 21 types by either test. E7-MPG:GP5+/6+RLB ratios were systematically above 1 for all HPV types and in all studies (with the one exception of HPV45 in Bhutan urine samples). The average E7-MPG:GP5+/6+RLB ratio across the 21 HPVs (i.e., the slope of the linear regression line) varied between 1.7 in Bhutan cell and urine samples to 2.3 in Rwandan urine samples. R2 values of these regression lines were all ≤0.4, and such poor fits suggest that E7-MPG:GP5+/6+RLB ratios differ by HPV type. Despite the differences between the studies in terms of the importance of HPV types relative to each other (e.g., high prevalence of HPV35 in Rwanda and of HPV31 in Mongolia), highly reproducible type-specific excesses for E7-MPG positivity in comparison to GP5+/6+RLB have been observed across all five studies. Most clearly, HPV53 and HPV68 showed high E7-MPG:GP5+/6+RLB ratios in all studies, while HPV16 and HPV45 showed consistently low ratios in all studies.
FIG 1.
Comparisons of type-specific positivity by E7-MPG and GP5+/6+RLB, separately for five study populations. For Mongolia, HPV6 and HPV11 are not included.
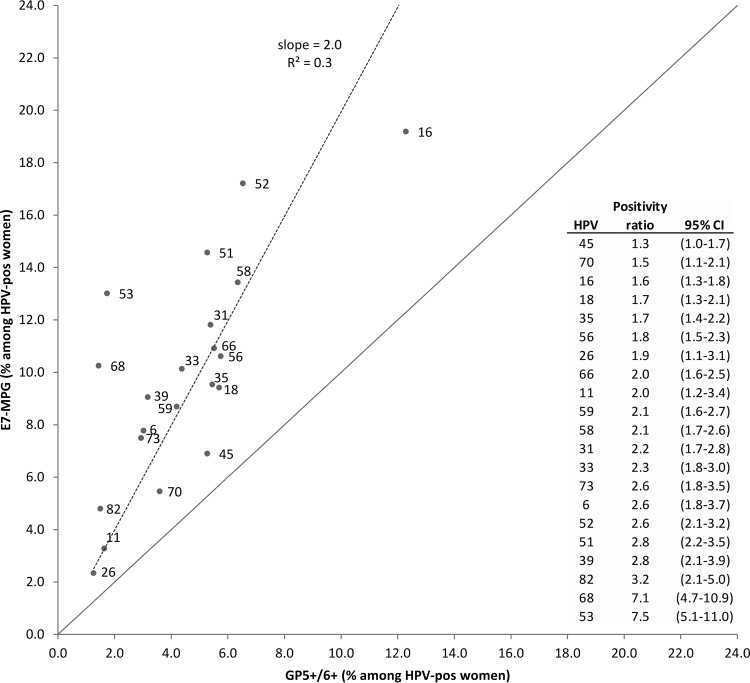
Given the strong reproducibility of type-specific E7-MPG:GP5+/6+RLB ratios across studies, pooled type-specific positivity by E7-MPG and GP5+/6+RLB was shown among all 1,668 HPV-positive women, as demonstrated by the data in Fig. 2. E7-MPG:GP5+/6+RLB ratios were 2.0 on average (i.e., slope of regression line). E7-MPG:GP5+/6+RLB ratios were all above 1, with 95% CIs excluding unity for all types with the exception of HPV45 (1.3; 95% CI, 1.0 to 1.7), and the ratios were highest for HPV53 (7.5), HPV68 (7.1), HPV82 (3.2), HPV39 (2.8), and HPV51 (2.8). HPV16 (1.6) and HPV18 (1.7) showed lower than average E7-MPG:GP5+/6+RLB ratios.
FIG 2.
Comparison of type-specific positivity by E7-MPG and GP5+/6+RLB for pooled results of the five study populations.
Median MFI values of infections detected by E7-MPG are shown in Table 2, compared by whether samples were positive or negative for the same HPV type by GP5+/6+RLB. For most HPV types, median MFI values were significantly higher among samples positive for the same type by GP5+/6+RLB than among those that were negative, suggesting that the chance of an infection being undetected by GP5+/6+RLB is related to a low viral DNA copy number. Notable exceptions were HPV53, -59, and -82, for which the chances of being missed by GP5+/6+RLB appeared to be independent of the MFI.
TABLE 2.
Median MFI values of E7-MPG-positive results compared by presence and absence of the same type detected by GP5+/6+RLB
| HPV type | E7-MPG positivea |
E7-MPG negative and GP5+/6+RLB positive (N) | ||||
|---|---|---|---|---|---|---|
| GP5+/6+RLB negativea |
GP5+/6+RLB positivea |
Wilcoxon P valueb | ||||
| Nc | Median E7-MPG MFI (IQd) | N | Median E7-MPG MFI (IQ) | |||
| HPV6 | 59 | 42 (20–83) | 36 | 156 (90–273) | <0.001 | 1 |
| HPV11 | 21 | 668 (157–964) | 19 | 1033 (733–1531) | 0.021 | 1 |
| HPV16 | 134 | 93 (11–438) | 186 | 307 (188–495) | <0.001 | 19 |
| HPV18 | 64 | 374 (55–793) | 93 | 1118 (720–1602) | <0.001 | 2 |
| HPV26 | 20 | 222 (21–491) | 19 | 403 (13–651) | 0.683 | 2 |
| HPV31 | 108 | 431 (312–693) | 89 | 624 (428–793) | <0.001 | 1 |
| HPV33 | 97 | 95 (5–392) | 72 | 335 (183–397) | 0.001 | 1 |
| HPV35 | 69 | 184 (104–277) | 90 | 282 (210–399) | <0.001 | 1 |
| HPV39 | 99 | 100 (41–218) | 52 | 386 (255–483) | <0.001 | 1 |
| HPV45 | 40 | 64 (38–140) | 75 | 203 (125–379) | <0.001 | 13 |
| HPV51 | 164 | 171 (90–251) | 79 | 201 (116–331) | <0.001 | 9 |
| HPV52 | 190 | 118 (40–204) | 97 | 135 (86–201) | 0.148 | 12 |
| HPV53 | 190 | 74 (37–209) | 27 | 80 (23–171) | 0.411 | 2 |
| HPV56 | 84 | 120 (32–302) | 93 | 447 (313–577) | <0.001 | 3 |
| HPV58 | 120 | 207 (78–405) | 104 | 289 (154–504) | <0.001 | 2 |
| HPV59 | 79 | 83 (52–129) | 66 | 88 (43–150) | 0.182 | 4 |
| HPV66 | 94 | 65 (14–142) | 88 | 228 (130–434) | <0.001 | 4 |
| HPV68 | 148 | 236 (48–564) | 23 | 903 (539–1329) | <0.001 | 1 |
| HPV70 | 38 | 66 (21–108) | 53 | 118 (46–244) | 0.005 | 7 |
| HPV73 | 79 | 162 (33–384) | 46 | 245 (110–567) | 0.007 | 3 |
| HPV82 | 62 | 280 (98–447) | 18 | 318 (243–382) | 0.791 | 7 |
Positive or negative for the specific HPV type in question.
Adjusted for study.
N, number of samples.
IQ, 25 to 75% interquartile interval.
In order to investigate potential masking of HPV types by competition for GP5+/6+ PCR primers in multiple infections, we investigated the probability of detecting at least one additional type by the E7-MPG test by GP5+/6+RLB status. Detection of additional type(s) by E7-MPG was significantly greater (P < 0.001) among GP5+/6+RLB-positive (690 of 1,342; 51.4%) than GP5+/6+RLB-negative (492 of 3,320; 14.8%) women (Table 3). The difference remained highly statistically significant (P < 0.001), even after adjustment for lifetime number of sexual partners in an attempt to account for the tendency of HPV types that share a sexual exposure to cluster in the same high-risk women.
TABLE 3.
GP5+/6+ RLB result and detection of at least one additional HPV type by E7-MPG
| GP5+/6+RLB resulta | Total no. of samples | No. (%) of samples with detection of at least one additional type by E7-MPG | P valueb |
|---|---|---|---|
| Negative | 3,320 | 492 (14.8) | <0.001 |
| Positive | 1,342 | 690 (51.4) |
Any of the 44 HPV types detected by GP5+/6+.
Adjusted for study (but the P value remained <0.001 in a model additionally adjusted for lifetime sexual partners).
DISCUSSION
We describe a novel approach to comparing the type-specific analytical performance of two widely used HPV DNA detection and genotyping methodologies. This was made possible by testing a large number of clinical samples, a large proportion or which were HPV positive, with the two methods. We confirmed previous smaller studies (7, 10) showing that E7-MPG is more analytically sensitive than GP5+/6+RLB while rarely not detecting GP5+/6+RLB-positive women. However, we went on to establish that the relative sensitivity of the two tests is highly HPV type specific and that these type-specific differences are remarkably consistent across studies and populations.
Highest E7 MPG:GP5+/6+RLB ratios were seen for HPV53 (7.5) and HPV68 (7.1), corroborating previous findings of GP5+/6+ PCR-related deficiencies in detecting these types. GP5+/6+ primers have long been known to be deficient in detection of HPV53 (18, 19), and a series of meta-analyses of global HPV type distribution (20–22) has always considered HPV53 to be undetectable by GP5+/6+ PCR. Underdetection of HPV68 has been reported for GP5+/6+ PCR in two independent comparisons against another HPV genotyping method, Papillocheck (23, 24), and for a modified GP5+/6+ PCR assay (25), which was thought to be related to a deficiency in detecting one of two HPV68 subtypes (23).
Schmitt et al. attempted to improve the suboptimal alignment of GP5+/6+ primers by developing additional broad-spectrum (BS) primers targeting the GP5+/6+ region (17). Types for which BSGP5+/6+ most improved detection over GP5+/6+ were HPV68 (BSGP5+/6+:GP5+/6+ ratio, 29.3) and HPV53 (15.1), followed by HPV82 (6.0), HPV52 (4.9), HPV51 (3.5), and HPV39 (2.4). These types closely match those that showed the greatest E7-MPG:GP5+/6+RLB ratios in the present study. The evaluation by Schmitt et al. was undertaken in a set of Mongolian women that overlap those represented in the present study, but independent studies in Bhutan and Rwanda showed the same finding and in both urine and cell samples.
HPV16 and HPV18 showed smaller than average differences between the two assays (ratios of 1.6 and 1.7, respectively), likely related to the fact that GP5+/6+ primers were originally designed to optimize the detection of these two potent carcinogens. The smallest difference (1.3) was observed for HPV45, and this may represent the minimum inherent difference in sensitivity between the two test technologies. These results confirm an increase in the analytical sensitivity of E7-MPG versus GP5+/6+RLB, probably combined with differences in resolution of the genotyping platforms used (i.e., visual confirmation of a positive spot for GP5+/6+RLB versus automated semiquantitative MFI output for E7-MPG).
For most HPV types, infections undetected by GP5+/6+ RLB were associated with low viral copy numbers (as measured by median MFI values), with the exception of some of those identified above with suboptimal PCR primer alignment (e.g., HPV53 and HPV82), as well as HPV59. As a general rule for any given type, the higher the E7-MPG:GP5+/6+RLB ratio, the stronger the relationship between infections undetected by GP5+/6+RLB and viral copy number. While HPV16 and -18 infections not detected by GP5+/6+ have previously been shown to be strongly related to low viral copy numbers (26), the relatively weak relationship to viral load for undetected HPV52 infections appears inconsistent with previous data from the Netherlands (26). This suggests that GP5+/6+ might be particularly insensitive to amplify HPV52 variants that are overrepresented outside Europe (27).
The probability of detecting an additional type by E7-MPG was higher when another type was already detected by GP5+/6+RLB. This phenomenon could, at least partly, represent the natural tendency of HPV types that share a sexual exposure to cluster in the same high-risk women (28). Although the lifetime number of partners was indeed a strong predictor of detecting an additional type by E7-MPG, adjustment for sexual behavior could not eliminate the strongly significant effect of the presence of another type detected by GP5+/6+RLB, suggesting that increased detection of multiple infections by E7-MPG is at least in part due to overcoming the masking effects of competition for consensus GP5+/6+ primers (7), a phenomena that has been described also for other consensus versus type-specific PCR primers (29). Even though there may be some lower-level masking of multiple infections even for type-specific PCR assays such as E7-MPG, due to competition for reagents (e.g., deoxynucleoside triphosphates [dNTPs], enzymes, and cofactors), the effect of eliminating vaccine types on the unmasking of other types needs to be considered in the choice of an HPV genotyping assay for monitoring HPV vaccine impact.
Discordant samples were strongly tended to be E7 MPG positive–GP5+/6+RLB negative for all types. Nevertheless, there were also a small number of GP5+/6+RLB-positive–E7-MPG-negative samples, most notably for HPV16 (n = 19) and HPV45 (n = 13) (Table 2). Upon attempts to sequence the rare E7-MPG-negative–GP5+/6+RLB-positive infections to explore for strange variants, however, we were mostly unable to reamplify DNA of the given type, suggesting poor-quality samples.
The consistency of type-specific E7-MPG:GP5+/6+RLB ratios across different populations and across different sample types provides confidence that findings can be extrapolated to other studies. Hence, it appears that the expected type-specific prevalence of one of the two tests can be reasonably estimated from the observed type-specific HPV prevalence of the other at the level of the general population. In contrast, however, given the features of suboptimal alignment and competition between HPV types for consensus GP5+/6+ PCR primers, simply applying higher MFI cutoffs to E7-MPG-positive samples would not allow expected GP5+/6+RLB positivity to be estimated at the level of the individual woman. Indeed, while the semiquantitative nature of E7-MPG enabled us to show that E7-MPG infections undetected by GP5+/6+RLB tended to reflect those with lower MFIs, the degree to which type-specific MFI values reflect true copy numbers of viral DNA is not known as we did not perform gold-standard real-time PCR.
While the higher analytical sensitivity of E7-MPG than that of GP5+/6+RLB is important to bear in mind when epidemiological studies are being compared or designed, it is likely to have an unfavorable impact on clinical performance in screening programs, which involve a balance between sensitivity to detect/predict cervical (pre)cancer (i.e., CIN2+) and specificity to avoid unnecessary interventions (30). Indeed, GP5+/6+-based PCR assays for 14 high-risk types have been widely validated against clinical outcomes in large population-based screening programs and show improved clinical performance in comparison to cytology (4, 5) and similar (23, 24) or better (26) clinical performance than other HPV tests. E7-MPG, on the other hand, has not been clinically evaluated and, indeed, was not originally designed for population-level screening.
Whether high analytical sensitivity of HPV testing is an advantage or a danger for certain epidemiological study designs, in particular for monitoring of HPV vaccines, remains unclear (8, 31). Among the urine samples reported here, estimates of vaccine effectiveness against HPV6/-11/-16/-18 in Rwanda and Bhutan were lower with E7-MPG than with GP5+/6+RLB (8), possibly due to increased detection of low-level HPV DNA that may have no clinical significance. On the other hand, tests with high analytical sensitivity produce a larger sample size of HPV-positive women to study, and these may eventually show that, in the long term, HPV vaccination programs with high coverage eradicate transmission even of these low-level infections. Both tests will continue to be used in parallel in the long-term monitoring of vaccine impact in Bhutan and Rwanda.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the technicians of the Molecular Pathology Unit of the Department of Pathology, VU University Medical Center, Amsterdam, and at the University of Antwerp for excellent technical assistance. We also thank Iacopo Baussano for his contribution to the study protocols in Bhutan and Rwanda.
G.M.C. and S.F. conceived the study. U.T., M.C.U., and B.D. were the principal investigators responsible for the collection of samples in Bhutan, Rwanda, and Mongolia, respectively. S.V. and V.T. performed data management and statistical analyses. P.J.F.S. and D.A.M.H. were responsible for GP5+/6+ HPV testing. T.G. and M.T. were responsible for E7-MPG testing. A.V. was responsible for protocols of urine collection and subsequent DNA extraction. G.M.C. wrote the first draft of the manuscript. All authors were involved in the interpretation of the analyses and gave input to the final manuscript.
P.J.F.S. has honoraria from the Speakers Bureau from Roche, Gen-Probe, Qiagen, Abbott, and Seegene and has a minority stake in Self-screen. D.A.M.H. has been on the speaker's bureau of Hologic/Gen-Probe, serves occasionally on the scientific advisory boards of AMGen and Pfizer, and has a minority stake in Self-screen; A.V. is cofounder and board member of Novosanis. No competing interests were disclosed by the other authors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00618-16.
REFERENCES
- 1.van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ. 2002. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol 40:779–787. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snijders PJ, van den Brule AJ, Schrijnemakers HF, Snow G, Meijer CJ, Walboomers JM. 1990. The use of general primers in the polymerase chain reaction permits the detection of a broad spectrum of human papillomavirus genotypes. J Gen Virol 71:173–181. doi: 10.1099/0022-1317-71-1-173. [DOI] [PubMed] [Google Scholar]
- 3.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. 1995. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol 76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 4.Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, Kenter GG, Cuzick J, Snijders PJ, Meijer CJ. 2012. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol 13:78–88. doi: 10.1016/S1470-2045(11)70296-0. [DOI] [PubMed] [Google Scholar]
- 5.Elfstrom KM, Smelov V, Johansson AL, Eklund C, Naucler P, Arnheim-Dahlstrom L, Dillner J. 2014. Long term duration of protective effect for HPV negative women: follow-up of primary HPV screening randomised controlled trial. BMJ 348:g130. doi: 10.1136/bmj.g130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eklund C, Zhou T, Dillner J. 2010. Global proficiency study of human papillomavirus genotyping. J Clin Microbiol 48:4147–4155. doi: 10.1128/JCM.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. 2010. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol 48:143–149. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschi S, Umulisa MC, Tshomo U, Gheit T, Baussano I, Tenet V, Tshokey T, Gatera M, Ngabo F, Van Damme P, Snijders PJ, Tommasino M, Vorsters A, Clifford GM. 2016. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int J Cancer 139:518–526. doi: 10.1002/ijc.30092. [DOI] [PubMed] [Google Scholar]
- 9.Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli UR, Jivarajani P, Verma Y, Zomawia E, Siddiqi M, Shastri SS, Jayant K, Malvi SG, Lucas E, Michel A, Butt J, Vijayamma JM, Sankaran S, Kannan TP, Varghese R, Divate U, Thomas S, Joshi G, Willhauck-Fleckenstein M, Waterboer T, Muller M, Sehr P, Hingmire S, Kriplani A, Mishra G, Pimple S, Jadhav R, Sauvaget C, Tommasino M, Pillai MR. 2016. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tshomo U, Franceschi S, Dorji D, Baussano I, Tenet V, Snijders PJ, Meijer CJ, Bleeker MC, Gheit T, Tommasino M, Clifford GM. 2014. Human papillomavirus infection in Bhutan at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis 14:408. doi: 10.1186/1471-2334-14-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngabo F, Franceschi S, Baussano I, Umulisa MC, Snijders PJF, Uyterlinde AM, Lazzarato F, Tenet V, Gatera M, Binagwaho A, Clifford GM. 2016. Human papillomavirus infection in Rwanda at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis 16:225. doi: 10.1186/s12879-016-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dondog B, Clifford GM, Vaccarella S, Waterboer T, Unurjargal D, Avirmed D, Enkhtuya S, Kommoss F, Wentzensen N, Snijders PJ, Meijer CJ, Franceschi S, Pawlita M. 2008. Human papillomavirus infection in Ulaanbaatar, Mongolia: a population-based study. Cancer Epidemiol Biomarkers Prev 17:1731–1738. doi: 10.1158/1055-9965.EPI-07-2796. [DOI] [PubMed] [Google Scholar]
- 13.Vorsters A, Van den Bergh J, Micalessi I, Biesmans S, Bogers J, Hens A, De Coster I, Ieven M, Van Damme P. 2014. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol Infect Dis 33:2005–2014. doi: 10.1007/s10096-014-2147-2. [DOI] [PubMed] [Google Scholar]
- 14.de Roda Husman AM, Snijders PJ, Stel HV, van den Brule AJ, Meijer CJ, Walboomers JM. 1995. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction. Br J Cancer 72:412–417. doi: 10.1038/bjc.1995.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs MV, Walboomers JM, Snijders PJ, Voorhorst FJ, Verheijen RH, Fransen-Daalmeijer N, Meijer CJ. 2000. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer 87:221–227. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Gheit T, Landi S, Gemignani F, Snijders PJ, Vaccarella S, Franceschi S, Canzian F, Tommasino M. 2006. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol 44:2025–2031. doi: 10.1128/JCM.02305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt M, Dondog B, Waterboer T, Pawlita M. 2008. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J Clin Microbiol 46:1050–1059. doi: 10.1128/JCM.02227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, Burk RD. 1997. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol 35:1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iftner T, Villa LL. 2003. Chapter 12: human papillomavirus technologies. J Natl Cancer Inst Monogr 31:80–88. [DOI] [PubMed] [Google Scholar]
- 20.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. 2005. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 14:1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 21.Clifford GM, Goncalves MA, Franceschi S. 2006. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 20:2337–2344. doi: 10.1097/01.aids.0000253361.63578.14. [DOI] [PubMed] [Google Scholar]
- 22.Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. 2013. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445:224–231. doi: 10.1016/j.virol.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Hesselink AT, Heideman DA, Berkhof J, Topal F, Pol RP, Meijer CJ, Snijders PJ. 2010. Comparison of the clinical performance of PapilloCheck human papillomavirus detection with that of the GP5+/6+-PCR-enzyme immunoassay in population-based cervical screening. J Clin Microbiol 48:797–801. doi: 10.1128/JCM.01743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbyn M, Depuydt C, Benoy I, Bogers J, Cuschieri K, Schmitt M, Pawlita M, Geraets D, Heard I, Gheit T, Tommasino M, Poljak M, Bonde J, Quint W. 2016. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol 76(Suppl 1):S14–S21. doi: 10.1016/j.jcv.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Vaccarella S, Soderlund-Strand A, Franceschi S, Plummer M, Dillner J. 2013. Patterns of human papillomavirus types in multiple infections: an analysis in women and men of the high throughput human papillomavirus monitoring study. PLoS One 8:e71617. doi: 10.1371/journal.pone.0071617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesselink AT, van Ham MA, Heideman DA, Groothuismink ZM, Rozendaal L, Berkhof J, van Kemenade FJ, Massuger LA, Melchers WJ, Meijer CJ, Snijders PJ. 2008. Comparison of GP5+/6+-PCR and SPF10-line blot assays for detection of high-risk human papillomavirus in samples from women with normal cytology results who develop grade 3 cervical intraepithelial neoplasia. J Clin Microbiol 46:3215–3221. doi: 10.1128/JCM.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan PK, Cheung TH, Tam AO, Lo KW, Yim SF, Yu MM, To KF, Wong YF, Cheung JL, Chan DP, Hui M, Ip M. 2006. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. Int J Cancer 118:243–245. doi: 10.1002/ijc.21299. [DOI] [PubMed] [Google Scholar]
- 28.Vaccarella S, Franceschi S, Snijders PJ, Herrero R, Meijer CJ, Plummer M. 2010. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV Prevalence Surveys. Cancer Epidemiol Biomarkers Prev 19:503–510. doi: 10.1158/1055-9965.EPI-09-0983. [DOI] [PubMed] [Google Scholar]
- 29.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. 2006. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol 44:3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snijders PJ, van den Brule AJ, Meijer CJ. 2003. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol 201:1–6. doi: 10.1002/path.1433. [DOI] [PubMed] [Google Scholar]
- 31.Cuschieri K, Kavanagh K, Sinka K, Robertson C, Cubie H, Moore C, Donaghy M. 2013. Effect of HPV assay choice on perceived prevalence in a population-based sample. Diagn Mol Pathol 22:85–90. doi: 10.1097/PDM.0b013e31827f3f7e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.