Abstract
Accurate analysis of female reproductive toxicity requires a thorough understanding the differences in and specifics of estrous or menstrual cycles between laboratory animals. There are some species differences such as the time of sex maturation, the length of the estrous or menstrual cycle, the length of the luteal phase, the number of dominant follicles or corpora lutea, the size of follicles, processes of luteinization, and hormonal changes during the estrous or menstrual cycle. Rodents have a short estrous cycle, and their ovarian cycling features are the same in both ovaries, which contain a large number of follicles and corpora lutea. The dog estrous cycle is much longer than those of other laboratory animals, and it includes a long anestrus phase. The duration of the menstrual cycle of monkeys is roughly 30 days, and their ovarian cycling features are different between the left and right ovaries. In both rodents and dogs, the theca cells invade the early luteum, mixing with granulosa cells during luteinization. However in monkeys, the theca layer dose not mix with the granulosa cells as it invaginates only slightly into the early luteum. In addition, we found that high progesterone levels after ovulation are sustained for a much shorter duration in rodents than in dogs and monkeys due to the comparatively rapid passage of the rodent luteal phase. Based on these species differences, animal species for use in ovarian toxicology studies need to be selected appropriately.
Keywords: estrous cycle, menstrual cycle, female, reproductive system, gonadotropin, ovarian hormone
Introduction
An estrous or menstrual cycle is crucial for procreation. However, cycles typically vary among species, with animals adopting the cycle most suited to a given environment or reproductive strategy. Laboratory animals are no exception; for example, small animals like rodents tend to have a short life span, tend to be relatively low on the food chain, and tend to have only a limited time to raise offspring. Therefore, these animals reach sex maturation early after birth, at approximately 5 weeks of age1,2,3, and have a short estrous cycle with no luteal phase, unless they become pregnant. Litters also tend to be relatively large, with approximately 10 to 14 offspring per litter4. In contrast, relatively large and long-lived animals, such as monkeys, take longer to sexually mature (approximately 2 to 3 years)3, have a long estrous cycle, and produce few offspring per pregnancy.
Dogs are sexually mature by 6 to 8 month age3 and can produce relatively large litters of 4 to 15. The estrous cycle of dogs is therefore monestral and nonseasonal1, 2. Reproduction patterns clearly vary throughout the animal kingdom.
Accurate analysis of female reproductive toxicity requires a thorough understanding the differences in and specifics of estrous or menstrual cycles between laboratory animals. Here, we describe the morphological features of the estrous cycle in rodents and beagle dogs and menstrual cycles in cynomolgus monkeys.
Species Differences of the Estrous or Menstrual Cycle
Rats
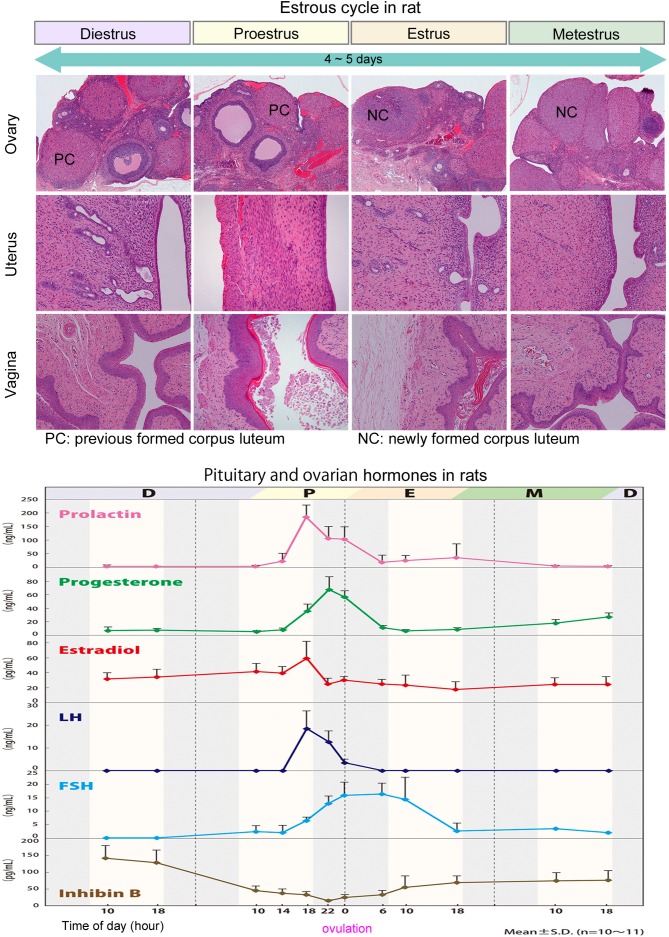
The estrous cycle of rats is shown in Fig. 1. The rodent estrous cycle repeats with a constant period of 4 to 5 days and includes the following phases: proestrus, estrus, metestrus, and diestrus1, 2, 5,6,7. Both ovaries exhibit the same morphological changes throughout the cycle and ovulate—bilaterally—at the same time. Since the ovulation number is relatively large in rodents (approximately 12 to 14 overall), a large number of follicles and corpora lutea can be observed in the ovaries.
Fig. 1.
Morphologic features of the ovary, uterus, and vagina, and pituitary and ovarian hormonal levels in each estrous phase in rats: D=diestrus, P=proestrus, E=estrus, M=metestrus.
In proestrus, follicles develop rapidly until ovulation at the end of proestrus. At this stage, two to four large follicles can be observed in a unilateral ovarian section. When estrus starts at ovulation, follicles are transformed into corpora lutea, the luteal cells of which are more basophilic and smaller than in later phases of the cycle. These corpora lutea begin to regress at metestrus and continue their regression into diestrus. This pattern of development and regression then repeats in the next cycle of estrous. Given that regressive corpora lutea may persist for some 12 to 14 days, ovaries of rodents tend to contain a large number of corpora lutea7, 8.
Dogs
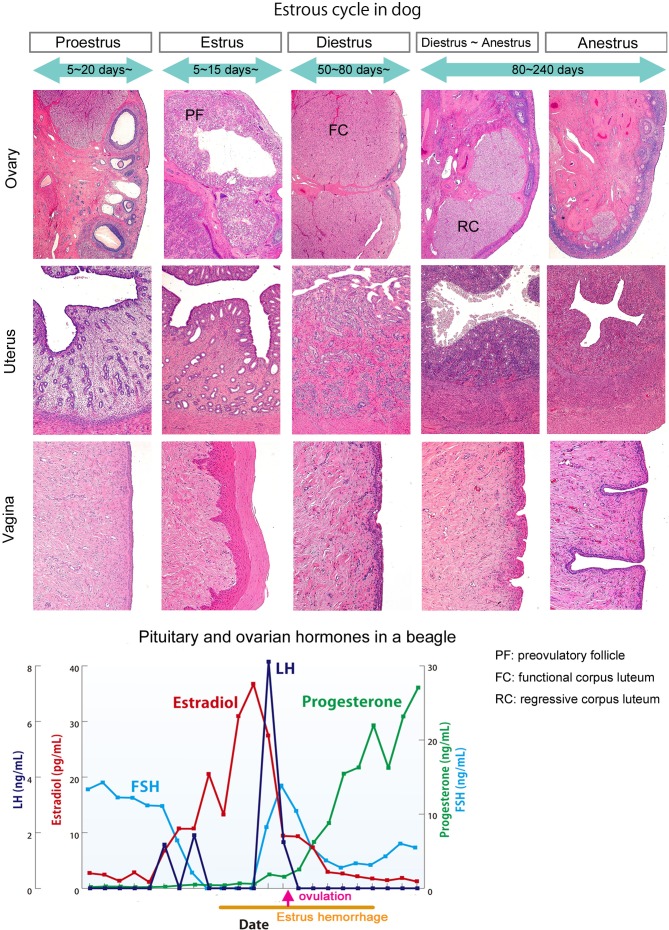
The estrous cycle of beagles is shown in Fig. 2. The dog estrous cycle is much longer than that of other laboratory animals and divided into four phases: proestrus (follicular phase), estrus and diestrus (luteal phase), and anestrus. Proestrus lasts for 5 to 10 days, estrus lasts for 5 to 15 days, and diestrus lasts for 50 to 80 days (metestrus). The long nonseasonal period between cycles is called anestrus and lasts for 80 to 240 days2. As in rodents, both ovaries exhibit the same morphological changes throughout the cycle and ovulate bilaterally at the same time. The ovulation number tends to range from 4 to 15.
Fig. 2.
Morphologic features of the ovary, uterus, and vagina and pituitary and ovarian hormonal levels in each estrus phase in a beagle dog.
In proestrus, two to four large follicles can be observed in a sagittal section of the unilateral ovary. The follicle wall begins to fold into the follicular antrum gradually in later phases of proestrus. In estrus, granulosa cells are transformed into luteal cells, and infolding of the follicle walls progresses. Luteinized follicles then rupture, and oocytes are excreted outside in the latter part of estrus. In early diestrus, the bilateral ovaries contain large corpora lutea. As the corpora lutea begin to regress in the latter phase of diestrus, the cytoplasm in luteal cells contains a number of vacuoles9, 10. In anestrus, corpora lutea gradually regress, shrinking in size and becoming vacuolar in the latter part of the anestrus phase9, 11, 12. Estrus hemorrhage occurs from proestrus to estrus due to endometrial edema and congestion.
Monkeys
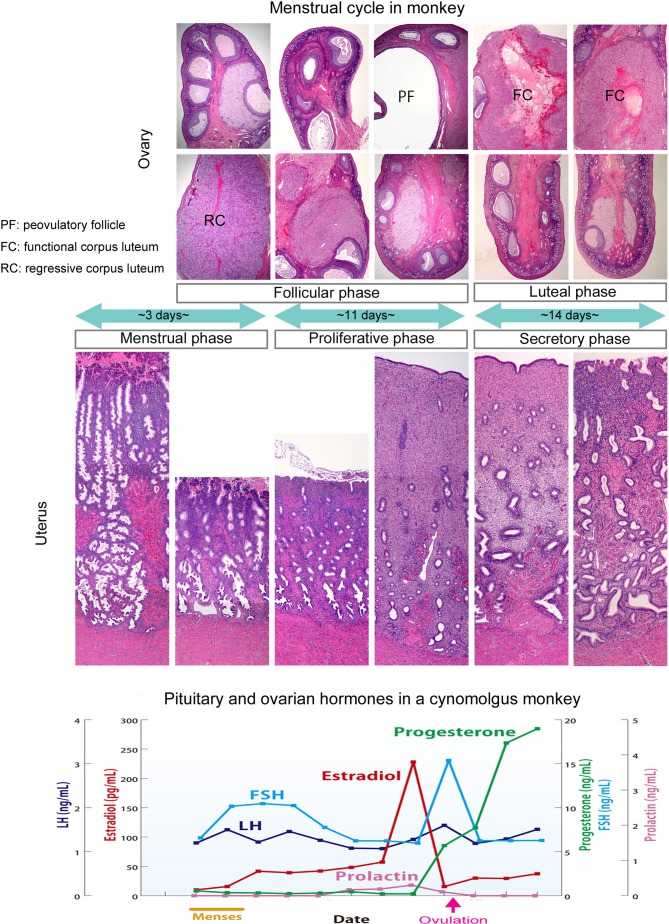
The menstrual cycle of cynomolgus monkeys is shown in Fig. 3. Sexual maturation is reached at the first menstrual bleeding, around 2 to 2.5 years old3, and regular menstrual cycles start around 3 years old13, 14. Ovulation in monkey ovaries is unilateral, alternating between the left and right ovary each cycle. While one ovary contains selectable follicles or active corpora lutea, the other contains atretic follicles and regressing corpora lutea. Generally, ovulation occurs not alternately but continuously in only one of the bilateral ovaries.
Fig. 3.
Morphologic features of the bilateral ovaries, uterus, and vagina, and pituitary and ovarian hormonal levels in each menstrual phase in a cynomolgus monkey.
The duration of the menstrual cycle is roughly 30 days2, 13, 15, with a rough interval between menses ranging from 14 to 66 days in our laboratory background data. However, this basic cycle can be easily influenced by stressful conditions. The follicle phase continues for approximately 14 days, and the luteal phase continues for another roughly 14 days after ovulation2, 13, 15. If pregnancy is not established, the corpora lutea begin to regress at 14 days after ovulation. Luteal cells contain small vacuoles, and pyknotic nuclei are observed in the regressing corpora lutea14, 17, 18. Without pregnancy, the endometrium is no longer needed and begins to necrose, resulting in menstrual bleeding14, 16, 17, 19.
Morphology During the Estrous or Menstrual Cycle
Follicle development in rats, dogs, and monkeys
Follicles develop in the following order: primordial, primary, secondary, small antral, and large antral stage (Graafian follicle)8, 9, 17, 18. All follicles in each stage are capable of regressing, except for the selectable follicle (dominant follicle)20, 21. The sizes of primordial to small antral follicles are roughly equivalent in rats, dogs, and monkeys, but large antral and preovulatory follicles subsequently grow to match the individual ovary size of each species (Fig. 4). While basal follicular growth until the secondary follicle does not depend largely on gonadotropins, follicular growth from the antral follicle does depend on gonadotropins, such as follicle-stimulating hormone (FSH)21. Follicle growth rates in dogs and monkeys are 6- to 7-fold that in rats.
Fig. 4.
Follicle development shown by the diameter of each class of follicles in rats, beagles, and monkeys: primordial=primordial follicle, primary small=small primary follicle, primary large=large primary follicle, secondary=secondary follicle, preantral=preantral follicle, antral small=small antral follicle, antral large=large antral follicle, preovulatory=preovulatory follicle (selectable follicle), mature corpus luteum=mature (functional) corpus luteum.
Luteinization in rats, dogs, and monkeys
Diagrams depicting the luteinization process for each species are shown in Fig. 5. Luteinization is the remodeling process that occurs following ovulation, in which cells transform rather dramatically from both a morphological and biochemical standpoint. Granulosa and theca cells, two follicle cell types, contribute to luteal formation. The selectable follicle is composed of granulosa cells surrounded by a layer of theca cells. Ovulatory stimulation induces these granulosa and theca cells to transform into luteal cells. While luteal cells were previously believed to be incapable of mitosis, recent studies have suggested that both proliferating and mature luteal cells mingle in early-stage corpora lutea22. Proliferating activity of early luteal cells begins to fade as the corpora lutea mature22. Luteinization additionally involves the invasion of extrafollicular elements, including theca cells, blood vessels, and reticuloendothelial components. Vascular invasion helps distinguish early corpora lutea from follicles. Processes occurring during luteinization vary by species22. For example, though Graafian follicles with abundant liquor folliculi in most animals start luteal formation by rupturing and folding the granulosa layer and expulsion of the liquor folliculi, luteal formation in dogs differs. In dogs, the theca and granulosa cell layer gradually begins to fold during pre-ovulation, before the surge in levels of luteinizing hormone (LH), and the early corpora lutea collapse and granulosa and theca cells transform into luteal cells after the LH surge10, 22. At this stage, in both rodents and dogs, the theca cells then invade the early luteum, with vessels and interstitial cells mixing together with granulosa cells. In monkeys, however, the theca layer only slightly invaginates into the granulosa layer, but the components never mix as in rodents and dogs; instead, only vessels and interstitial cells invade the granulosa-luteum22.
Fig. 5.
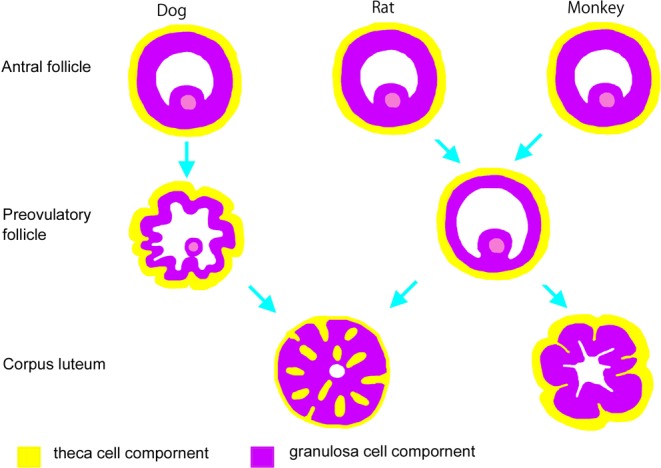
Schematic description of variations in luteinization between rats, beagles, and monkeys. In the dog, folding of theca cells and the granulosa cell layer starts during pre-ovulation. In dogs and rodents, theca cells invade into the early luteum, with vessels and interstitial cells mixed together with granulosa cells. In monkeys, the theca layer slightly invaginates into the granulosa layer, but the components never mix.
Hormonal Changes in the Estrous or Menstrual Cycle
Rats
Changes in levels of hormones during the estrous cycle in rats are shown in Fig. 1. These data were collected from 10 Sprague Dawley rats in our laboratory. Estrogen levels were elevated throughout proestrus. LH and prolactin (PRL) surges are observed in the late afternoon on the day of proestrus, followed by a short luteal phase of estrus2. The preovulatory peak in PRL levels can lead to luteolysis after metestrus2, 23. FSH levels increase relative to the time of the LH peak and then increase again after ovulation during the morning on the day of estrus. Inhibin levels are reduced around the time of follicular ovulation. During development and ovulation of follicles, estrogen levels inform follicle maturation to the pituitary gland, while inhibin levels inform the number of developing follicles to the pituitary gland5. Progesterone levels are transiently elevated at the time of transformation of newly formed corpora lutea but rapidly reduced when corpora lutea begin to regress.
Dogs
Changes in levels of hormones during the estrous cycle in a beagle are shown in Fig. 2. Serum gonadotropin data were obtained over a period of 33 days before and after estrus hemorrhage in a single beagle dog in our laboratory. Hormonal levels in the month containing the estrus phase are graphed in Fig. 2. Early follicular development requires FSH stimulation, after which the developed follicles begin to secrete estradiol and FSH levels are then reduced via negative feedback from estradiol. A surge in LH levels was observed the day after the peak of estradiol levels, and then FSH levels rapidly increased again. Progesterone levels gradually increase in the estrus phase during luteinization of granulosa cells. Previous reports have suggested that the decrease in the estrogen-to-progesterone ratio triggers the preovulatory surge release of LH10, 24.
Monkeys
Changes in levels of hormones during the menstrual cycle in a cynomolgus monkey are shown in Fig. 3. Serum hormone data were obtained over a period of 33 days from the start of the menstrual phase in a single cynomolgus monkey in our laboratory. The early follicular phase is roughly equivalent to the menstrual phase. The corpora lutea start to regress at the end of the luteal phase, with progesterone and estradiol levels low in the early follicular phase and FSH levels gradually increasing to induce development of the early follicles. After that, developed Graafian follicles begin to secrete estradiol in the latter half of the follicular phase, which reduces FSH levels due to negative feedback. Under these conditions of low FSH levels, less-developed follicles undergo atresia, and the largest grown follicle becomes the selectable (dominant) follicle, which produces estradiol. Once estradiol levels exceed a certain threshold, positive feedback leads to LH and FSH release. This surge in LH and FSH secretion induces ovulation, and progesterone levels increase rapidly as the granulosa cells start to luteinize and release progesterone. These longitudinal hormonal changes closely resemble those observed in humans19, 20, 25.
Conclusion
Appropriate selection of animal species for use in ovarian toxicology studies is important. Given the relatively short rodent estrous cycle of 4 to 5 days, the cycle occurs approximately 7 times during a 4-week toxicology study. Additionally, ovarian morphological changes are easy to observe, as ovarian cycling features are the same in both ovaries, which both contain a large number of follicles and corpora lutea. In contrast, the estrous cycle in dogs includes a long anestrus phase. Given the relatively short period of toxicology studies, dogs are inappropriate for use in such studies. Monkey ovarian toxicity is important to assess, as the morphological and hormonal changes in the reproductive system and menstrual cycle phases closely resemble those of humans. However, most toxicology studies involving monkeys only use 3 or 4 animals per dosing group, and menstrual cycles may come as infrequently as once or not at all during a 4-week toxicology study. We therefore recommend that researchers take the following steps in toxicology studies using monkeys: 1, select animals that have regular menstrual cycles; 2, conduct comparatively long-term studies; 3, time the start of test article administration to a certain phase in the menstrual cycle; 4, measure sex hormone levels; and 5, examine the largest histological section area possible containing corpora lutea or large follicles.
Acknowledgments
We are grateful to Ms. Kinuko Zaizen and Ms. Junko Hangai for measuring hormone levels.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Greaves P. Female genital tract. In: Histopathology of Preclinical Toxicity Studies, 4th ed. Greaves P (ed). Academic Press, Amsterdam. 667–723. 2012. [Google Scholar]
- 2.Andersson H, Rehm S, Stanislaus D, and Wood CE. Scientific and regulatory policy committee (SRPC) paper: assessment of circulating hormones in nonclinical toxicity studies III. female reproductive hormones. Toxicol Pathol. 41: 921–934. 2013. [DOI] [PubMed] [Google Scholar]
- 3.Beckman DA, and Feuston M. Landmarks in the development of the female reproductive system. Birth Defects Res B Dev Reprod Toxicol. 68: 137–143. 2003; (Part B). [DOI] [PubMed] [Google Scholar]
- 4.Liberati TA, Roe BJ, and Feuston MH. An oral (gavage) control embryo-fetal development study in the Wistar Hannover rat. Drug Chem Toxicol. 25: 109–130. 2002. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida M. Reproductive system. In: Ito’s Toxicologic Pathology. M Takahashi, and S Fukushima (eds). Maruzen, Japan. 321–334. 2013. [Google Scholar]
- 6.Yuan Y, and Foley GL. Female reproductive system. In: Handobook of Toxicologic Pathology, 2nd ed., Vol. 2. WM Haschek, CG Rousseaux, and MS Wallig (eds).Academic press, London. 847–894. 2002. [Google Scholar]
- 7.Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 36: 375–384. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M, Sanbuissyo A, Hisada S, Takahashi M, Ohno Y, and Nishikawa A. Morphological characterization of the ovary under normal cycling in rats and its viewpoints of ovarian toxicity detection. J Toxicol Sci. 34(Suppl 1): SP189–SP197. 2009. [DOI] [PubMed] [Google Scholar]
- 9.Chandra SA, and Adler RR. Frequency of different estrous stages in purpose-bred beagles: a retrospective study. Toxicol Pathol. 36: 944–949. 2008. [DOI] [PubMed] [Google Scholar]
- 10.Concannon P, Hansel W, and Mcentee K. Changes in LH, progesterone and sexual behavior associated with preovulatory luteinization in the bitch. Biol Reprod. 17: 604–613. 1977. [DOI] [PubMed] [Google Scholar]
- 11.Concannon PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci. 124: 200–210. 2011. [DOI] [PubMed] [Google Scholar]
- 12.Sato J, Doi T, Wako Y, Hamamura M, Kanno T, Tsuchitani M, and Narama I. Histopathology of incidental findings in beagles used in toxicity studies. J Toxicol Pathol. 25: 103–134. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Esch E, Cline JM, Buse E, Wood CE, De Ruk EPCT, and Weinbauer GF. Summary comparison of female reproductive system in human and the cynomolgus monkey (Macaca fascicularis). Toxicol Pathol. 36: 171S–172S. 2008. [Google Scholar]
- 14.Watanabe D, Hoshiya T, Sato J, Yamaguchi Y, Horiguchi K, Nagashima Y, Okaniwa A, and Yoshikawa H. Changes in the reproductive organs depending on phases of reproductive cycle and aging in female cynomolgus monkeys. J Toxicol Pathol. 19: 169–177. 2006. [Google Scholar]
- 15.Yoshida T, Nakajima M, Hiyaoka A, Suzuki MT, Cho F, and Honjo S. [Menstrual cycle lengths and the estimated time of ovulation in the cynomolgus monkey (Macaca fascicularis)]. Jikken Dobutsu. 31: 165–174. 1982. [DOI] [PubMed] [Google Scholar]
- 16.Sato J, Doi T, Kanno T, Wako Y, Tsuchitani M, and Narama I. Histopathology of incidental findings in cynomolgus monkeys ( macaca fascicularis ) used in toxicity studies. J Toxicol Pathol. 25: 63–101. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koering MJ. Cyclic changes in ovarian morphology during the menstrual cycle in Macaca mulatta. Am J Anat. 126: 73–101. 1969. [DOI] [PubMed] [Google Scholar]
- 18.Buse E, Zoller M, and van Esch E. The macaque ovary, with special reference of the cynomolgus macaque (Macaca fascicularis). Toxicol Pathol. 36: 24S–66S. 2008. [Google Scholar]
- 19.van Esch E, Buse E, Weinbauer GF, and Cline JM. The macaque endometrium, with special reference to the cynomolgus monkey (Macaca fascicularis). Toxicol Pathol. 36: 67S–100S. 2008. [Google Scholar]
- 20.Weinbauer GF, Niehoff M, Niehaus M, Srivastav S, Fuchs A, Van Esch E, and Cline JM. Physiology and endocrinology of the ovarian cycle in macaque. Toxicol Pathol. 36(7S): 7S–23S. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gougeon A. Dynamics of human follicular growth: morphologic, dynamic, and functional aspects. In: The Ovary, 2nd ed. PCK Leung, and EY Adashi (eds). Academic Press, Amsterdam. 25–43. 2004. [Google Scholar]
- 22.Murphy BD. Luteinization. In: The Ovary, 2nd ed. PCK Leung, and EY Adashi (eds). Academic Press, Amsterdam. 185–199. 2004. [Google Scholar]
- 23.Gaytán F, Bellido C, Morales C, and Sánchez-Criado JE. Luteolytic effect of prolactin is dependent on the degree of differentiation of luteal cells in the rat. Biol Reprod. 65: 433–441. 2001. [DOI] [PubMed] [Google Scholar]
- 24.Concannon PW. Endocrinologic control of normal canine ovarian function. Reprod Domest Anim. 44(Suppl 2): 3–15. 2009. [DOI] [PubMed] [Google Scholar]
- 25.Kierszenbaum AL. Follicle development and menstrual cycle. In: Histology and Cell Biology. An Introduction to Pathology, 2nd ed. AL Kierszanbaum (ed). Mosby, Canada. 613–634. 2007. [Google Scholar]