Abstract
Background
Helminth infections, such as soil‐transmitted helminths, schistosomiasis, onchocerciasis, and lymphatic filariasis, are prevalent in many countries where human immunodeficiency virus (HIV) infection is also common. There is some evidence from observational studies that HIV and helminth co‐infection may be associated with higher viral load and lower CD4+ cell counts. Treatment of helminth infections with antihelminthics (deworming drugs) may have benefits for people living with HIV beyond simply clearance of worm infections.
This is an update of a Cochrane Review published in 2009 and we have expanded it to include outcomes of anaemia and adverse events.
Objectives
To evaluate the effects of deworming drugs (antihelminthic therapy) on markers of HIV disease progression, anaemia, and adverse events in children and adults.
Search methods
In this review update, we searched online for published and unpublished studies in the Cochrane Library, MEDLINE, EMBASE, CENTRAL, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICRTP), ClinicalTrials.gov, and the WHO Global Health Library up to 29 September 2015. We also searched databases listing conference abstracts, scanned reference lists of articles, and contacted the authors of included studies.
Selection criteria
We searched for randomized controlled trials (RCTs) that compared antihelminthic drugs with placebo or no intervention in HIV‐positive people.
Data collection and analysis
Two review authors independently extracted data and assessed trials for eligibility and risk of bias. The primary outcomes were changes in HIV viral load and CD4+ cell count, and secondary outcomes were anaemia, iron deficiency, adverse events, and mortality events. We compared the effects of deworming using mean differences, risk ratios (RR), and 95% confidence intervals (CIs). We assessed the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Eight trials met the inclusion criteria of this review, enrolling a total of 1612 participants. Three trials evaluated the effect of providing antihelminthics to all adults with HIV without knowledge of their helminth infection status, and five trials evaluated the effects of providing deworming drugs to HIV‐positive individuals with confirmed helminth infections. Seven trials were conducted in sub‐Saharan Africa and one in Thailand.
Antihelminthics for people with unknown helminth infection status
Providing antihelminthics (albendazole and praziquantel together or separately) to HIV‐positive adults with unknown helminth infection status may have a small suppressive effect on mean viral load at six weeks but the 95% CI includes the possibility of no effect (difference in mean change −0.14 log10 viral RNA/mL, 95% CI −0.35 to 0.07, P = 0.19; one trial, 166 participants, low quality evidence).
Repeated dosing with deworming drugs over two years (albendazole every three months plus annual praziquantel), probably has little or no effect on mean viral load (difference in mean change 0.01 log10 viral RNA, 95% CI: −0.03 to −0.05; one trial, 917 participants, moderate quality evidence), and little or no effect on mean CD4+ count (difference in mean change 2.60 CD4+ cells/µL, 95% CI −10.15 to 15.35; P = 0.7; one trial, 917 participants, low quality evidence).
Antihelminthics for people with confirmed helminth infections
Treating confirmed helminth infections in HIV‐positive adults may have a small suppressive effect on mean viral load at six to 12 weeks following deworming (difference in mean change −0.13 log10 viral RNA, 95% CI −0.26 to −0.00; P = 0.04; four trials, 445 participants, low quality evidence). However, this finding is strongly influenced by a single study of praziquantel treatment for schistosomiasis. There may also be a small favourable effect on mean CD4+ cell count at 12 weeks after deworming in HIV‐positive populations with confirmed helminth infections (difference in mean change 37.86 CD4+ cells/µL, 95% CI 7.36 to 68.35; P = 0.01; three trials, 358 participants, low quality evidence).
Adverse events and mortality
There is no indication that antihelminthic drugs impart additional risks in HIV‐positive populations. However, adverse events were not well reported (very low quality evidence) and trials were underpowered to evaluate effects on mortality (low quality evidence).
Authors' conclusions
There is low quality evidence that treating confirmed helminth infections in HIV‐positive adults may have small, short‐term favourable effects on markers of HIV disease progression. Further studies are required to confirm this finding. Current evidence suggests that deworming with antihelminthics is not harmful, and this is reassuring for the routine treatment of confirmed or suspected helminth infections in people living with HIV in co‐endemic areas.
Further long‐term studies are required to make confident conclusions regarding the impact of presumptively deworming all HIV‐positive individuals irrespective of helminth infection status, as the only long‐term trial to date did not demonstrate an effect.
11 April 2019
No update planned
Research area no longer active
This is no longer a current research question. All eligible published studies found in the last search (29 Sep, 2015) were included
Keywords: Adult; Humans; Endemic Diseases; Anthelmintics; Anthelmintics/therapeutic use; CD4 Lymphocyte Count; Disease Progression; HIV Infections; HIV Infections/immunology; HIV Infections/parasitology; HIV Infections/virology; HIV‐1; HIV‐1/genetics; Health Resources; Helminthiasis; Helminthiasis/complications; Helminthiasis/drug therapy; Poverty Areas; RNA, Viral; RNA, Viral/analysis; Randomized Controlled Trials as Topic; Viral Load
Plain language summary
Antihelminthics in helminth endemic areas: effects on HIV infection
This Cochrane Review summarizes trials that evaluated the benefits and potential risks of providing deworming drugs (antihelminthics) to people infected with human immunodeficiency virus (HIV). After we searched for relevant trials up to 29 September 2015 we included eight trials that enrolled 1612 participants.
What are deworming drugs and why might they delay HIV disease progression
Deworming drugs are used to treat a variety of human helminth infections, such as soil‐transmitted helminths, schistosomiasis, onchocerciasis, and lymphatic filariasis. In areas where these infections are common, the World Health Organization currently recommends that targeted populations are routinely treated every six to 12 months without prior confirmation of an individual's infection status. The use of empiric therapy, or treating all at‐risk populations presumptively, is preferred to test‐and‐treat strategies because deworming drugs are inexpensive and well tolerated. Additionally, a strategy of testing before treatment is considered less cost‐effective given that available diagnostic tests are relatively expensive and can exhibit poor sensitivity.
Helminth infections are known to affect the human immune system. In people with HIV, some studies have suggested that helminth infections may reduce the number of CD4+ cells (which are a critical part of the immune response to HIV) and compromise a person's ability to control HIV viral replication. Thus, treatment of helminth infections could have important benefits for people living with HIV beyond the benefits observed in the general population as a result of deworming.
What the evidence in this review suggests
Treating all HIV‐positive adults with deworming drugs without knowledge of their helminth infection status may have a small suppressive effect on viral load at six weeks (low quality evidence), but repeated dosing over two years appears to have little or no effect on either viral load (moderate quality evidence) or CD4+ cell count (low quality evidence). These findings are based on two included studies.
Providing deworming drugs to HIV‐positive adults with diagnosed helminth infection may result in a small suppressive effect on mean viral load at six to 12 weeks (low quality evidence) and a small favourable effect on mean CD4+ cell count at 12 weeks (low quality evidence). However, these findings are based on small studies and are strongly influenced by a single study of praziquantel for schistosomiasis. Further studies from different settings and populations are needed for confirmation.
Adverse events were not well reported (very low quality evidence), and trials were too small to evaluate the effects on mortality (low quality evidence). However there is no suggestion that deworming drugs are harmful for HIV‐positive individuals.
Summary of findings
Summary of findings for the main comparison. Summary of findings for participants with unknown helminth infection status.
| Deworming drugs compared with placebo for people with HIV and an unknown helminth infection status | ||||
|
Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole or praziquantel or a combination) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 weeks after a single dose | 166 (1 trial) |
⊕⊕⊝⊝1,2,3,4 low | |
| in the control group, the mean change in viral load was an increase of 0.09 log10 viral RNA |
On average, with deworming, there was a suppressive effect on mean viral load of 0.14 log10 viral RNA (0.35 benefit to 0.07 harm) |
|||
| At 2 years after multiple doses | 917 (1 trial) |
⊕⊕⊕⊝1,2,5,6 moderate | ||
| In the control group, the mean viral load increased by 0.03 log10 viral RNA |
On average, with deworming, there was a suppressive effect on mean viral load of 0.01 log10 viral RNA (0.03 benefit to 0.05 harm) |
|||
| CD4+ cell count | At 2 years after multiple doses | 917 (1 trial) |
⊕⊕⊝⊝1,2,4,5 low | |
| In the control group, the mean CD4+ cell count reduced by 37.3 CD4+ cells/µL |
On average, with deworming, there was a favourable effect on mean CD4+ cell count of 2.60 CD4+ cells/µL (15.35 benefit 10.15 harm) |
|||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; RNA: ribonucleic acid; HIV: human immunodeficiency virus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
1No serious risk of bias: this single trial was at low risk of selection bias. 2No serious inconsistency: this was not applicable as there was only a single trial. 3We downgraded by 1 for serious indirectness: this single trial is based upon a specific sample of pregnant women treated with albendazole or praziquantel, or both. The overall finding of an effect cannot be easily generalized to all populations or settings. 4We downgraded by 1 for imprecision: the 95% CI includes potentially clinically important differences as well as no effect. Further larger studies are needed. 5We downgraded by 1 for serious indirectness: this trial was conducted in three sites in rural and urban Kenya. Helminth infection was expected to be high but was not assessed at baseline. The findings are not easily generalized to all helminth endemic settings. 6No serious imprecision: the 95% CI includes no effect but is narrow around the estimate and excludes clinically important differences.
Summary of findings 2. Summary of findings for participants with confirmed helminth infections.
| Deworming drugs compared with placebo for people with HIV and confirmed helminth infections | ||||
|
Participant or population: HIV‐positive people with proven helminth infection Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||
| Placebo | Deworming drugs | |||
| Viral load | At 6 to 12 weeks | 445 (4 trials) |
⊕⊕⊝⊝ low1,2,3,4 | |
| In the control groups, the mean change in viral load ranged from a 0.13 decrease to an increase of 0.21 log10 viral RNA |
On average, with deworming, there was a small suppressive effect on mean viral load of 0.13 log10 viral RNA (0.26 benefit to 0.00 benefit) |
|||
| CD4+ cell count | At 6 to 12 weeks | 358 (3 trials) |
⊕⊕⊝⊝ low2,5,6,7 | |
| In the control groups, the mean change in CD4+ cell count ranged from a decrease of 68 to an increase of 45 CD4+ cells/µL |
On average, with deworming, there was favourable effect on mean CD4+ cell count of 37.86 CD4+ cells/µL (7.36 benefit to 68.35 benefit) |
|||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; RNA: ribonucleic acid; HIV: human immunodeficiency virus. | ||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||
1We downgraded by 1 for serious risk of bias: of the five studies, only one had CIs that excluded the possibility of no effect and this study was at high risk of selection bias. 2No serious inconsistency: statistical heterogeneity was low. 3We downgraded by 1 for serious indirectness: the only trial for which the CIs excluded the possibility of no effect administered praziquantel to people with schistosomiasis. The overall finding of an effect cannot be easily generalized to all deworming drugs, helminth infections, or settings. 4No serious imprecision: the overall 95% CI is wide and includes both clinically important effects and no effect. However, as potential harm is excluded, we did not further downgrade the evidence. 5No serious risk of bias: one trial was at high risk of selection bias, but excluding this trial did not substantially change the result. 6We downgraded by 1 for serious indirectness: the included trials are from a very limited number of settings and participants. The overall finding is not easily generalized to all deworming drugs, helminth infections, or settings, and further trials are needed. 7We downgraded by 1 for imprecision: the 95% CI is wide and includes potentially clinically important differences as well as no effect. Further larger studies are needed.
Summary of findings 3. Summary of findings for secondary outcomes.
| Deworming drugs compared with placebo for people with HIV and both known and unknown helminth infection status | |||||
|
Participant or population: HIV‐positive people Settings: urban and rural areas co‐endemic for helminths and HIV Intervention: deworming drugs (albendazole, praziquantel, albendazole and praziquantel, or diethylcarbamazine) Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Albendazole | ||||
| Iron deficiency (serum ferritin) | log10 mean increase of 0.04 µg/L | log10 mean increase of 0.07 µg/L | Deworming drugs associated with a 0.03 higher µg/L log10 mean ferritin measure (0.30 lower to 0.35 higher) | 16 (1 trial) |
⊕⊝⊝⊝1,2,3,4 very low |
| Anaemia (serum haemoglobin) | Increase in 0.15 g/dL | Decrease in 0.10 g/dL | Deworming drugs associated with a 0.25 lower g/dL haemoglobin (0.58 lower to 0.08 higher) |
130 (1 trial) |
⊕⊝⊝⊝2,4,5,6 very low |
| Mortality | 41 per 1000 | 52 per 1000 |
RR 0.77 (0.52 to 1.14) |
1627 (5 trials) |
⊕⊕⊝⊝7,8,9,10 low |
| Adverse events | 32 per 1000 | 35 per 1000 |
RR 1.23 (0.53 to 2.83) |
1649 (7 trials) |
⊕⊝⊝⊝4,9,11,12 very low |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; HIV: human immunodeficiency virus. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1No serious risk of bias: this single trial was at risk of selection bias. However, the outcome is an objective laboratory measure. We did not downgrade the quality of the evidence. 2No serious inconsistency: not applicable as this is a single trial. 3We downgraded by 2 for serious indirectness: this single trial is based upon a very small sample size of people treated with diethylcarbamazine for lymphatic filariasis. The overall finding of an effect can not be easily generalized to all deworming drugs, helminth infections, or settings. 4We downgraded by 1 for imprecision: the 95% CI is wide and includes potentially clinically important differences as well as no effect. Larger studies are needed. 5We downgraded by 1 for serious risk of bias: this single trial was at high risk of selection bias. 6Downgraded by 1 for serious indirectness: this single trial is based upon a very small sample size of people treated with praziquantel for schistosomiasis. The overall finding of an effect can not be easily generalized to all deworming drugs or helminth infections or settings. 7No serious risk of bias: although some of the trials are at risk of selection bias the outcome is an objective measure. We did not downgrade the evidence. 8No serious inconsistency: statistical heterogeneity was low. 9No serious indirectness: these trials took place in different settings, with different drugs, and with different helminth infections present. However, effects of treatment were consistent across the trials. 10We downgraded by 2 for imprecision: the 95% CI is wide and includes potentially clinically important differences as well as no effect. Additionally the trials were not powered to detect changes in the rare outcome of mortality. A trial in which 3% of controls died would require a sample size of 7648 participants to attain 80% power to detect a 33% reduction in mortality. 11We downgraded by 2 for serious risk of bias: it is unclear how some of the trial authors defined adverse events and thus there is risk of bias in outcome assessment. 12No serious inconsistency: however, the analysis has an I² statistic value of 34%, which may represent low to moderate heterogeneity.
Background
Description of the condition
Helminths, which include the soil‐transmitted helminths (STH), schistosomiasis, onchocerciasis, and lymphatic filariasis (LF), infect nearly one‐quarter of the world’s population (WHO 2014). Communities with the highest helminth prevalence are often also areas with high prevalence of human immunodeficiency virus (HIV), and over half of HIV‐positive people living in helminth endemic areas are estimated to be co‐infected with at least one helminth infection (UNAIDS 2007).
Helminth infections have a significant effect on the human immune system, and it is hypothesized that helminth infections may modulate the host's ability to control the HIV virus (Lawn 2001; Modjarrad 2010). More specifically, studies have shown that amongst HIV‐positive individuals with helminth co‐infections.
There may be a more rapid decline in the CD4+ T‐cells responsible for immunologic function in HIV‐positive people, and increased cellular susceptibility to HIV infection (Eggena 2005; Shapira‐Nahor 1998).
CD4+ and CD8 T‐cells and monocytes may exhibit increased expression of HIV chemokine co‐receptors (Chachage 2014; Lawn 2001; Kalinkovich 1999; Kalinkovich 2001; Secor 2003).
There may be increased suppression of the antiviral T helper (Th)1 lymphocyte due to helminth‐associated Th2 lymphocyte propagation (Borkow 2006; Brown 2006; Kinter 2007).
There may be higher levels of eosinophilia, increased immunoglobulin (Ig) E levels, and stimulation of other immunosuppressive cytokine responses (Bentwich 1996; Blish 2010).
In HIV‐positive individuals, co‐infection with helminths may lead to the accelerated destruction of the host immune system and earlier onset of acquired immunodeficiency syndrome (AIDS)‐defining illnesses and death (Nesheim 2007). Even amongst HIV‐positive patients treated with antiretroviral (ART) drugs for HIV, helminth infections may adversely influence HIV clinical outcomes (Ivan 2015). Given these profound effects on host immunity, helminth infections have been suggested to play an important role in the pathogenesis of HIV in Africa (Bentwich 1995; Fincham 2003).
Description of the intervention
Current World Health Organization (WHO) guidelines recommend that high‐risk populations living in endemic areas participate in regular mass drug administration with antihelminthic medicines (deworming drugs) without the need for prior confirmatory diagnostic testing. This is also known as preventive chemotherapy. In settings where community prevalence of STH infections exceed 20%, the WHO recommends annual treatment with albendazole or mebendazole for all preschool‐age children, school‐age children, and pregnant women in their second and third trimester (WHO 2006).
The rationale for mass treatment rather than a test and treat approach is the safety of the drugs, low cost of the drugs (often donated by pharmaceutical companies), the relatively high cost of diagnostic testing, and the high prevalence of infection in some areas. The cost of these programmes is estimated to be as low as USD 0.25 per treatment, including delivery costs (Bundy 2009; Partnership for Child Development 1998). Mass deworming programs are scaling up rapidly in part due to the WHO NTD Roadmap for Implementation and the 2012 WHO‐endorsed London Declaration, which calls for the control or elimination of 10 neglected tropical diseases including STHs, schistosomiasis, LF, and onchocerciasis by 2020 (Uniting to Combat NTDs 2012).
In research settings deworming treatment may require prior confirmatory testing or treatment may be delivered empirically as customary in preventative chemotherapy campaigns. For example, specific age groups may be targeted and recruited to receive deworming medications during community‐based household recruitment, at HIV clinics, at tuberculosis (TB) clinics, or during antenatal care.
How the intervention might work
If helminth co‐infection plays a significant detrimental role in HIV disease progression it is possible that effective treatment or prevention of helminth infections could slow the progression of HIV (Kallestrup 2005 ZWE; Wolday 2002). Relatively modest increases in viral load (0.3 to 0.5 log10 copies/mL) may increase the annual risk of progression to an AIDS‐defining illness or death by as much as 25% to 44% (Modjarrad 2008). Mathematical modelling suggests that a reduction in HIV‐1 ribonucleic acid (RNA) levels of 0.5 log10 copies/mL could slow the onset of AIDS by 3.5 years and could delay the need for ART medications by almost a full year (Gupta 2007). The potential increases in HIV RNA associated with helminth co‐infection suggest that for every 100 HIV‐positive individuals with an STH infection in sub‐Saharan Africa, there could be 3.1 (95% CI: 0.1 to 14.9) excess HIV‐1 transmission events. The trend is similar for other helminth infections, with 8.5 (95% CI 0.2 to 38.6) excess HIV transmission events attributed to schistosomiasis and 13.3 (95% CI 0.3 to 89.2) to filariasis (Baggaley 2015).
Observational studies of the effects of deworming drugs on markers of HIV disease progression have had conflicting results. Several studies have noted delays in HIV disease progression following deworming (Brown 2005; Ivan 2015; Lankowski 2014; Mulu 2013; Muok 2013; Wolday 2002 ), while others have reported no association (Brown 2004; Elliott 2003; Hosseinipour 2007; Kleppa 2014; Lawn 2000; Modjarrad 2005). In the previous version of this systematic review we found some evidence from randomized controlled trials of short term benefits with deworming but also the need for further larger studies (Walson 2009).
Why it is important to do this review
Given that a substantial number of individuals targeted by helminth control programmes may be exposed to or infected with HIV, it is important to establish the safety of deworming drugs in HIV‐positive populations and the potential for drug interactions, particularly as an increased incidence of drug reactions has been observed in patients with advanced HIV infection (Gordin 1984; Nunn 1991). Available data from a recent systematic review also suggested the potential for an interaction between both mebendazole and albendazole and nucleoside reverse transcriptase inhibitors appears low, although no formal pharmacokinetic studies exist. The review also reported that interactions may exist between praziquantel and protease inhibitors via enzyme inhibition (Seden 2013).
Since the publication of our initial Cochrane Review of this topic in 2009 (Walson 2009), deworming campaigns have been dramatically scaled up and it has become increasingly important to understand not only the effects of deworming on HIV disease progression but also the safety of treating these unique patient populations. In this update, we have expanded the scope of this Cochrane Review to include trials that evaluate the impact of deworming on anaemia and iron deficiency, as well as markers of HIV disease progression and safety.
Objectives
To evaluate the effects of deworming drugs (antihelminthic therapy) on markers of HIV disease progression, anaemia, and adverse events in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) or quasi‐RCTs.
Types of participants
Adults and children (older than one year of age) infected with human immunodeficiency virus (HIV)‐1 or HIV‐2 with and without documented helminth co‐infection.
Relevant helminth‐infections included schistosomiasis (Schistosomiasis mansoni and Schistosomiasis haematobium), STH (Strongyloides stercoralis, Ancylostoma duodenale,Necator americanus, Trichuris trichiura, Ascaris lumbricoides, and Trichostrongylus), lymphatic filariasis (LF) (Wuchereria bancrofti,Brugia malayi, and Brugia timori), onchocerciasis (Onchocerca volvulus), and other filariasis (Mansonella streptocerca).
For trials that included both HIV‐positive and uninfected participants, we contacted the trial authors and requested data relevant to the HIV‐positive participants only.
Types of interventions
Intervention
Any antihelminthic drug therapy recommended by World Health Organization (WHO) guidelines for use in the eradication of helminth infections in humans. This included the benzimidazoles (albendazole or mebendazole), ivermectin, praziquantel, diethylcarbamazine (DEC), bithionol, oxamniquine, pyrantel, and nitazoxanide (WHO 2006).
Control
Placebo or no treatment. For the outcome of adverse events, we also considered alternative antihelminthic drugs.
Types of outcome measures
Primary outcomes
Change in plasma viral load (log10copies viral RNA/mL).
Change in CD4+ T‐cell count (cells/µL).
Secondary outcomes
Change in CD8+ T‐cell count (cells/µL).
CD4+/CD8+ T‐cell ratio.
Adverse events associated with antihelminthic therapy (if reported in the included trials).
Iron deficiency (as defined by the trial authors, based on a biomarker of iron status and tests, at 34 weeks gestation or later in pregnant populations).
Anaemia (defined as haemoglobin (Hb) below 110 g/L, adjusted for altitude and smoking as appropriate).
Death.
Search methods for identification of studies
Electronic searches
In coordination with a the Information Specialist of the Cochrane Infectious Diseases Group (CIDG), we searched MEDLINE online (1980 to 2015), EMBASE (1980 to 2015), CENTRAL (1980 to 2015), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (all dates), and clinicaltrials.gov (1980 to 2015) using the strategies documented in Table 4, Table 5, Table 6, Table 7, and Table 8, respectively. As this is a review update, we also searched MEDLINE, EMBASE, CENTRAL, and AIDSEARCH databases using the exact search terms utilized in the previously published in Walson 2009 up to 29 September 2015 (Table 9; Table 10; Table 11; Table 12). We conducted a separate search in the WHO Global Health Library, which accesses AIM (AFRO), LILACS (AMRO/PAHO), IMEMR (EMRO), IMSEAR (SEARO), WPRIM (WPRO), WHOLIS (KMS), and SciELO resources (Table 13).
1. Cochrane librarian search strategy for MEDLINE.
| Number | Search terms |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #3 | Search (helminths[mh] OR helminth*[tiab] OR nematode*[tiab] OR worm*[tiab] OR parasites[mh] OR parasit*[tiab] OR round worm*[tiab] OR roundworm*[tiab] OR hookworm*[tiab] OR hook worm*[tiab] OR ancylostoma*[tiab] OR cestode*[tiab] OR tapeworm*[tiab] OR tape worm*[tiab] OR trematode*[tiab] OR fluke*[tiab] OR whipworm*[tiab] OR whip worm*[tiab] OR trichuris[tiab] OR ascaris[tiab] OR enterobi*[tiab] OR strongyloide*[tiab] OR mansonell*[tiab] OR taenia[tiab] schistosom*[tiab] OR necator*[tiab] OR paragonim*[tiab] OR hymenolepis[tiab] OR fasciol*[tiab] OR filariasis[tiab] OR trichostrongl*[tiab] OR microfilaria[tiab] OR parasitic diseases[mh:noexp] OR helminthiasis[mh] OR intestinal diseases, parasitic[mh]) |
| #4 | Search (benzimidazoles OR albendazole OR mebendazole OR ivermectin OR praziquantel OR diethylcarbamazine OR bithionol OR oxamniquine OR pyrantel OR nitazoxanide OR anthelmintic OR anthelmintics OR anthelminthic OR anthelminthics OR “anti helminthic” OR “anti helminthics” OR “anti helmintic” OR “anti helmintics” OR antihelminthic OR antihelminthics OR antihelmintic OR antihelmintics) |
| #5 | Search (#1 AND #2 AND #3 AND #4) |
| #6 | Search (((#1 AND #2 AND #3 AND #4))) AND ("1980/01/01"[Date ‐ Publication] : "2015/09/29"[Date ‐ Publication]) |
2. Cochrane librarian search strategy for EMBASE.
| Number | Search terms |
| #1 | 'human immunodeficiency virus infection' exp OR 'human immunodeficiency virus infection':ab,ti OR 'hiv infection':ab,ti OR 'hiv infections':ab,ti OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR hiv:ab,ti OR 'hiv 1':ab,ti OR 'hiv 2':ab,ti OR 'human immune deficiency virus':ab,ti OR 'human immuno deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno deficiency syndrome':ab,ti OR 'acquired immune deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl*NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (crossNEXT/1 over*):ab,ti |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #5 | #3 AND #4 |
| #6 | #3 NOT #5 |
| #7 | #2 NOT #6 |
| #8 | 'helminth'/exp OR helminth*:ab,ti OR nematode*:ab,ti OR worm*:ab,ti OR 'parasite'/exp OR parasit*:ab,ti OR roundAND worm*:ab,ti OR roundworm*:ab,ti OR hookworm*:ab,ti OR hookAND worm*:ab,ti OR ancylostoma*:ab,ti OR cestode*:ab,ti OR tapeworm*:ab,ti OR tapeAND worm*:ab,ti OR trematode*:ab,ti OR fluke*:ab,ti OR whipworm*:ab,ti OR whipAND worm*:ab,ti OR trichuris:ab,ti OR ascaris:ab,ti OR enterobi*:ab,ti OR strongyloide*:ab,ti OR mansonell*:ab,ti OR taenia:ab,ti AND schistosom*:ab,ti OR necator*:ab,ti OR paragonim*:ab,ti OR hymenolepis:ab,ti OR fasciol*:ab,ti OR filariasis:ab,ti OR trichostrongl*:ab,ti OR microfilaria:ab,ti OR 'parasitic diseases'/exp OR 'helminthiasis'/de OR 'intestine infection'/de |
| #9 | 'benzimidazoles'/de OR benzimidazolesOR 'albendazole'/de OR albendazoleOR 'mebendazole'/de OR mebendazoleOR 'ivermectin'/de OR ivermectinOR 'praziquantel'/de OR praziquantelOR 'diethylcarbamazine'/de OR diethylcarbamazineOR 'bithionol'/de OR bithionolOR 'oxamniquine'/de OR oxamniquineOR 'pyrantel'/de OR pyrantelOR 'nitazoxanide'/de OR nitazoxanideOR 'anthelmintic'/de OR anthelminticOR 'anthelmintics'/de OR anthelminticsOR 'anthelminthic'/de OR anthelminthicOR anthelminthicsOR 'anti helminthic'OR 'anti helminthics'OR 'anti helmintic'OR 'anti helmintics'OR antihelminthicOR antihelminthicsOR 'antihelmintic'/de OR antihelminticOR 'antihelmintics'/de OR antihelmintics |
| #10 | #1 AND #7 AND #8 AND #9 |
| #11 | #1 AND #7 AND #8 AND #9 AND [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
3. Cochrane librarian search strategy for CENTRAL.
| Number | Search terms |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | [mh helminths] or helminth*:ti,ab,kw or meatode*:ti,ab,kw or worm*:ti,ab,kw or [mh parasites] or parasit*:ti,ab,kw or round worm:ti,ab,kw or roundworm:ti,ab,kw or hook worm*:ti,ab,kw or hookworm*:ti,ab,kw or ancylostoma*:ti,ab,kw or cestode*:ti,ab,kw or tapeworm*:ti,ab,kw or tape worm*:ti,ab,kw or trematode*:ti,ab,kw or fluke*:ti,ab,kw or whipworm*:ti,ab,kw or whip worm*:ti,ab,kw or trichuris:ti,ab,kw or ascaris:ti,ab,kw or enterobi*:ti,ab,kw or strongyloide*:ti,ab,kw or mansonell*:ti,ab,kw or taenia:ti,ab,kw schistosom*:ti,ab,kw or necator*:ti,ab,kw or paragonim*:ti,ab,kw or hymenolepis:ti,ab,kw or fasciol*:ti,ab,kw or filariasis:ti,ab,kw or trichostrongl*:ti,ab,kw or microfilaria:ti,ab,kw or [mh ^"parasitic diseases"] or [mh helminthiasis] or [mh ^"intestinal diseases, parasitic"] (Word variations have been searched) |
| #8 | benzimidazoles or albendazole or mebendazole or ivermectin or praziquantel or diethylcarbamazine or bithionol or oxamniquine or pyrantel or nitazoxanide or anthelmintic or anthelmintics or anthelminthic or anthelminthics or "anti helminthic" or "anti helminthics" or "anti helmintic" or "anti helmintics" or antihelminthic or antihelminthics or antihelmintic or antihelmintics (Word variations have been searched) |
| #9 | #6 and #7 and #8 Publication Year from 1980 to 2015, in Trials |
4. Cochrane librarian search strategy for the WHO ICTRP.
| Number | Search terms |
| #1 | HIV AND HELMINTH |
5. Cochrane librarian search strategy for clinicaltrials.gov.
| Number | Search terms |
| #1 | HIV AND HELMINTH | Interventional Studies | received from 01/01/1980 to 09/29/2015 |
6. Search terms used in 2009 newly applied in MEDLINE.
| Number | Search terms |
| #1 | “HIV Infections”[MeSH] OR “HIV”[MeSH] OR hiv [tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immunodeficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immunodeficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR “Sexually Transmitted Diseases, Viral”[MeSH:NoExp] |
| #2 | randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) |
| #3 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CESTODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #4 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #5 | #3 AND #4 |
| #6 | #1 AND #2 AND #5 |
| #7 | #1 AND #2 AND #5 Limits: Publication Date from 1980 to 2015/09/29 |
7. Search terms used in 2009 newly applied in EMBASE.
| Number | Search terms |
| #1 | ((’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’) OR (’human immunodeficiency virus infection’/exp OR ’human immunodeficiency virus infection’)) OR ((’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’) OR (’human immunodeficiency virus’/exp OR ’human immunodeficiency virus’)) OR (hiv:ti OR hiv:ab) OR (’hiv‐1’:ti OR ’hiv‐1’:ab) OR (’hiv‐2’:ti OR ’hiv‐2’:ab) OR (’human immunodeficiency virus’:ti OR ’human immunodeficiency virus’:ab) OR (’human immuno‐deficiency virus’:ti OR ’human immuno‐deficiency virus’:ab) OR (’human immunedeficiency virus’:ti OR ’human immunedeficiency virus’:ab) OR (’human immune‐deficiency virus’:ti OR ’human immune‐deficiency virus’:ab) OR (’acquired immune‐deficiency syndrome’:ti OR ’acquired immune‐deficiency syndrome’:ab) OR (’acquired immunedeficiency syndrome’:ti OR ’acquired immunedeficiency syndrome’:ab) OR (’acquired immunodeficiency syndrome’:ti OR ’acquired immunodeficiency syndrome’:ab) OR (’acquired immuno‐deficiency syndrome’:ti OR ’acquired immuno‐deficiency syndrome’:ab) |
| #2 | random:ti OR random:ab OR factorial:ti OR factorial*:ab OR 'cross over':ti OR 'cross over':ab OR crossover:ti OR crossover:ab OR placebo:ti OR placebo:ab OR (double:ti AND blind:ti) OR (doubl:ab AND blind*:ab) OR (single:ti AND blind:ti) OR (single:ab AND blind:ab) OR assign:ti OR assign:ab OR allocat:ti OR allocate:ab OR volunteer:ti OR volunteer:ab OR 'crossover procedure'/exp OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial' |
| #3 | 'helminths' OR 'roundworm' OR 'round worm' OR 'round‐worm' OR roundworms OR 'round worms' OR 'round‐worms' OR nematodes OR 'nematode' OR 'cestode' OR cestodes OR 'tapeworm' OR 'tape worm' OR 'tape‐worm' OR tapeworms OR 'tape worms' OR 'tape‐worms' OR 'trematode' OR trematodes OR 'fluke' OR flukes OR 'worm' OR worms OR 'parasite' OR 'parasites' OR 'ascaris' OR 'trichuris' OR 'enterobius' OR strongyloide OR stronglyloides OR 'ancylostoma' OR ancylostomas OR 'necator' OR necators OR 'hymenolepis' OR 'paragonimus' OR 'fasciola' OR 'taenia' OR 'hookworm' OR 'hook worm' OR 'hook‐worm' OR hookworms OR 'hook worms' OR 'hook‐worms' OR 'whipworm' OR 'whip worm' OR 'whip‐worm' OR whipworms OR 'whip worms' OR 'whip‐worms' OR shistosomiasis OR 'mansonella' OR 'filariasis' OR 'microfilaria' OR 'trichostrongylus' OR trichostronglylosis OR stronglyloidea OR pargonimiasis |
| #4 | ((’benzimidazoles’/exp OR ’benzimidazoles’) OR (’benzimidazoles’/exp OR ’benzimidazoles’)) OR ((’albendazole’/exp OR ’albendazole’) OR (’albendazole’/exp OR ’albendazole’)) OR ((’mebendazole’/exp OR ’mebendazole’) OR (’mebendazole’/exp OR ’mebendazole’)) OR ((’ivermectin’/exp OR ’ivermectin’) OR (’ivermectin’/exp OR ’ivermectin’)) OR ((’praziquantel’/exp OR ’praziquantel’) OR (’praziquantel’/exp OR ’praziquantel’)) OR ((’diethylcarbamazine’/exp OR ’diethylcarbamazine’) OR (’diethylcarbamazine’/exp OR ’diethylcarbamazine’)) OR ((’bithionol’/exp OR ’bithionol’) OR (’bithionol’/exp OR ’bithionol’)) OR ((’oxamniquine’/exp OR ’oxamniquine’) OR (’oxamniquine’/exp OR ’oxamniquine’)) OR ((’pyrantel’/exp OR ’pyrantel’) OR (’pyrantel’/exp OR ’pyrantel’)) OR ((’nitazoxanide’/exp OR ’nitazoxanide’) OR (’nitazoxanide’/exp OR ’nitazoxanide’)) |
| #5 | #3 OR #4 |
| #6 | #1 AND #2 AND #5 [1‐1‐1980]/sd NOT [29‐09‐2015]/sd |
8. Search terms used in 2009 newly applied in CENTRAL.
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) |
| #2 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS OR HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #3 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 from 1980 to 2015 |
9. Search terms used in 2009 newly applied in AIDSEARCH.
| Number | Search terms |
| #1 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐ BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (“CLINICAL TRIAL”) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND*)) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN) |
| #3 | #1 AND #2 |
| #4 | HELMINTHS OR ROUNDWORM OR ROUND WORM OR ROUND‐WORM OR ROUNDWORMS OR ROUND WORMS OR ROUND‐WORMS OR NEMATODES OR NEMATODE OR CESTODE OR CES‐TODES OR TAPEWORM OR TAPE WORM OR TAPE‐WORM OR TAPEWORMS OR TAPE WORMS OR TAPE‐WORMS OR TREMATODE OR TREMATODES OR FLUKE OR FLUKES OR WORM OR WORMS OR PARASITE OR PARASITES OR ASCARIS OR TRICHURIS OR ENTEROBIUS OR STRONGYLOIDE OR STRONGYLOIDES OR ANCYLOSTOMA OR ANCYLOSTOMAS OR NECATOR OR NECATORS |
| #5 | HYMENOLEPIS OR PARAGONIMUS OR FASCIOLA OR TAENIA OR HOOKWORM OR HOOK WORM OR HOOK‐WORM OR HOOKWORMS OR HOOK WORMS OR HOOK‐WORMS OR WHIPWORM OR WHIP WORM OR WHIP‐WORM OR WHIPWORMS OR WHIP WORMS OR WHIP‐WORMS OR SCHISTOSOMIASIS OR MANSONELLA OR FILARIASIS OR MICROFILARIA OR TRICHOSTRONGYLUS OR TRICHOSTRONGYLOSIS OR STRONGYLOIDEA OR PARAGONIMIASIS |
| #6 | BENZIMIDAZOLES OR ALBENDAZOLE OR MEBENDAZOLE OR IVERMECTIN OR PRAZIQUANTEL OR DIETHYLCARBAMAZINE OR BITHIONOL OR OXAMNIQUINE OR PYRANTEL OR NITAZOXANIDE |
| #7 | #4 OR #5 OR #6 |
| #8 | #7 AND #3 |
10. Search strategy for the WHO Global Health Library.
| Number | Search terms |
| #1 | Helminth* AND "HIV Infections" received from 01/01/1980 to 09/29/2015 |
We did not apply any language or date restrictions.
Searching other resources
Reference lists
We examined all references cited by included trials to identify any further studies for inclusion.
Data collection and analysis
Selection of studies
Two review authors (ARM and PB) independently read the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches and discarded any irrelevant reports. When there was uncertainty as to the relevance of the study, we maintained the citation for further review. Two review authors (ARM and PB) then independently evaluated the full‐text articles of all identified citations to establish relevance of the article according to the pre‐specified criteria.
We reviewed the studies for relevance based on study design, types of participants, exposures, and outcome measures. ARM and PB independently applied the inclusion criteria for this Cochrane Review update. There were three differences that required a third review author (JW) to resolve.
Data extraction and management
Two review authors (ARM and PB) independently extracted data from the included trials using standardized data extraction forms for randomized trials, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We extracted the following characteristics from each included trial.
Administrative details: identification; author(s); published or unpublished; year of publication; year in which study was conducted; funding source.
Details of study: study design; randomization method; duration; completeness of follow‐up; country and location of the study; setting (for example, urban or rural, hospital or clinic); method(s) of recruitment; number of participants by trial arm.
Characteristics of participants: age; gender; socioeconomic status; HIV clinical staging (if available); antiretroviral (ART) status.
Details of intervention: medication; dose; duration; number of treatments; control group.
Details of outcomes: primary outcome; unit of measurement; change in viral load; change in CD4+ count; change in rate of clinical HIV disease progression (changes in the WHO or Centers for Disease Control and Prevention (CDC) staging); nutritional indicators; adverse events; mortality.
Assessment of risk of bias in included studies
Two review authors (ARM and PB) independently evaluated the methodological quality of included clinical trials using the Cochrane 'Risk of bias' assessment tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We followed the guidance to assess whether adequate steps were taken to reduce the risk of bias across five domains: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; and incomplete outcome data.
For sequence generation and allocation concealment, we reported the methods used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion of participants lost to follow‐up. We categorized our 'Risk of bias' judgements as either 'low', 'high', or 'unclear'. Where risk of bias was unclear, we attempted to contact the trial authors for clarification and resolved any differences of opinion through discussion.
Measures of treatment effect
We summarized dichotomous outcomes using risk ratios (RR) with 95% confidence interval (CIs). We summarized continuous outcomes using mean differences with 95% CIs. For some studies we utilized only data from HIV‐positive participants and the trial authors provided the raw data accordingly.
HIV disease progression is associated with a decline in CD4+ cell counts and an increase in viral load. A favourable effect of deworming drugs on CD4+ cell count therefore refers to either a larger increase in CD4+ cell count or a smaller depletion in CD4+ cell count. A suppressive effect on viral load refers to either a reduction in viral load, or a smaller increase in viral load.
Unit of analysis issues
For trials with repeat outcome measurements over time, we utilized and reported the study's final outcome measure reflecting the whole follow‐up time for each individual participant. For studies with 2 x 2 factorial designs in which there were more than one treatment group we chose not to combine experimental intervention groups into a single group and risk loss of information regarding the effects of different antihelminthic treatments. Rather, we chose to present the interventions separately and divide the total number of participants in the control group to account for multiple comparisons in meta‐analyses. The means and standard deviations were left unchanged as recommended in Section 16.5.4 of the Cochrane Handbook (Higgins 2011). This method only partially overcomes the unit‐of‐analysis error (because the resulting comparisons remain correlated). However, an advantage of this approach is that it allows for investigations of heterogeneity across intervention arms.
Dealing with missing data
We contacted trial authors where there were missing or unclear data. We considered missing data resulting from losses to follow‐up to be missing at random unless there were indications otherwise.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by inspecting the forest plots (to detect overlapping CIs) and the I² statistic with a level of 50% to denote moderate levels of heterogeneity, as well as by applying the Chi² test with a P value of 0.10 to indicate statistical significance, as described in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We could not perform an assessment of the likelihood of reporting bias due to an insufficient number of included trials.
Data synthesis
We pooled the results from included RCTs in six different meta‐analyses, using a random‐effects method, due to the heterogeneity of the interventions (DerSimonian 1986). We performed pooled meta‐analyses to assess the outcomes of change in mean plasma viral load in populations with unknown helminth infection status, change in mean CD4+ cell count in populations with unknown helminth infection status, change in mean plasma viral load in helminth‐infected populations only, change in mean CD4+ cell count in helminth‐infected populations only, difference in adverse events, and difference in mortality events.
We assessed the quality of evidence by outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. We used GRADEprofiler Guideline Development Tool (GDT) software (GRADEpro GDT 2015) to import data from Review Manager (RevMan) (RevMan 2014) to create three 'Summary of findings' tables. For each of the review outcomes we created a summary of the intervention effect and a measure of quality using the GRADE approach whereby the quality of evidence received a grade of either 'very low', 'low', 'moderate', or 'high', by outcome. We assessed the quality of the evidence based on five criteria: risk of bias, imprecision, indirectness, inconsistency, and publication bias.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses with respect to trials in which participants had confirmed helminth infections, as well as trials in which participants had unknown infection status. We planned to investigate statistical heterogeneity by conducting subgroup analysis with respect to antihelminthic treatment used, participant age, ARV initiation status, and geographic location. However, we could not do so because of the limited number of studies included for meta‐analyses.
Sensitivity analysis
We assessed the robustness of the results by conducting a sensitivity analysis against the 'Risk of bias' criteria.
Results
Description of studies
Results of the search
In this Cochrane Review update, we identified 312 references using the CIDG Information Specialist's search criteria described above and an additional 1936 unique references by applying the search criteria utilized in the 2009 Cochrane Review (Walson 2009). Of the 2248 citations screened, we excluded 2203 citations due to irrelevance to antihelminthics, no inclusion of HIV‐positive participants, or both. We retrieved and formally reviewed 42 full‐text articles for eligibility (see Figure 1 for the study flow diagram).
1.
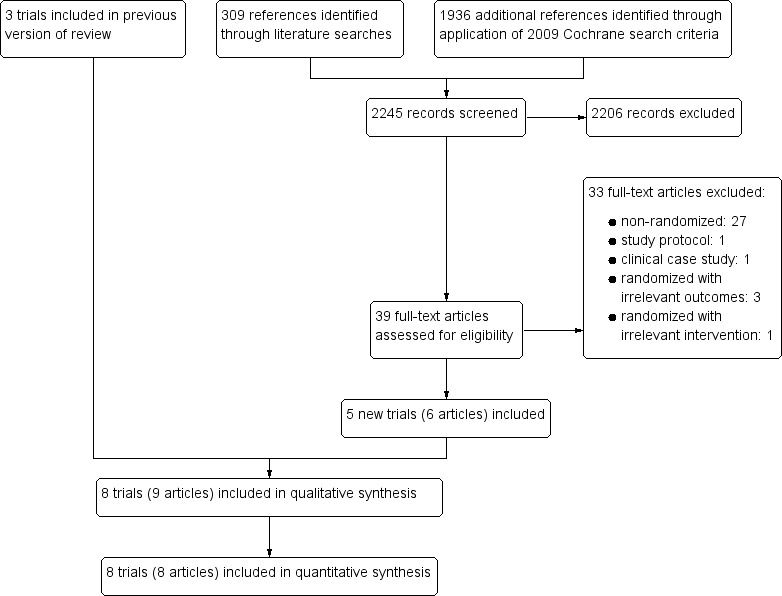
Study flow diagram.
Included studies
Eight randomized controlled trials (RCTs) met the inclusion criteria of this Cochrane Review. Three were included in the 2009 version of this Cochrane Review (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN) and we added five new trials, due to more recent publication dates or the expanded scope of the protocol (Abate 2014 ETH; Kelly 1996 ZMB; Suputtamongkol 2011 THA; Walson 2012 KEN; Webb 2012 UGA).
Seven trials were conducted in sub‐Saharan Africa, and one in Thailand (Suputtamongkol 2011 THA). All eight trials included adults infected with HIV‐1 or HIV‐2, and one trial was limited to pregnant adult women (Webb 2012 UGA). One small trial from Zambia recruited only adults with chronic diarrhoea (Kelly 1996 ZMB). There were no trials in preschool or school‐age children.
The RCTs aimed to evaluate a range of primary outcomes: changes in tuberculosis (TB) morbidity factors (Abate 2014 ETH), diarrhoea duration (Kelly 1996 ZMB), clearance of Strongyloides infection (Suputtamongkol 2011 THA), CD4+/CD8+ cell ratios (Nielsen 2007 TZA), CD4+ cell count (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN), and HIV‐1 RNA viral load (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA).
In two trials, HIV‐positive participants were not taking antiretroviral (ART) drugs (Walson 2008 KEN; Walson 2012 KEN), in one trial ART use was limited to prevention of mother‐to‐child transmission of HIV (Webb 2012 UGA), and in one trial ART use was very high (Abate 2014 ETH). Four trials did not specify if the HIV‐positive individuals were currently taking ART (Kallestrup 2005 ZWE; Kelly 1996 ZMB; Nielsen 2007 TZA; Suputtamongkol 2011 THA).
Three trials evaluated deworming drugs provided presumptively to participants with unknown helminth infection status: Walson 2012 KEN administered albendazole (400 mg) every three months and praziquantel (40 mg/kg) annually for two years; Webb 2012 UGA administered either albendazole (800 mg), praziquantel (40 mg/kg), or both to pregnant women and followed up for six weeks and at delivery; and Kelly 1996 ZMB administered albendazole (800 mg) twice daily for two weeks to adults with chronic diarrhoea (see Table 14 and Table 15).
11. Characteristics of trials in which participants had unknown helminth infection status.
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Kelly 1996 ZMB | Zambia | Urban | Not stated | HIV‐infected adults with persistent diarrhoea | > 18 years | 174 | 174 | Not stated (probably low) | Unknown |
| Webb 2012 UGA | Uganda | Urban | 2005 | HIV‐infected pregnant women | Not stated | 264 | 264 | < 3% | 67% had at least 1 helminth species |
| Walson 2012 KEN | Kenya | Urban/rural | 2011 | HIV‐infected, not on ART | > 18 years | 948 | 948 | None at baseline | Unknown |
Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral.
12. Description of interventions.
| Trial | Intervention | Control | Outcomes | Timing | ||
| Drug | Dose | Frequency | ||||
| Walson 2008 KEN | Albendazole | 400 mg | Once daily for 3 days | Placebo | Viral load CD4 Adverse events Mortality events |
12 weeks |
| Abate 2014 ETH | Albendazole | 400 mg | Once daily for 3 days | Placebo | CD4 Adverse events Mortality events |
12 weeks |
| Kallestrup 2005 ZWE | Praziquantel | 40 mg/kg | Once only | No intervention | Viral load CD4 |
12 weeks |
| Nielsen 2007 TZA | Diethylcarbamazine | 6 mg/kg | Once only | Placebo | Viral load CD4 Adverse events |
12 weeks |
| Kelly 1996 ZMB | Albendazole | 800 mg | Twice daily for 14 days | Placebo | Adverse events Mortality events |
6 months |
| Suputtamongkol 2011 THA | Ivermectin | 200 mg/kg | Single or double dose 2 weeks apart | Albendazole | Adverse events | 1 year |
| Webb 2012 UGA | Albendazole Praziquantel |
400 mg 40 mg/kg |
Once only Once only |
Placebo | Viral load Adverse events Mortality events |
6 weeks |
| Walson 2012 KEN | Albendazole Praziquantel |
400 mg 25 mg/kg |
Every 3 months Annually |
No intervention | Viral load CD4 Adverse events Mortality events |
2 years |
Five RCTs treated HIV‐positive adults with confirmed helminth infections; two trials treated soil‐transmitted helminth (STH) infections with albendazole (400 mg) once daily for three days (Abate 2014 ETH; Walson 2008 KEN), one study treated schistosomiasis with praziquantel (40 mg/kg) once only (Kallestrup 2005 ZWE), one study treated STH or schistosomiasis with albendazole, praziquantel, or both (Webb 2012 UGA), and one trial treated lymphatic filariasis (LF) with diethylcarbamazine (DEC) (Nielsen 2007 TZA). These trials followed participants for six to 12 weeks (see Table 16 and Table 15).
13. Characteristics of trials in which participants had confirmed helminth infections.
| Trial | Country | Setting | Year enrolment completed | Inclusion criteria | Age | Total randomized | Number of HIV‐positive participants | Taking ART | Number co‐infected with helminths at baseline |
| Walson 2008 KEN | Kenya | Urban/rural | 2007 | HIV‐infected with at least one helminth co‐infection | > 18 years | 234 | 234 | None at baseline | All |
| Abate 2014 ETH | Ethiopia | Urban | 2012 | Newly diagnosed TB participants with helminth co‐infection | 15 to 60 years | 140 | 32 | 94% of intervention group, 100% of controls | All |
| Kallestrup 2005 ZWE | Zimbabwe | Rural | 2003 | Adults infected with schistosomiasis | > 18 years | 287 | 130 | Not stated | All |
| Nielsen 2007 TZA | Tanzania | Rural | 2002 | HIV‐infected adults | 22 to 70 years | 34 | 34 | Not stated | 18 |
| Suputtamongkol 2011 THA | Thailand | Urban | 2009 | Adults with characteristic strongyloides infection | > 18 years | 100 | 10 | Not stated | All |
Abbreviations: HIV: human immunodeficiency virus; ART: antiretroviral; TB: tuberculosis.
Five trials measured and reported HIV‐1 RNA viral load (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA), and four trials reported CD4+ cell count (Kallestrup 2005 ZWE; Nielsen 2007 TZA; Walson 2008 KEN; Walson 2012 KEN). One study reported CD4+/CD8+ cell ratio, seven trials reported adverse events, one trial reported measures of iron deficiency and anaemia respectively, and six trials reported mortality.
We requested unpublished data from all but one trial author (Kelly 1996 ZMB), and we included these data in the analyses.
Excluded studies
We excluded 33 studies due to their observational designs or irrelevance to the intervention or outcomes of interest. See the 'Characteristics of excluded studies' table. We identified two ongoing or planned RCTs, and have provided descriptions of these trials in Table 17.
14. Ongoing or planned RCTs.
| Trial name | Relevant intervention | Relevant outcome | Target population | Location(s) | Estimated completion date |
| Reduction of EArly mortaLITY in HIV‐infected Adults and Children Starting Antiretroviral Therapy (REALITY) | Immediate enhanced opportunistic infections (OI) prophylaxis with isoniazid/pyridoxine and cotrimoxazole, plus 12 weeks fluconazole, 5 days azithromycin, and a single dose of albendazole versus cotrimoxazole prophylaxis alone for the first 12 weeks followed by isoniazid and any prophylaxis and/or treatment prescribed at screening | • Change in CD4 count • Adverse events |
HIV‐infected individuals ages ≥ 5 years | Kenya, Malawi, Uganda, Zimbabwe | February 2016 |
| Can Anthelminthic Treatment Delay the Progression of HIV? Randomised Open‐label Trial Testing Presumptive Anthelminthic Treatment on Progression of HIV in ART‐naïve HIV‐positive Patients in a Rural African Setting With Presumed High Prevalence of Helminth Infections | Standard HIV care with provision of praziquantel, albendazole, and ivermectin at baseline, after 6 months, and after 12 months versus standard HIV care with no anthelminthic treatment | • Change in viral load • Change in CD4 count • Adverse event |
HIV‐infected individuals aged ≥ 18 years | Tanzania | Terminated prematurely due to recruitment difficulties |
Abbreviations: RCT: randomized controlled trial; HIV: human immunodeficiency virus; ART: antiretroviral.
Risk of bias in included studies
We have presented a summary of our 'Risk of bias' assessments in Figure 2 and Figure 3.
2.
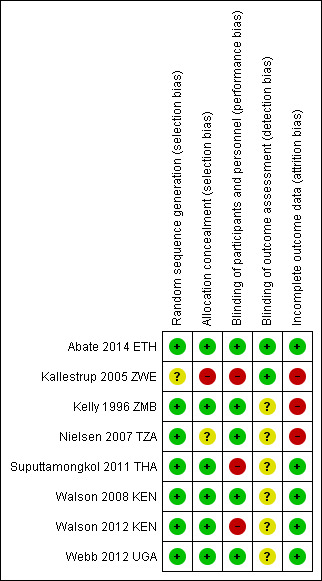
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial.
3.
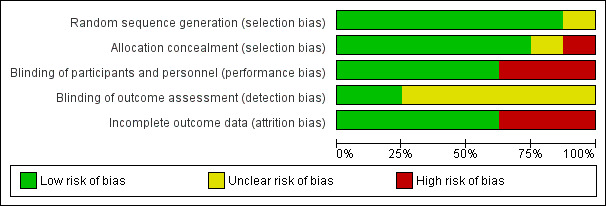
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
Allocation
Five trials adequately described both random sequence generation and allocation concealment and we judged them at low risk of selection bias (Abate 2014 ETH; Suputtamongkol 2011 THA; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA). One trial did not describe allocation concealment and was at unclear risk of selection bias (Nielsen 2007 TZA), and one stated that allocation was 'open' and was at high risk of selection bias (Kallestrup 2005 ZWE). All included trials appeared to have similar reported baseline characteristics between randomization groups.
Blinding
Five trials used placebos and we judged them to be at low risk of performance bias (systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest) (Abate 2014 ETH; Kelly 1996 ZMB; Nielsen 2007 TZA; Walson 2008 KEN; Webb 2012 UGA). We judged the remaining trials to be at high risk of performance bias (Kallestrup 2005 ZWE; Suputtamongkol 2011 THA; Walson 2012 KEN). In addition, individual participants who received more frequent treatment doses had more opportunities to interface with health facilities as an inherent aspect of the intended trial design and may have non‐differentially had access to improved care.
Only two trials adequately described blinding of outcomes assessment to prevent detection bias (Abate 2014 ETH; Kallestrup 2005 ZWE). The remaining trials were at unclear risk of bias. Two trials utilized diagnostic methods with low sensitivity, such as duplicate Kato‐Katz from a single stool sample (Walson 2008 KEN; Webb 2012 UGA), which might affect the total sample size of helminth‐infected participants, but not the fidelity of the intervention or outcome measures.
Incomplete outcome data
Attrition bias may have been an issue in one included trial, Nielsen 2007 TZA, where there appeared to be differential loss to follow‐up between the groups. In addition, two trials reported that less than 80% of the established participant cohort were followed‐up (Kallestrup 2005 ZWE; Kelly 1996 ZMB), although Kallestrup 2005 ZWE reported that reasons for study drop‐out were evenly distributed, with the exception of a single group which had a higher number of losses to follow‐up due to migration.
Selective reporting
We could not perform an assessment of the likelihood of reporting bias due to an insufficient number of included trials.
Five trials performed and reported an intention‐to‐treat analysis (Abate 2014 ETH; Kallestrup 2005 ZWE; Walson 2008 KEN; Walson 2012 KEN; Webb 2012 UGA). The three remaining trials performed outcome comparisons only for participants for whom follow‐up data were available or relevant (Kelly 1996 ZMB; Nielsen 2007 TZA; Suputtamongkol 2011 THA).
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison: antihelminthic drugs versus placebo or no intervention
Primary outcomes: markers of HIV disease progression
Participants with unknown helminth infection status
Two trials evaluated the effects of antihelminthic drugs versus placebo on HIV disease progression amongst HIV‐positive adults in sub‐Saharan Africa with unknown helminth infection status. One trial administered either albendazole (800 mg), praziquantel (40 mg/kg), or both, to pregnant women and followed up for six weeks (Webb 2012 UGA), and one administered albendazole (400 mg) every three months and praziquantel (40 mg/kg) annually for two years (Walson 2012 KEN).
Viral load
In the single included trial of pregnant women, the mean viral load at six weeks was lower with each of the interventions, but the confidence intervals (CIs) included the possibility of no difference (difference in mean change −0.14 log10 viral RNA, 95% CI −0.35 to 0.07; P = 0.19, one trial, 166 participants, Analysis 1.1).
1.1. Analysis.
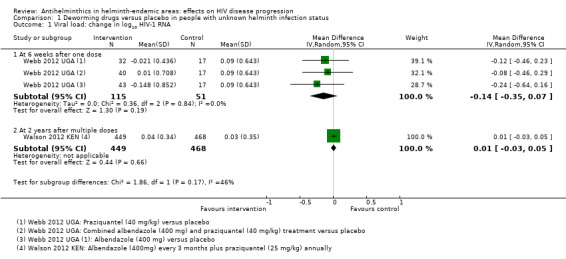
Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 1 Viral load: change in log10 HIV‐1 RNA.
In the larger trial from Kenya, there was no substantial difference in mean change in viral load between treatment and control groups after two years of repeated drug administration (difference in mean change 0.01 log10 viral RNA, 95% CI −0.03 to 0.05; P = 0.66, one trial, 917 participants, Analysis 1.1).
CD4+ cell count
Only the long‐term trial from Kenya reported mean change in CD4+ cell counts. There was no substantial difference in mean change in CD4+ count between the treatment and control groups over two years of repeated drug administration (difference in mean change 2.60 cells/µL/year, 95% CI −10.15 to 15.35; P = 0.7, one trial, 917 participants, Analysis 1.2).
1.2. Analysis.
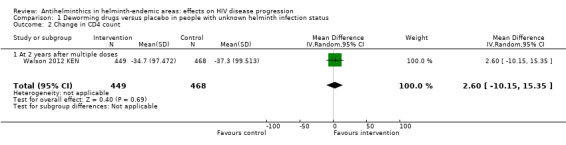
Comparison 1 Deworming drugs versus placebo in people with unknown helminth infection status, Outcome 2 Change in CD4 count.
Participants with confirmed helminth infections
Five trials treated HIV‐positive adults known to be infected with helminths; two trials treated STH infection with albendazole (400 mg) once daily for three days (Abate 2014 ETH; Walson 2008 KEN), one trial treated schistosomiasis with praziquantel (40 mg/kg) once only (Kallestrup 2005 ZWE), and one trial treated LF with DEC (Nielsen 2007 TZA). These trials reported these outcomes at 12 weeks. One trial also provided outcome data at six weeks for participants infected with schistosomiasis and STHs (Webb 2012 UGA).
Viral load
In a pooled analysis across four trials with different helminth infections and different drug regimens, there was an overall suppressive effect on viral load after six to 12 weeks in the intervention groups compared to controls (difference in mean change −0.13 log10 viral RNA, 95% CI −0.26 to −0.00; P = 0.04, four trials, 445 participants, Analysis 2.1). However the CIs of the individual trials are wide and the CIs of all but one trial, Kallestrup 2005 ZWE, included the possibility of no effect. In this trial of HIV and schistosomiasis co‐infected participants, those treated with praziquantel had little change in mean plasma viral load at 12 weeks (−0.001 log10 viral RNA), while the mean viral load substantially increased in the untreated group (0.21 log10 viral RNA, P = 0.03). This trial was at high risk of selection bias, and exclusion of this trial broadens the CI of the pooled estimate to allow the possibility of no difference.
2.1. Analysis.
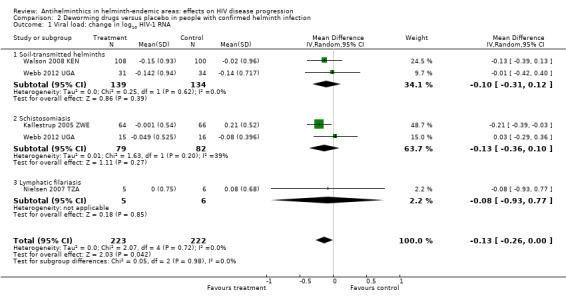
Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 1 Viral load: change in log10 HIV‐1 RNA.
CD4+ cell count
In three trials mean CD4+ cell count declined in both treated and control groups over 12 weeks, with a smaller decrease in the treated groups (Analysis 2.2). However in one trial of TB‐infected individuals who initiated both TB treatment and ART at baseline, CD4+ cell counts increased in both treated and control groups with a larger increase in those treated with deworming drugs (difference in mean change 43.00 cells/µL, 95% CI 1.14 to 84.86; one trial, 208 participants). In the pooled analysis, the average change in CD4+ cell count was more favourable in the group treated with deworming drugs, and the CI excludes the possibility of no effect (difference in mean change 37.86 cells/µL, 95% CI 7.36 to 68.35; P = 0.01; three trials, 358 participants, Analysis 2.2).
2.2. Analysis.
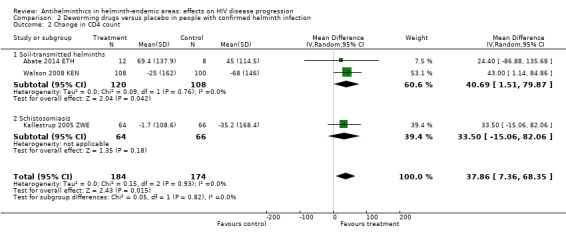
Comparison 2 Deworming drugs versus placebo in people with confirmed helminth infection, Outcome 2 Change in CD4 count.
Nielsen 2007 TZA presented results as declines in CD4+ per cent, and thus we did not include the results in the pooled meta‐analysis (Analysis 2.2).
Secondary outcomes
Adverse events
Seven trials commented on adverse events, of which five reported that no adverse events occurred (Abate 2014 ETH; Nielsen 2007 TZA; Suputtamongkol 2011 THA; Walson 2008 KEN; Webb 2012 UGA).
Kelly 1996 ZMB administered albendazole twice daily for two weeks to adults with chronic diarrhoea, and reported seven adverse events in the treatment group and three in the control group. Of the seven adverse events with albendazole, one participant had a cutaneous reaction that resolved within one week of discontinuing the drug, and four complained of dizziness, headache, cough, and difficulty swallowing. The trial authors noted that the symptoms were indistinguishable from symptoms of the underlying illness. Walson 2012 KEN administered praziquantel annually and albendazole every three months for two years, and reported 16 severe adverse events in the treatment group and 18 in the control group. The trial authors determined that none of the adverse events were directly related to the study drug.
The pooled analysis of these two trials does not suggest a significant increase in relative risk for adverse events in HIV‐positive individuals who receive antihelminthics relative to those who do not (RR 1.23, 95% CI 0.53 to 2.83; P = 0.63, seven trials, 1649 participants, Analysis 3.2).
3.2. Analysis.
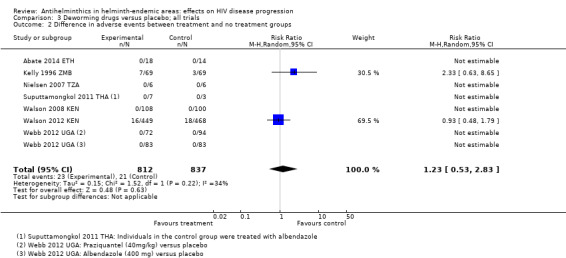
Comparison 3 Deworming drugs versus placebo; all trials, Outcome 2 Difference in adverse events between treatment and no treatment groups.
Mortality
Six included trials recorded mortality events, of which five trials observed at least one death (Abate 2014 ETH; Kelly 1996 ZMB; Suputtamongkol 2011 THA; Walson 2008 KEN; Walson 2012 KEN). In the pooled analysis, treatment was associated with a point estimate of a 33% reduction in mortality, but the CIs included no effect (RR 0.77, 95% CI 0.52 to 1.14; P = 0.19, five trials, 1627 participants, Analysis 3.3). Most deaths occurred in the oldest study where participants were chronically unwell (had chronic diarrhoea) and were probably not on ART (Kelly 1996 ZMB). None of the deaths appeared to be directly associated with the antihelminthic interventions, as reported by the trial authors.
3.3. Analysis.
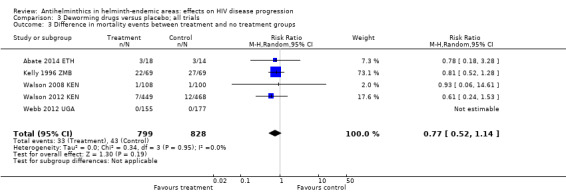
Comparison 3 Deworming drugs versus placebo; all trials, Outcome 3 Difference in mortality events between treatment and no treatment groups.
Anaemia and iron deficiency
Only one trial captured data regarding changes in serum haemoglobin levels (Kallestrup 2005 ZWE). The trial authors reported a mean difference in haemoglobin of −0.25 g/dL (95% CI −0.58 to −0.08) in the HIV‐positive participants that received praziquantel relative to the untreated participants over three months follow‐up (unpublished data).
A secondary analysis of the primary Nielsen 2007 TZA trial examined the effect of antihelminthic treatment on micronutrient indicators in LF and HIV co‐infected individuals (Nielsen 2009). The study found that serum ferritin levels increased in co‐infected individuals 12 weeks after treatment. The study authors observed a log mean increase of 0.07 µg/L in the treatment group, while they noted a log mean increase of 0.04 µg/L in the non‐treated group. The mean difference in log ferritin levels between baseline and 12 weeks follow‐up in the treated group relative to the control group was thus 0.03 µg/L (95% CI −0.30 to 0.35) (unpublished data provided by the study authors). As the study authors did not observe the same increase in ferritin in the HIV‐positive LF‐uninfected groups, the study authors hypothesize that the serum ferritin changes may be related to the immune mechanisms involved in killing of filaria.
Discussion
Summary of main results
In human immunodeficiency virus (HIV)‐infected antiretroviral (ART)‐naive adults with unknown helminth infection status, provision of deworming drugs (albendazole and praziquantel together or separately) may have a small suppressive effect on viral load at six weeks. However, these data come from a small single trial and the 95% confidence interval (CI) includes the possibility of no effect (low quality evidence). Repeated dosing over two years appears to have little or no effect in HIV‐positive ART‐naive adults (moderate quality evidence).
Treating helminth infections in HIV‐positive individuals with confirmed helminth infections may have a small suppressive effect on mean viral load at six to 12 weeks (low quality evidence). However, this finding is strongly influenced by a single study of praziquantel for schistosomiasis and further studies from different settings and populations are needed for validation. There may also be a small favourable effect on mean CD4+ cell count at 12 weeks.
Most of the included trials did not consistently or rigorously report adverse events (very low quality evidence) and trials were underpowered to evaluate effects on mortality (low quality evidence). However, there is no indication of excessive risk associated with deworming HIV‐positive populations.
Overall completeness and applicability of evidence
Despite the publication of five new RCTs since the previous edition of this Cochrane Review, Walson 2009, there is still insufficient evidence to make definitive conclusions about the effects of deworming drugs on markers of HIV disease progression. The eight trials included evaluate different drugs used to treat different helminth infections, which limits our ability to make broad generalizations.
For preventive chemotherapy programmes, in which deworming drugs are provided presumptively to all individuals, this Cochrane Review includes only two relevant trials and only one that reported long‐term effects. In the short‐term trial there is a suggestion of a short‐term benefit to deworming, however the trial is too small to prove an effect. Similarly, while the single long‐term trial found no evidence of benefit, further trials from other settings with different helminth endemicities are necessary before the possibility of long‐term beneficial effects can be fully excluded.
Only short‐term evidence is available when we limit the analysis to HIV‐positive adults with confirmed helminth infections. The finding of a suppressive effect on viral load in this group is highly reliant on a single trial of praziquantel for schistosomiasis. While the effect estimate of the pooled analysis is small (−0.13 log10), mathematical modelling exercises estimate that a decrease in viral load of 0.3 log10 in heterosexual couples could decrease HIV transmission by an average of 20% and AIDS‐related illnesses or death by 25% (Modjarrad 2008).
It is also important to note that none of the trials included children, and only one trial included a significant number of people taking ART (Abate 2014 ETH). These are therefore important groups for further research.
While overall few adverse events were reported in the included trials (23 in total in treated participants and 21 in total in non‐treated participants), it is important to note that only three trials defined adverse events, one with a detailed definition of qualifying adverse events (Kelly 1996 ZMB), and two with more generalized definitions (Suputtamongkol 2011 THA; Webb 2012 UGA). Only one trial reported how adverse events were monitored, using patient record‐keeping cards and house visits (Kelly 1996 ZMB). Despite these biases, most randomized controlled trials (RCTs) reported no adverse events following antihelminthic therapy and there is no evidence of excessive harm associated with deworming in HIV‐positive populations. To validate review findings, larger RCTs should be conducted with well‐documented and active adverse event identification, as well as frequent follow‐up points to assess how deworming may affect HIV‐positive populations of all ages over time.
Quality of the evidence
We have presented the GRADE assessment of the quality of evidence in the included trials in the 'Summary of findings' tables (Table 1; Table 2; Table 3). The overall assessment of quality ranges from moderate (representing a moderate level of certainty in the result) to very low (where we are very uncertain of the result).
The primary reason for downgrading the quality of evidence was 'indirectness', where we had too few trials to make broad generalizations about the potential effects. This is a particular problem for trials that evaluate deworming in populations with unknown helminth infection status as helminth endemicity varies substantially across settings, undoubtedly influencing observed findings. Although we had more data from trials that evaluated deworming drugs in participants with documented helminth infections, the substantial heterogeneity in populations and deworming drugs used again decreased our confidence in making broad generalizations. The second most common reason for downgrading the quality of evidence was 'imprecision' in effect estimates. Many of the included trials were small, and had wide 95% CIs, which included the possibility of both clinically important effects and no effect.
Potential biases in the review process
Judd Walson is a co‐author of this Cochrane Review and was Primary Investigator on two of the included RCTs. However, he was not involved in conducting any of the 'Risk of bias' assessments or in data extraction for this Cochrane Review.
There were not enough trials available in the published literature to be able to assess for publication bias.
Agreements and disagreements with other studies or reviews
The first review on this topic, Walson 2007a, identified only one RCT for inclusion that evaluated the potential benefit of treating helminth co‐infection in HIV‐1‐infected individuals (Kallestrup 2005 ZWE). Subsequently two additional RCTs were published (Nielsen 2007 TZA; Walson 2008 KEN), and a Cochrane Review of all three RCTs concluded that treatment of helminth co‐infection appeared to result in favourable effects on markers of HIV‐1 disease progression (Walson 2009). Specifically, the pooled analysis of the data suggested that treatment of helminth co‐infection may attenuate increases in HIV‐1 RNA and result in slower decline in CD4+ counts.
The findings of this Cochrane Review update are consistent with the previous edition (Walson 2009). However, we used the GRADE approach to assess the quality of evidence, which caused us to downgrade the quality of the evidence. As we stratified by helminth infection status, we also separately provide evidence for individuals who were helminth‐infected or of unknown infection status.
Authors' conclusions
Implications for practice.
There is low quality evidence that treating confirmed helminth infections in HIV‐positive adults has a short‐term favourable effect on markers of HIV disease progression. Further studies are necessary to confirm this finding. However, there is no evidence that deworming is harmful. This is reassuring for the routine treatment of confirmed or suspected helminth infections in people living with HIV in co‐endemic areas.
Further long‐term studies would be required to make conclusions about the likely impact of a policy of deworming all HIV‐positive individuals irrespective of helminth infection status (i.e. preventive chemotherapy) as the only trial to date did not demonstrate an effect.
Given the large favourable effect of ART on HIV disease progression, the additive effect of regular deworming, if any, is unknown.
Implications for research.
Further long‐term studies are required to make conclusions regarding the likely impact of deworming all HIV‐positive individuals irrespective of helminth infection status, as the only trial to date did not demonstrate an effect.
Future RCTs should consider measuring both helminth infection status and long‐term HIV disease progression outcomes repeatedly between baseline and end line to understand how disease progression changes over differential amounts of follow‐up time. These RCTs should have well‐documented and active adverse event identification. Given that deworming is often targeted at preschool and school‐age children, it is also important to identify the benefit in HIV‐positive children.
What's new
| Date | Event | Description |
|---|---|---|
| 11 April 2016 | New citation required and conclusions have changed | In this review update we included eight trials: three were in the 2009 version of this Cochrane review, Walson 2009, and we included five new trials. We assessed the quality of the evidence using GRADE criteria, and produced 'Summary of findings' tables. |
| 11 April 2016 | New search has been performed | We included eight trials in this review update. Bradley R Herrin and Grace John‐Stewart stepped down from the review author team, and Arianna Rubin Means, Paul Burns, and David Sinclair joined the review author team. We amended the title of this review from 'Deworming helminth co‐infected individuals for delaying HIV disease progression' to 'Antihelminthics in helminth‐endemic areas: effects on HIV disease progression'. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 19 August 2014 | Amended | Update started. |
Acknowledgements
We thank Dr. Juan Pablo Peña‐Rosas for his helpful insight and feedback throughout the duration of this project, and Joy Oliver for her guidance in the systematic search process. In addition, we acknowledge Ebba Abate, Per Kallestrup, Emily Webb, Yupin Suputtamongkol Krista Yuhas, and Nina Nielsen, who provided additional unpublished data to include in this Cochrane Review update.
The editorial base of the Cochrane Infectious Diseases Group (CIDG) is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). David Sinclair is supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Data and analyses
Comparison 1. Deworming drugs versus placebo in people with unknown helminth infection status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Viral load: change in log10 HIV‐1 RNA | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 weeks after one dose | 1 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.07] |
| 1.2 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2 Change in CD4 count | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
| 2.1 At 2 years after multiple doses | 1 | 917 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐10.15, 15.35] |
Comparison 2. Deworming drugs versus placebo in people with confirmed helminth infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Viral load: change in log10 HIV‐1 RNA | 4 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 1.1 Soil‐transmitted helminths | 2 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.31, 0.12] |
| 1.2 Schistosomiasis | 2 | 161 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.36, 0.10] |
| 1.3 Lymphatic filariasis | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 2 Change in CD4 count | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 37.86 [7.36, 68.35] |
| 2.1 Soil‐transmitted helminths | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 40.69 [1.51, 79.87] |
| 2.2 Schistosomiasis | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 33.5 [‐15.06, 82.06] |
Comparison 3. Deworming drugs versus placebo; all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in log10 HIV RNA, by antihelminthic | 5 | 1534 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.01] |
| 1.1 Albendazole | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.02] |
| 1.2 Praziquantel | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.32, ‐0.03] |
| 1.3 Diethylcarbamazine | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.93, 0.77] |
| 1.4 Albendazole and praziquantel | 2 | 1008 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2 Difference in adverse events between treatment and no treatment groups | 7 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.53, 2.83] |
| 3 Difference in mortality events between treatment and no treatment groups | 5 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.14] |
3.1. Analysis.
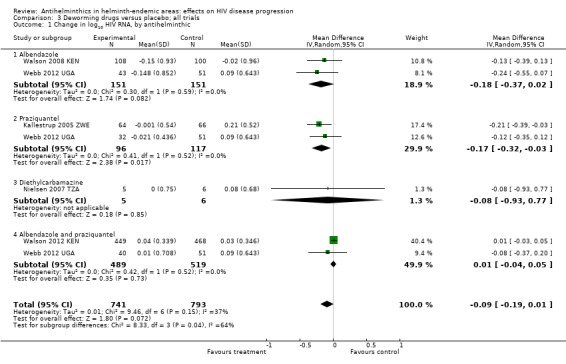
Comparison 3 Deworming drugs versus placebo; all trials, Outcome 1 Change in log10 HIV RNA, by antihelminthic.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abate 2014 ETH.
| Methods | Trial design: randomized, double‐blind, placebo‐controlled trial. Participants: adults with and without HIV infection who were found to be co‐infected with soil‐transmitted helminths (STHs) and tuberculosis (TB). Data of the HIV seropositive cohort only are included in this analysis Length of follow‐up: relevant outcomes (CD4+ T cell count) were recorded at baseline and after three months. Monitoring and diagnostics: all helminth co‐infections were diagnosed using triplicate stool samples, direct microscopy, and Kato‐Katz techniques. Participants were routinely tested for human immunodeficiency virus (HIV) at TB treatment centres using rapid test kits, Stat‐Pak, and Unigold. |
|
| Participants | Number of participants: a total of 140 helminth‐positive TB participants were enrolled and randomized. 72 participants were randomized to the treatment arm and 68 to the placebo arm. We included only data from the 18 HIV‐positive participants in the treatment arm and 14 HIV‐positive participants in the placebo arm in this Cochrane Review.
Inclusion criteria: newly diagnosed TB participants (15 to 60 years of age) presenting at a university referral hospital and co‐infected with an STH infection. Exclusion criteria: participants that required hospital admission, were pregnant, infected with Schistosoma spp., displayed symptoms of active helminth infection, or displayed signs of any concomitant chronic or infectious disease other than TB/HIV. |
|
| Interventions | Intervention: albendazole treatment (400 mg/day) for 3 consecutive days. All helminth‐positive TB participants, including the placebo group, received deworming treatment at week 12. Randomization occurred 2 weeks following TB treatment. Control: identical placebo tablets. |
|
| Outcomes | Outcomes included in this review: change in CD4+ T cells after three months, adverse events, and mortality events. Other trial outcomes: the primary outcome of the trial was change in TB score after 2 months. Other secondary outcomes were sputum smear conversion after 2 months, changes in the chest x‐ray pattern at week 12, IgE and eosinophil responses, as well as changes in the frequency of Tregs and IFN‐c, IL‐5, and IL‐10 producing peripheral blood mononuclear cells (PBMCs) after 3 months. |
|
| Notes | Location: Gondar, Ethiopia. Participant helminth status: ascertained. Participant antiretroviral (ART) status: 94% and 100% of participants were on ART in the intervention and placebo groups, respectively. Note: this is the only trial in which all participants had a major co‐infection beyond the helminth and HIV relationship of interest, which might limit comparability to other findings in this review. Author contact: we requested additional data for the HIV cohort alone from the trial authors, who provided the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were generated in a block size of eight by the Addis Continental Institute of Public Health, Ethiopia." |
| Allocation concealment (selection bias) | Low risk | "All tablets looked identical and were assigned a treatment code by the manufacturer.The treatment allocated for each patient was concealed in an individual envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both the investigators and clinic staff were blinded to the treatment given." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The treatment code was kept in a sealed envelope at the manufacturer and opened after the last patient had been to a follow‐up visit and the data had been analysed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No risk of attrition; 15% lost to follow‐up with 72 participants in the intervention group and 68 participants in the control group. |
Kallestrup 2005 ZWE.
| Methods | Trial design: randomized, unblinded, controlled trial. Participants: adults with and without HIV‐1 infection who were found to be infected with schistosomes. We only included data from the HIV seropositive cohort in this analysis. Length of follow‐up: HIV‐1 RNA and CD4+ T cell counts were measured at baseline and after 3 months. Monitoring and diagnostics: from Kallestrup 2005; Status for HIV was determined by a rapid HIV‐1/2 test in the field on a dry blood spot and two enzyme‐linked immunosorbent assays on serum. Infection with Schistosoma haematobium was diagnosed by microscopic identification and quantification of fixed‐volume urine samples filtered on Nytrel filters. Diagnosis of infection with Schistosoma mansoni and other helminth eggs or parasites was assessed by the modified formol‐ether concentration technique on 1 g of stool from each participant. |
|
| Participants | Number of participants: 287 individuals were enrolled of whom we included 130 with HIV‐1 and schistosome co‐infection in this analysis. 64 participants received early praziquantel treatment and 66 received delayed treatment. Inclusion criteria: HIV‐1 and schistosomiasis co‐infection, HIV‐negative schistosomiasis infected, HIV‐1 negative but schistosomiasis infected, or neither infection. Exclusion criteria: pregnant women and participants presenting with clinical signs/symptoms of TB, terminal stages of schistosomiasis, or severe anaemia. |
|
| Interventions | Intervention: participants received a single oral dose of praziquantel (40 mg/kg) at enrolment or after a delay of 3 months. Control: participants received a single oral dose of praziquantel (40 mg/kg) after a delay of 3 months following enrolment. |
|
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels, CD4+ T cell count, and haemoglobin levels between individuals randomized to early versus delayed treatment. Other trial outcomes: none |
|
| Notes | Location: Shamva District, Zimbabwe. Participant helminth status: ascertained. Participant ART status: not stated. Author contact: we requested additional haemoglobin data from the trial authors for this update, who provided the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact random sequence generation used unclear. From Kallestrup 2005: "Allocation of participants to these 3 control groups was done randomly: 1 participant with schistosomiasis was selected for every 2 coinfected participants, and 1 HIV‐1–positive participant or 1 healthy participant was selected for every 4 coinfected participants." |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not done. "On inclusion, all participants infected with schistosomes within each HIV‐1 group were openly randomised". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded; "On inclusion, all participants infected with schistosomes within each HIV‐1 group were openly randomised into 2 equally sized groups". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | From Kallestrup 2005: "At all stages, when performing parasitological examinations, the technician was blinded to clinical and serological information". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is a risk of attrition as there was < 80% follow‐up. |
Kelly 1996 ZMB.
| Methods | Trial design: randomized double blind placebo controlled trial. Participants: HIV‐1 or HIV‐2 seropositive adults with persistent diarrhoea. Length of follow‐up: adverse and mortality events were recorded over 6 months of follow‐up. Monitoring and diagnostics: ELISA tests were used to determine HIV seropositivity. Home care staff noted any potential adverse effects. |
|
| Participants | Number of participants: 174 participants were initially randomized but only 138 participants were followed‐up after 1 month and considered correctly randomized, with 69 in the intervention group and 69 in the placebo‐controlled group. Inclusion criteria: HIV‐positive adults with persistent diarrhoea (defined as loose but not bloody stools 3 or more times a day for 3 weeks or longer). Exclusion criteria: participants were excluded if they had received antibiotics in the preceding week, or were deteriorating clinically (Karnofsky score ≤ 20). |
|
| Interventions | Intervention: 800 mg albendazole twice daily for 14 days for treatment of persistent diarrhoea in HIV‐positive participants. Control: identical placebos twice daily for 14 days. |
|
| Outcomes | Outcomes included in this review: incidence of adverse events and mortality. Incidence of adverse events defined as exacerbated diarrhoea, cutaneous reaction, dizziness, headache, cough, and difficulty swallowing. Other trial outcomes: proportion of time periods during which diarrhoea was experienced after completion of treatment and proportion of participants with full remission after completion of treatment. |
|
| Notes | Location: 3 urban hospitals/health centres in Zambia. Participant helminth status: not ascertained. Participant ART status: not specified, but the study took place before treatment was widely available in Zambia. Author contact: we did not contact the trial authors to provide additional information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the patients were randomised by allocation of a study pack containing either albendazole or placebo, which had been prepared in London according to a randomisation code." |
| Allocation concealment (selection bias) | Low risk | "The code (constructed so that the numbers of patients randomised to albendazole and placebo balanced every 20 patients) was kept in London during the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Each pack contained 112 tablets of albendazole or placebo, which were indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is a risk of attrition as there was < 80% follow‐up. |
Nielsen 2007 TZA.
| Methods | Trial design: randomized double‐blind placebo‐controlled cross‐over trial. Participants: HIV‐positive individuals with or without Wuchereria bancrofti co‐infection. Length of follow‐up: all subjects were followed‐up at 1, 12, 13, and 24 weeks after the 1st round of treatment. The data presented in the published manuscript considered the 12‐week visit as the initial visit and the 24‐week visit as the final visit. Individuals treated at the initial visit with diethylcarbamazine (DEC) were then considered as the 'placebo' arm and those who were not treated until the 12‐week visit were the 'treatment' arm. For the purposes of this analysis, we considered only individuals with confirmed HIV‐1 infection who were also filariasis‐infected at the baseline visit. We considered individuals treated with DEC at the initial visit to be in the 'treatment' arm and those who did not receive treatment to be in the 'placebo' arm. We reported outcomes as indicated for the 12‐week visit. As all participants were treated with DEC by the 12 week visit, we did not include data from the 24‐week follow‐up period. Monitoring and diagnostics: diagnosis of infection with lymphatic filariasis (LF) infection with W. bancrofti was performed using immunochromatographic tests (ICT) followed by ELISA testing for circulating filarial antigens (CFA) in serum samples. Participants were also screened for malaria and intestinal helminth eggs. |
|
| Participants | Number of participants: the trial authors screened 858 adults and 34 HIV‐1 infected individuals were enrolled and randomized in the trial, of which 27 were followed‐up. Eighteen were co‐infected with W. bancrofti, and 16 were not co‐infected. In the co‐infected group, 10 participants were randomized to early treatment and 8 participants were randomized to delayed treatment. Twelve of these participants were followed‐up, 6 in each treatment group. The HIV RNA from one of the participants in the delayed treatment arm could not be amplified. Inclusion criteria: HIV‐positive individuals without clinical manifestations of HIV, with and without LF infection. Exclusion criteria: none specified. |
|
| Interventions | Intervention: DEC (6 mg/kg) at randomization. Control: equivalent placebo and treatment after a delay of 3 months. |
|
| Outcomes | Outcomes included in this review: plasma HIV‐1 RNA levels, CD4+ cell count, CD4 percent, serum concentrations of ferritin, adverse events. Other trial outcomes: CD4/CD8 ratio between individuals randomized to early versus delayed treatment (3 months later) (Nielsen 2007 TZA). Baseline and change in serum retinol, β‐carotene, α‐tocopherol, and the acute phase reactant a‐1 antichymotrypsin after 3 months (Nielsen 2009). |
|
| Notes | Location: Northeastern Tanzania Participant helminth status: ascertained Participation ART status: the trial authors did not specify if any participants were receiving ART treatment at the start or during the trial. Author contact: we requested additional CD4 and HIV‐1 RNA data from the trial authors, who provided the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomised (1:1). "The identical DEC and placebo tablets were packed in containers with different color codes (“red” or “blue”). Individuals were randomised (1:1) to receive treatment in the order of “red” followed by “blue” or “blue” followed by “red” by using a list of random numbers.The selected study participants were listed and numbered from 1 to 34, and the first 17 numbers (between 1 and 34) encountered in the table (when starting on a randomly chosen figure) were assigned to receive treatment in the order red–blue, and the remaining 17 received treatment in the order blue–red." |
| Allocation concealment (selection bias) | Unclear risk | This was not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study personnel and participants were blinded to treatment assignment throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There is a risk of attrition as there was < 80% follow‐up. However, this reflects 7 participants who were lost to follow‐up and therefore excluded from the analyses. The excluded participants did not significantly differ at baseline from the included participants. |
Suputtamongkol 2011 THA.
| Methods | Trial design: randomized, non‐blinded study. Participants: adult participants with chronic strongyloidiasis. Length of follow‐up: participants were followed up 2 weeks after treatment initiation, then 1 month, 3 months, 6 months, 9 months, and 1 year post‐treatment. Monitoring and diagnostics: infection with Strongyloides stercoralis was ascertained using the direct smear, formol‐ether concentration method, and modified Koga agar plate culture method. |
|
| Participants | Number of participants: 90 adult participants with chronic Strongyloides infection were recruited. There were 10 participants with HIV co‐infections, with 3 HIV‐positive participants randomized to albendazole, 2 HIV‐positive participants randomized to single dose ivermectin, and 5 HIV‐positive participants randomized to double dose ivermectin. Inclusion criteria: adult participants with characteristic rhabditiform larvae of S. stercoralis present on faecal microscopy. Exclusion criteria: history of allergic reaction to either study medication, treatment within the month prior to the trial with any drug known to have anti‐Strongyloides activity, pregnancy, or lactation, and any suggestion of disseminated strongyloidiasis. |
|
| Interventions | Intervention: Group 1: ivermectin delivered as a single dose of 200 µg/kg; Intervention. Group 2: 2 doses of ivermectin (200 µg/kg) delivered 2 weeks apart. For the purpose of this analysis we considered both groups that received ivermectin together. Control: participants received 7 days of albendazole (800 mg per day). |
|
| Outcomes | Outcomes included in this review: incidence of adverse events defined as "symptoms or signs that developed after the study drug administration and had not been reported prior to the administration of the first dose of the antihelmintic." Other trial outcomes: treatment cure (defined as clinical improvement (if symptomatic before treatment) and the absence of rhabditiform larvae in the stool at day 14 of treatment and throughout the follow‐up period) and treatment failure (defined as the presence of larvae two weeks after initiation of treatment or the reappearance of larvae during follow‐up). |
|
| Notes | Location: Siriraj Hospital, Thailand Participant helminth status: ascertained Participant ART status: it was unspecified if any individuals were receiving ART treatment at the start or during the trial. Author contact: we requested additional data regarding the incidence of adverse events in the HIV‐positive participants specifically from the trial authors, who provided this information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated, simple, random allocation sequences were prepared for 3 study groups by the investigator team." |
| Allocation concealment (selection bias) | Low risk | "These were sealed in an opaque envelope and numbered. The investigator assigned study participants to their respective treatment group after opening the sealed envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "prospective open‐label, randomised, controlled study". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 10% loss to follow‐up; "Ten patients were excluded from analysis because they did not receive or complete the study treatment (3 in albendazole group, 2 in ivermectin‐II group), or they were lost to follow‐up immediately after treatment (3 in albendazole group, 1 each in ivermectin‐I and ivermectin‐II respectively)." |
Walson 2008 KEN.
| Methods | Trial design: randomized double‐blind placebo‐controlled trial. Partcipants: HIV‐1 positive adults with evidence of co‐infection with albendazole‐treatable STHs. Length of follow‐up: all subjects were followed‐up at 12 weeks post‐randomization. Monitoring and diagnostics: helminth diagnosis was performed using stool samples processed and evaluated with wet preparation, Kato‐Katz, and formol‐ether concentration techniques. HIV‐1 was diagnosed using Determine™ rapid test qualitative immunoassay. The CD4 lymphocyte count was determined using Multiset™ software on a FACSCalibur machine. Plasma HIV‐1 RNA was quantified using the Gen‐Probe HIV‐1 viral load assay. |
|
| Participants | Number of participants: the trial screened 1551 adults attending HIV care clinics and 299 were infected with at least 1 helminth species. Regarding enrolment, 234 ART‐naive individuals were enrolled, of whom 208 HIV and STH co‐infected (hookworm, Ascaris, Trichuris, or Strongyloides) individuals were included in the final analysis, 108 were randomized to receive early treatment and 100 to receive placebo. Inclusion criteria: HIV‐1 seropositive adults, not pregnant, and ineligible for initiation of ART based on WHO guidelines (CD4 < 200 cells/mm³, any stage 4 and some stage 3 disease). Exclusion criteria: ever used ART drugs, took medicine for helminth infection in the preceding 6 months, evidence of active TB or TB treatment in the past 3 months, and clinical signs of severe anaemia. |
|
| Interventions | Intervention: albendazole (400 mg per day) for 3 days versus placebo at enrolment. Control: placebo at enrolment. After a delay of 3 months, all participants showing evidence of helminth infection were treated with albendazole, regardless of randomization arm. |
|
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels and CD4+ count between individuals randomized to early versus delayed treatment (3 months later) and adverse events. Other trial outcomes: none. |
|
| Notes | Location: 10 sites throughout Kenya. Participant helminth status: ascertained. Participant ART status: treatment naive. Author contact: the trial author provided additional CD4 and HIV‐1 RNA data for inclusion in this Cochrane Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to two groups using a 1:1 allocation scheme with block randomisation of 30 patients and following a random‐allocation list generated independently." |
| Allocation concealment (selection bias) | Low risk | "Pre‐labeled, sequentially numbered treatment packs were used. Both the active drug (albendazole) and an identical appearing placebo were provided by the drug manufacturer." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators, clinic staff and patients were blinded to study‐group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No risk of attrition; 0.5% lost to follow‐up, with 4 in the intervention arm and 5 in the control arm. |
Walson 2012 KEN.
| Methods | Trial design: non‐blinded randomized study. Partcipants: HIV‐positive ART naive adults in Kenya. Length of follow‐up: CD4+ cell counts measured every 6 months and plasma RNA measured every 12 months. Participants were followed for 24 months. Monitoring and diagnostics: HIV serological testing was conducted with Determine™ rapid tests, CD4 cell counts using a FACSCalibur™, plasma HIV RNA assays with the COBAS® Amplicor assay. |
|
| Participants | Number of participants: 979 individuals were screened for enrolment in the trial and 917 individuals were enrolled and eligible, with 449 participants randomized to the treatment group and 468 randomized to the control group. All participants were administered cotrimoxazole prophylaxis. Inclusion criteria: adults ≥ 18 years, HIV seropositive, and do not meet criteria for ART initiation (on the basis of documented WHO disease stage and CD4+ cell count within the previous 3 months and a clinical assessment at enrolment). Exclusion criteria: pregnancy, reports of taking antihelminthics in the previous 6 months, reports of having previously received ART ((except for the prevention of mother‐to‐child transmission). |
|
| Interventions | Intervention: empiric deworming with repeat single‐dose albendazole (400 mg) given every 3 months plus single dose praziquantel (25 mg/kg) given annually. Participants were excluded if they missed two or more consecutive doses of study drug. Control: standard of care. |
|
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels, CD4+ cell count, non‐traumatic death, and adverse events between individuals randomized to treatment versus no intervention. Other trial outcomes: time to CD4+ count of < 350 cells/μL and first occurrence of any of the following: CD4+ count < 350 cells/μL, first reported use of ART (excluding that used for the prevention of mother‐to‐child transmission), or non‐traumatic death. Secondary analyses included time to death and ART initiation separately. |
|
| Notes | Location: 3 sites in Kenya Participant helminth status: helminth status of participants was not evaluated at baseline. Participant ART status: treatment naive Author contact: the trial author provided additional CD4+ and HIV‐1 RNA data for inclusion in this Cochrane Review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer‐generated randomisation sequence, we assigned participants (1:1) to either the treatment group or control group." |
| Allocation concealment (selection bias) | Low risk | "We used a computerised database to ensure that treatment allocation was not disclosed to study staff and participants until randomisation was complete." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no risk of attrition; 4.2% of participants were lost to follow‐up with 16 in the intervention arm and 24 in the control arm. |
Webb 2012 UGA.
| Methods | Trial design: 2 x 2 randomized double‐blind placebo controlled trial. Partcipants: HIV‐positive ART‐naive pregnant women. Length of follow‐up: viral load was measured 6 weeks post‐treatment and at delivery. However, this analysis included data at 6 weeks post‐treatment only in order to minimize the effects of prenatal HIV care provided to study participants. Monitoring and diagnostics: stool samples were processed and examined for helminth ova using a duplicate Kato‐Katz method and by charcoal culture for Strongyloides. |
|
| Participants | Number of participants: 2507 women were enrolled in the parent study (EMaBS), of whom 299 tested positive for HIV. Of these, 222 participants were randomized and followed‐up to 6 weeks post‐enrolment. Sixty‐four participants were randomized to single‐dose albendazole, 54 participants to praziquantel, 67 participants to both albendazole and praziquantel, and 70 participants to placebo only. Inclusion criteria: pregnant women presenting at the government‐funded antenatal clinic at Entebbe General Hospital, who were resident in the study area, planning to deliver in the hospital, willing to know their HIV status, and in the 2nd or 3rd trimester of pregnancy. Exclusion criteria: evidence of possible helminth‐induced pathology (haemoglobin < 8 g/dL, clinically apparent severe liver disease, diarrhoea with blood in stool), history of adverse reaction to antihelminthics, prior enrolment in an earlier pregnancy, or abnormal pregnancy as assessed by the midwife. |
|
| Interventions | Intervention 1: albendazole (400 mg). Intervention 2: praziquantel (40 mg/kg). Intervention 3: both albendazole (400 mg) and praziquantel (40 mg/kg). Control: equivalent placebos. |
|
| Outcomes | Outcomes included in this review: changes in plasma HIV‐1 RNA levels 6 weeks post‐treatment, adverse events (defined as post‐treatment hospitalizations), and mortality. Other trial outcomes: primary outcomes for EMaBS were response to immunisation and incidence of infectious diseases in the offspring, and vertical transmission of HIV (Webb 2011). Secondary outcomes in this analysis included viral load at delivery. |
|
| Notes | Location: Entebbe, Uganda. Participant helminth status: ascertained. Participant ART status: ART naive. However, in accordance with guidelines at the time, HIV‐positive women were counselled and given intrapartum and neonatal single dose nevirapine for prevention of mother‐to‐child HIV transmission. After enrolment, women received standard antenatal care, including haematinics, tetanus immunization, and intermittent presumptive treatment for malaria. Author contact: we requested additional HIV‐1 RNA and adverse events data from the trial authors, who provided this information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were then randomised in a 1:1:1:1 ratio to single‐dose albendazole 400 mg or matching placebo and praziquantel 40 mg/kg or matching placebo in a 2×2 factorial design. The randomisation code was generated by the trial statistician in blocks of 100." |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes containing the study intervention were prepared by colleagues at the Medical Research Council Unit in Entebbe with no other involvement in the trial. Treatments were allocated in numerical order by trained interviewer‐counsellors and taken under observation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All participants and staff were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unclear whether or not the assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is no risk of attrition; 10% of participants were lost to follow‐up. |
Abbreviations: antiretroviral treatment (ART), circulating filarial antigens (CFA), diethylcarbamazine (DEC), human immunodeficiency virus (HIV), immunochromatographic tests (ICT), soil‐transmitted helminths (STHs), tuberculosis (TB)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blish 2010 | This manuscript presents further analyses using data already presented in Walson 2008 KEN |
| Brown 2004 | Observational, prospective cohort study. |
| Brown 2005 | Observational, prospective cohort study. |
| Elliott 2003 | Observational, prospective cohort study. |
| Elliott 2007 | Study protocol for included study Webb 2012 UGA. |
| Erikstrup 2008 | This manuscript presents further analyses using data already presented in Kallestrup 2005 ZWE. |
| Esan 2013 | A RCT with non‐relevant intervention (that is, iron supplementation and multivitamins) to assess effects of anaemia in children 6 to 59 months of age. |
| Finkelstein 2012 | Observational, prospective cohort study. |
| Fischer 1995 | Observational, prospective cohort study. |
| Gallagher 2005 | Observational, retrospective cohort study. |
| Ganley‐Leal 2006 | Observational, prospective cohort study. |
| Geelhoed 2006 | Observational, prospective case‐control study, with irrelevant outcomes to this Cochrane Review. |
| Heath 1996 | Case study of a single participant. |
| Hosseinipour 2007 | Observational, cross‐sectional study. |
| Ivan 2015 | Observational, cross‐sectional study. |
| Joseph 2004 | Observational, prospective cohort study. |
| Kallestrup 2006 | This manuscript presents further analyses using data already presented in Kallestrup 2005 ZWE. |
| Karanja 1998 | Observational, prospective cohort study. |
| Kassu 2003 | Observational, prospective cohort study. |
| Kipp 2005 | Observational, prospective cohort study. |
| Kleppa 2014 | Observational, case‐control study. |
| Lankowski 2014 | Observational retrospective cohort study. |
| Lawn 2000 | Observational, prospective cohort study with no comparison group. |
| McElroy 2005 | Observational, cross‐sectional study. |
| Modjarrad 2005 | Observational, prospective cohort study. |
| Mulu 2013 | Observational, prospective cohort study. |
| Muok 2013 | Observational, prospective cohort study |
| Mwanakasale 2003 | Observational, prospective cohort study. |
| Ndibazza 2012 | A RCT with irrelevant outcomes to this Cochrane Review. The study authors hypothesize that antihelminthic treatment in pregnancy and early childhood would improve responses to immunization and modulate disease incidence in early childhood. |
| Watanabe 2007 | Observational, prospective cohort study. |
| Wolday 2002 | Observational, prospective cohort study. |
| Zinyama 2009 | Observational, prospective cohort study. |
| Zulu 2002 | Observational, prospective cohort study. |
Abbreviations: RCT: randomized controlled trial.
Differences between protocol and review
In this review update we utilized the GRADE criteria, produced 'Summary of findings' tables, and stratified outcomes according to whether HIV‐positive participants had confirmed helminth infections or were of unknown helminth infection status.
Contributions of authors
ARM and JW contributed to the protocol design of this updated review. ARM and PB screened the identified abstracts and manuscripts. ARM, PB, DS, and JW all assisted in the analysis of the findings and preparation of the final manuscript.
Sources of support
Internal sources
Cochrane Infectious Diseases Group, UK.
Liverpool School of Tropical Medicine, UK.
External sources
-
Evidence and Programme Guidance team, Department of Nutrition for Health and Development, World Health Organization (WHO), Switzerland.
We thank the Evidence and Programme Guidance team, Department of Nutrition for Health and Development, WHO for providing partial financial support for the update of this Cochrane Review.
-
Department for International Development, UK.
Grant: 5242.
Declarations of interest
The senior author of this review (JW) is also the primary investigator of two of the included trials. In alignment with Cochrane recommendations, JW was not involved in the extraction of data from these trials. JW is the principle investigator and ARM is a research scientist on a grant from the Bill & Melinda Gates Foundation, which is funding clinical trials related to the elimination of soil‐transmitted helminths.
David Sinclair is supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
PB has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Abate 2014 ETH {published and unpublished data}
- Abate E, Elias D, Getachew A, Alemu S, Diro E, Britton S, et al. Effects of albendazole on the clinical outcome and immunological responses in helminth co‐infected tuberculosis patients: a double blind randomised clinical trial. International Journal for Parasitology 2014;45(2‐3):133‐40. [DOI] [PubMed] [Google Scholar]
Kallestrup 2005 ZWE {published and unpublished data}
- Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, Dam GJ, et al. Schistosomiasis and HIV‐1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV‐1 RNA load. The Journal of Infectious Diseases 2005;192(11):1956‐61. [DOI] [PubMed] [Google Scholar]
Kelly 1996 ZMB {published data only}
- Kelly P, Lungu F, Keane E, Baggaley R, Kazembe F, Pobee J, et al. Albendazole chemotherapy for treatment of diarrhoea in patients with AIDS in Zambia: a randomized double blind controlled trial. BMJ 1996;312(7040):1187‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2007 TZA {published and unpublished data}
- Nielsen NO, Simonsen PE, Dalgaard P, Krarup H, Magnussen P, Magesa S, et al. Effect of diethycarbamazine on HIV load, CD4%, and CD4/CD8 ratio in HIV‐infected adult Tanzanians with or without lymphatic filariasis: randomized double‐blind and placebo‐controlled cross‐over trial. American Journal of Tropical Medicine and Hygiene 2007;77(3):507‐13. [PubMed] [Google Scholar]
- Nielsen NO, Simonsen PE, Kaestel P, Krarup H, Magnussen P, Magesa S, et al. Micronutrient status indicators in individuals single‐ or double‐infected with HIV and Wuchereria bancrofti before and after DEC treatment. Tropical Medicine & International Health 2009;14(1):44‐53. [DOI] [PubMed] [Google Scholar]
Suputtamongkol 2011 THA {published and unpublished data}
- Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, et al. Efficacy and safety of single and double doses of ivermectin versus 7‐day high dose albendazole for chronic strongyloidiasis. PLoS Neglected Tropical Diseases 2011;5(5):e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2008 KEN {published and unpublished data}
- Walson JL, Otieno PA, Mbuchi M, Richardson BA, Lohman‐Payne B, Macharia SW, et al. Albendazole treatment of HIV‐1 and helminth co‐infection: a randomized, double‐blind, placebo‐controlled trial. AIDS 2008;22(13):1601‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2012 KEN {published and unpublished data}
- Walson J, Singa B, Sangaré L, Naulikha J, Piper B, Richardson B, et al. Empiric deworming to delay HIV disease progression in adults with HIV who are ineligible for initiation of antiretroviral treatment (the HEAT study): a multi‐site, randomised trial. The Lancet Infectious diseases 2012;12(12):925‐32. [PUBMED: 22971323] [DOI] [PubMed] [Google Scholar]
Webb 2012 UGA {published and unpublished data}
- Webb EL, Kyosiimire‐Lugemwa J, Kizito D, Nkurunziza P, Lule S, Muhangi L, et al. The effect of anthelmintic treatment during pregnancy on HIV plasma viral load: results from a randomized, double‐blind, placebo‐controlled trial in Uganda. Journal of Acquired Immune Deficiency Syndromes 2012;60(3):307‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Blish 2010 {published data only}
- Blish CA, Sangaré L, Herrin BR, Richardson BA, John‐Stewart G, Walson JL. Changes in plasma cytokines after treatment of Ascaris lumbricoides infection in individuals with HIV‐1 infection. The Journal of Infectious Diseases 2010;201(12):1816‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown M, Kizza M, Watera C, Quigley MA, Rowland A, Highes P, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. The Journal of Infectious Diseases 2004;190(10):1869‐79. [DOI] [PubMed] [Google Scholar]
Brown 2005 {published data only}
- Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, Whitworth JAG, et al. Treatment of Schistosoma mansoni infection increases helminth‐specific type 2 cytokine responses and HIV‐1 loads in coinfected Ugandan adults. Journal of Infectious Diseases 2005;191(10):1648‐57. [DOI] [PubMed] [Google Scholar]
Elliott 2003 {published data only}
- Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV‐1 infected Ugandan adults. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(1):103‐8. [DOI] [PubMed] [Google Scholar]
Elliott 2007 {published data only}
- Elliott AM, Kizza M, Quigley MA, Ndibazza J, Nampijja M, Muhangi L, et al. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double‐blind, placebo‐controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447]. Clinical Trials 2007;4(1):42‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erikstrup 2008 {published data only}
- Erikstrup C, Kallestrup P, Zinyama‐Gutsire RB, Gomo E, Dam GJ, Deelder AM, et al. Schistosomiasis and infection with human immunodeficiency virus 1 in rural Zimbabwe: Systemic inflammation during co‐infection and after treatment for schistosomiasis. American Journal of Tropical Medicine and Hygiene 2008;79(3):331‐7. [PubMed] [Google Scholar]
Esan 2013 {published data only}
- Esan MO, Hensbroek MB, Nkhoma E, Musicha C, White SA, Ter Kuile FO, et al. Iron supplementation in HIV‐infected Malawian children with anemia: a double‐blind, randomized, controlled trial. Clinical Infectious Diseases 2013;57(11):1626‐34. [DOI] [PubMed] [Google Scholar]
Finkelstein 2012 {published data only}
- Finkelstein JL, Mehta S, Duggan CP, Spiegelman D, Aboud S, Kupka R, et al. Predictors of anaemia and iron deficiency in HIV‐infected pregnant women in Tanzania: a potential role for vitamin D and parasitic infections. Public Health Nutrition 2012;15(5):928‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 1995 {published data only}
- Fischer P, Kipp W, Kabwa P, Buttner DW. Onchocerciasis and human immunodeficiency virus in western Uganda: prevalences and treatment with ivermectin. American Journal of Tropical Medicine and Hygiene 1995;53(2):171‐8. [PubMed] [Google Scholar]
Gallagher 2005 {published data only}
- Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, et al. The effects of maternal helminth and malaria infections on mother‐to‐child HIV transmission. AIDS 2005;19(16):1849‐55. [DOI] [PubMed] [Google Scholar]
Ganley‐Leal 2006 {published data only}
- Ganley‐Leal LM, Mwinzi PN, Cetre‐Sossah CB, Andove J, Hightower AW, Karanja DM, et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infection and Immunity 2006;74(4):2169‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geelhoed 2006 {published data only}
- Geelhoed D, Agadzi F, Visser L, Ablordeppey E, Asare K, O'Rourke P, et al. Severe anemia in pregnancy in rural Ghana: a case‐control study of causes and management. Acta Obstetricia et Gynecologica Scandinavica 2006;85(10):1165‐71. [DOI] [PubMed] [Google Scholar]
Heath 1996 {published data only}
- Heath T, Riminton S, Garsia R, MacLeod C. Systemic strongyloidiasis complicating HIV: a promising response to ivermectin. International Journal of STD & AIDS 1996;7(4):294‐6. [DOI] [PubMed] [Google Scholar]
Hosseinipour 2007 {published data only}
- Hosseinipour MC, Napravnik S, Joaki G, Gama S, Mbeye N, Banda B, et al. HIV and parasitic infection and the effect of treatment among adult outpatients in Malawi. The Journal of Infectious Diseases 2007;195(9):1278‐82. [DOI] [PubMed] [Google Scholar]
Ivan 2015 {published data only}
- Ivan E, Crowther NJ, Mutimura E, Rucogoza A, Janssen S, Njunwa KK, et al. Effect of deworming on disease progression markers in HIV‐1‐infected pregnant women on antiretroviral therapy: a longitudinal observational study from Rwanda. Clinical Infectious Diseases 2015;60(1):135‐42. [DOI] [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph S, Jones FM, Laidlaw ME, Mohamed G, Mawa PA, Namujju PB, et al. Impairment of the Schistosoma mansoni‐specific immune responses elicited by treatment with praziquantel in Ugandans with HIV‐1 coinfection. The Journal of Infectious Diseases 2004;190(3):613‐8. [DOI] [PubMed] [Google Scholar]
Kallestrup 2006 {published data only}
- Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Dam GJ, Gerstoft J, et al. Schistosomiasis and HIV in rural Zimbabwe: efficacy of treatment of schistosomiasis in individuals with HIV coinfection. Clinical Infectious Diseases 2006;42(12):1781‐9. [DOI] [PubMed] [Google Scholar]
Karanja 1998 {published data only}
- Karanja DM, Boyer AE, Strand M, Colley DG, Nahlen BL, Ouma JH, et al. Studies on schistosomiasis in western Kenya: II. Efficacy of praziquantel for treatment of schistosomiasis in persons coinfected with human immunodeficiency virus‐1. American Journal of Tropical Medicine and Hygiene 1998;59(2):307‐11. [DOI] [PubMed] [Google Scholar]
Kassu 2003 {published data only}
- Kassu A, Tsegaye A, Wolday D, Petros B, Aklilu M, Sanders EJ, et al. Role of incidental and/or cured intestinal parasitic infections on profile of CD4+ and CD8+ T cell subsets and activation status in HIV‐1 infected and uninfected adult Ethiopians. Clinical Experimental Immunology 2003;132(1):113‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kipp 2005 {published data only}
- Kipp W, Bamhuhiiga J, Rubaale T, Kabagambe G. Adverse reactions to the ivermectin treatment of onchocerciasis patients: does infection with the human immunodeficiency virus play a role?. Annals of Tropical Medicine and Parasitology 2005;99(4):395‐402. [DOI] [PubMed] [Google Scholar]
Kleppa 2014 {published data only}
- Kleppa E, Ramsuran V, Zulu S, Karlsen GH, Bere A, Passmore JA, et al. Effect of female genital schistosomiasis and anti‐schistosomal treatment on monocytes, CD4+ T‐cells and CCR5 expression in the female genital tract. PLoS One 2014;9(6):e98593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lankowski 2014 {published data only}
- Lankowski AJ, Tsai AC, Kanyesigye M, Bwana M, Haberer JE, Wenger M, et al. Empiric deworming and CD4 count recovery in HIV‐infected Ugandans initiating antiretroviral therapy. PLoS Neglected Tropical Diseases 2014;8(8):e3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2000 {published data only}
- Lawn SD, Karanja DM, Mwinzi P, Andove J, Colley DG, Folks TM, et al. The effect of treatment of schistosomiasis on blood plasma HIV‐1 RNA concentration in coinfected individuals. AIDS 2000;14(16):2437‐43. [DOI] [PubMed] [Google Scholar]
McElroy 2005 {published data only}
- McElroy MD, Elrefaei M, Jones N, Ssali F, Mugyenyi P, Barugahare B, et al. Coinfection with Schistosoma mansoni is associated with decreased HIV‐specific cytolysis and increased IL‐10 production. The Journal of Immunology 2005;174(8):5119‐23. [DOI] [PubMed] [Google Scholar]
Modjarrad 2005 {published data only}
- Modjarrad K, Zulu I, Redden DT, Njobvu L, Lane HC, Bentwich Z, et al. Treatment of intestinal helminths does not reduce plasma concentrations of HIV‐1 RNA in coinfected Zambian adults. The Journal of Infectious Diseases 2005;192(7):1277‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulu 2013 {published data only}
- Mulu A, Maier M, Liebert UG. Deworming of intestinal helminths reduces HIV‐1 subtype C viraemia in chronically co‐infected individuals. International Journal of Infectious Diseases 2013;17(10):e897–901. [DOI] [PubMed] [Google Scholar]
Muok 2013 {published data only}
- Muok EM, Simiyu EW, Ochola EA, Ng'ang'a ZW, Secor WE, Karanja DM, et al. Association between CD4+ T‐lymphocyte counts and fecal excretion of Schistosoma mansoni eggs in patients coinfected with S. mansoni and human immunodeficiency virus before and after initiation of antiretroviral therapy. American Journal of Tropical Medicine and Hygiene 2013;89(1):42‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwanakasale 2003 {published data only}
- Mwanakasale V, Vounatsou P, Sukwa TY, Ziba M, Ernest A, Tanner M. Interactions between Schistosoma haematobium and human immunodeficiency virus type 1: the effects of coinfection on treatment outcomes in rural Zambia. American Journal of Tropical Medicine and Hygiene 2003;69(4):420‐8. [PubMed] [Google Scholar]
Ndibazza 2012 {published data only}
- Ndibazza J, Mpairwe H, Webb EL, Mawa PA, Nampijja M, Muhangi L, et al. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLoS One 2012;7(12):e50325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2007 {published data only}
- Watanabe K, Mwinzi PN, Black CL, Muok EM, Karanja DM, Secor WE, et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. American Journal of Tropical Medicine and Hygiene 2007;77(4):676‐82. [PMC free article] [PubMed] [Google Scholar]
Wolday 2002 {published data only}
- Wolday D, Mayaan S, Mariam ZG, Berhe N, Seboxa T, Britton S, et al. Treatment of intestinal worms is associated with decreased HIV plasma viral load. Journal of Acquired Immune Deficiency Syndromes 2002;31(1):56‐62. [DOI] [PubMed] [Google Scholar]
Zinyama 2009 {published data only}
- Zinyama‐Gutsire R, Gomo E, Kallestrup P, Erikstrup C, Ullum H, Butterworth AE, et al. Downregulation of MIP‐1alpha/CCL3 with praziquantel treatment in Schistosoma haematobium and HIV‐1 co‐infected individuals in a rural community in Zimbabwe. BMC Infectious Diseases 2009;9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zulu 2002 {published data only}
- Zulu I, Veitch A, Sianongo S, McPhail G, Feakins R, Farthing MJ, et al. Albendazole chemotherapy for AIDS‐related diarrhoea in Zambia‐‐clinical, parasitological and mucosal responses. Alimentary Pharmacology & Therapeutics 2002;16(3):595‐601. [DOI] [PubMed] [Google Scholar]
Additional references
Baggaley 2015
- Baggaley RF, Hollingsworth TD. Brief report: HIV‐1 transmissions during asymptomatic infection: exploring the impact of changes in HIV‐1 viral load due to coinfections. Journal of Acquired Immune Deficiency Syndromes 2015;68(5):594‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bentwich 1995
- Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunology Today 1995;16(4):187‐91. [DOI] [PubMed] [Google Scholar]
Bentwich 1996
- Bentwich Z, Weisman Z, Moroz C, Bar‐Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections?. Clinical and Experimental Immunology 1996;103(2):239‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borkow 2006
- Borkow G, Bentwich Z. HIV and helminth co‐infection: is deworming necessary?. Parasite Immunology 2006;28(11):605‐12. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown M, Mawa PA, Kaleebu P, Elliott AM. Helminths and HIV infection: epidemiological observations on immunological hypotheses. Parasite Immunology 2006;28(11):613‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bundy 2009
- Bundy DAP, Kremer M, Bleakley H, Jukes MCH, Miguel E. Deworming and development: asking the right questions, asking the questions right. PLoS Neglected Tropical Diseases 2009;3(1):e362. [10.1371/ journal.pntd.0000362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chachage 2014
- Chachage M, Podola L, Clowes P, Nsojo A, Bauer A, Mgaya O, et al. Helminth‐associated systemic immune activation and HIV co‐receptor expression: response to albendazole/praziquantel treatment. PLoS Neglected Tropical Diseases 2014;8(3):e2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐89. [DOI] [PubMed] [Google Scholar]
Eggena 2005
- Eggena MP, Barugahare B, Okello M, Mutyala S, Jones N, Ma Y, et al. T cell activation in HIV‐seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. The Journal of Infectious Diseases 2005;191(5):694‐701. [DOI] [PubMed] [Google Scholar]
Fincham 2003
- Fincham JE, Markus MB, Adams VJ. Could control of soil‐transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Tropica 2003;86(2‐3):315‐33. [DOI] [PubMed] [Google Scholar]
Gordin 1984
- Gordin FM, Simon GL, Wofsy CB, Mills J. Adverse reactions to trimethoprim‐sulphamethoxazole in patients with the acquired immunodeficiency syndrome. Annals of Internal Medicine 1984;100(4):495–9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software] McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Gupta 2007
- Gupta SB, Jacobson LP, Margolick JB, Rinaldo CR, Phair JP, Jamieson BD, et al. Estimating the benefit of an HIV‐1 vaccine that reduces viral load set point. The Journal of Infectious Diseases 2007;195(4):546‐50. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kalinkovich 1999
- Kalinkovich A, Weisman Z, Bentwich Z. Chemokines and chemokine receptors: role in HIV infection. Immunology Letters 1999;68(2‐3):281‐7. [DOI] [PubMed] [Google Scholar]
Kalinkovich 2001
- Kalinkovich A, Borkow G, Weisman Z, Tsimanis A, Stein M, Bentwich Z. Increased CCR5 and CXCR4 expression in Ethiopians living in Israel: environmental and constitutive factors. Clinical Immunology 2001;100(1):107‐17. [DOI] [PubMed] [Google Scholar]
Kallestrup 2005
- Kallestrup P, Zinyama R, Gomo E, Butterworth A, Dam GJ, Erikstrup C, et al. Schistosomiasis and HIV‐1 infection in rural Zimbabwe: implications of coinfection for excretion of eggs. Journal of Infectious Diseases 2005;191(8):1311–20. [DOI] [PubMed] [Google Scholar]
Kinter 2007
- Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, et al. CD25+ regulatory T cells isolated from HIV‐infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV‐specific CD8+ T cells in vitro. AIDS Research and Human Retroviruses 2007;23(3):438‐50. [DOI] [PubMed] [Google Scholar]
Lawn 2001
- Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clinical Microbiology Reviews 2001;14(4):753‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Modjarrad 2008
- Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS 2008;22(16):2179‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Modjarrad 2010
- Modjarrad K, Vermund SH. Effect of treating co‐infections on HIV‐1 viral load: a systematic review. The Lancet. Infectious Diseases 2010;10(7):455‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nesheim 2007
- Nesheim SR, Kapogiannis BG, Soe MM, Sullivan KM, Abrams E, Farley J, et al. Trends in opportunistic infections in the pre‐ and post‐highly active antiretroviral therapy eras among HIV‐infected children in the Perinatal AIDS Collaborative Transmission Study, 1986‐2004. Pediatrics 2007;120(1):100‐9. [DOI] [PubMed] [Google Scholar]
Nunn 1991
- Nunn P, Kibuga D, Gathua S, Brindle R, Imalingat A, Wasunna K, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV‐1 seropositive patients treated for tuberculosis. The Lancet 1991;377(8742):627–30. [DOI] [PubMed] [Google Scholar]
Partnership for Child Development 1998
- Partnership for Child Development. Cost of school‐based treatment in Tanzania. Health Policy and Planning 1998;13(4):384‐96. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Secor 2003
- Secor WE, Shah A, Mwinzi PM, Ndenga BA, Watta CO, Karanja DM. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and monocytes of patients with Schistosoma mansoni infection. Infection and Immunity 2003;71(11):6668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seden 2013
- Seden K, Khoo S, Back D, Prevatt N, Lamorde M, Byakika‐Kibwika P, et al. Drug–drug interactions between antiretrovirals and drugs used in the management of neglected tropical diseases: important considerations in the WHO 2020 Roadmap and London Declaration on Neglected Tropical Diseases. AIDS 2013;27(5):675‐86. [DOI] [PubMed] [Google Scholar]
Shapira‐Nahor 1998
- Shapira‐Nahor O, Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Shapiro M, et al. Increased susceptibility to HIV‐1 infection of peripheral blood mononuclear cells from chronically immune‐activated individuals. AIDS 1998;12(13):1731‐3. [PubMed] [Google Scholar]
UNAIDS 2007
- UNAIDS. 2007 AIDS Epidemic Update. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf (accessed 1 August 2015).
Uniting to Combat NTDs 2012
- Uniting to Combat NTDs. The London Declaration. http://unitingtocombatntds.org/sites/default/files/resource_file/london_declaration_on_ntds.pdf (accessed 1 August 2015).
Webb 2011
- Webb EL, Mawa PA, Ndibazza J, Kizito D, Namatovu A, Kyosiimire‐Lugemwa J, et al. Effect of single‐dose anthelmintic treatment during pregnancy on an infant’s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double‐blind, placebo‐controlled trial. The Lancet 2011;377(9759):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2014
- WHO. Soil‐transmitted helminth infections: fact sheet. April 2014. http://www.who.int/mediacentre/factsheets/fs366/en/ (accessed February 2015).
References to other published versions of this review
Walson 2007a
- Walson JL, John‐Stewart G. Treatment of helminth co‐infection in individuals with HIV‐1: a systematic review of the literature. PLoS Neglected Tropical Diseases 2007;1(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2007b
- Walson JL, John‐Stewart G. Treatment of helminth co‐infection in HIV‐1 infected individuals in resource‐limited settings. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006419] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2008
- Walson JL, John‐Stewart G. Treatment of helminth co‐infection in HIV‐1 infected individuals in resource‐limited settings. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006419.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walson 2009
- Walson JL, Herrin BR, John‐Stewart G. Deworming helminth co‐infected individuals for delaying HIV disease progression. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006419.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


