Abstract
Obesity is a major public health problem that is reaching pandemic proportion. Currently two thirds of the American population is either overweight or obese and worldwide, 39% of the population is overweight and 13% are considered obese [1,2]. This rapid rise in obesity is associated with increased in diabetes mellitus type 2 (DM2), hypertension (HTN), chronic kidney disease (CKD) and cardiovascular diseases (CVD), the major killer of adults in the USA. Parallel to this epidemic is the rapid rise of sleep disorders such as Obstructive Sleep Apnea (OSA). These disorders lead to increased morbidity and mortality and generally go undiagnosed and undertreated, particularly among minority groups. Accumulating evidence indicates common pathophysiologic background underlying all of these related disorders. Among these include: increased inflammation, increased oxidative stress, endothelial dysfunction, dyslipidemia and hypercoagulability. We discuss the rising epidemic of sleep disorders and its interrelationship with DM2, HTN, CVD and renal disease highlighting the racial disparity in diagnosis and treatment of these disorders that disproportionately affects minority populations. We also discuss the various treatment modalities and the cutting edge developments in this field.
Keywords: Sleep disorders, Cardio-renal disease, Populations
Introduction
With the rise in obesity in the USA and around the world, there has been a rapid increase in the prevalence of DM2, HTN, CVD and (CKD). Alarming recent reports indicate that 21 million people or 9.3% of the USA population have diabetes, and of those, 8.1 million were undiagnosed [3]. HTN is a very common disease in patients who are obese, particularly those with diabetes occurring in up to 71% in this population [3]. Furthermore, DM2 is by far the most common cause of chronic kidney disease (CKD) and End Stage Renal Disease (ESRD) in the USA.
Sleep disorders including OSA are quite common among obese populations and appear to share some of the underlying pathophysiologic mechanisms as DM2, HTN, CVD and CKD.
It is estimated that OSA affects 3–7% of the USA population [4] and 70% of patients with OSA are either overweight or obese [5] DM2 is present in up to 60% of patients with OSA as well as HTN, that affects 50% of this population [6,7] More recently, CKD has been linked to OSA and it is estimated that up to 50% of patients with ESRD have OSA which is strikingly higher than the general population [8].
Racial and Ethnic Differences in Sleep Disorders
Sleep disorders disproportionately affect minority populations. The recently published Multi-ethnic study of atherosclerosis (MESA) which objectively and subjectively screened patients and documented a higher prevalence of sleep disorders in blacks, hispanics and asians compared to caucasians. Of the four ethnic groups, blacks demonstrated the highest levels of sleep disturbance, shorter sleep duration, worse sleep quality and daytime sleepiness [10]. After adjusting for age, sex, location and BMI, these relationships remained evident. Although minority groups had higher levels of sleep-disturbance compared to caucasians, minorities were less likely to have been formally diagnosed [10]. This may be a result of poor access to health care services. The difference in the prevalence of sleep disorders in minority groups is likely secondary to a multitude of environmental and genetic variations across different ethnicities. Further research is necessary to identify these differences in order to better strategize for screening and therapeutic modalities tailored to improve recognition of sleep disorders and improve outcomes among these vulnerable populations [10].
Diabetes and Sleep Disorders
Diabetes is a major health concern around the world, and the prevalence of DM2 has almost doubled in the past 30 years to about 8.7% of the population worldwide [11]. DM2 is more prevalent in patients with OSA and depending on the severity OSA, the prevalence of DM2 ranges between 29–60% [6]. One breakthrough study demonstrated that patients with a higher respiratory disturbance index had a higher degree of glucose intolerance and insulin resistance when looking at fasting glucose levels and 2-hour oral glucose tolerance tests [12]. Although DM2 and OSA share many of the same risk factors such as obesity and metabolic syndrome, there is evidence that OSA can increase risk of diabetes independent of age, sex and BMI [12,13] Animal studies have demonstrated that intermittent hypoxia leads to insulin resistance in non-obese mice demonstrating that there may be a pathophysiologic mechanism independent of obesity [14].
Although there is clinical evidence that there is a relationship between DM2 and OSA/SDB, there are very few studies that demonstrate a causal relationship between the two. There is emerging evidence that intermittent hypoxia increases sympathetic activity leading to decreased insulin sensitivity and impaired glucose tolerance [15]. Sleep fragmentation was also shown to decrease insulin sensitivity through increased adrenocortical activity. Patients with more fragmented sleep were found to have higher levels of morning cortisol and increased sympathetic nervous system activity indirectly measured through assessment of heart rate variability [16]. Another hypothesis is related to disturbances in specific stages of sleep secondary to OSA. Selectively disrupting “slow wave sleep” has been shown to cause variable hormonal disturbances, specifically, decreased insulin sensitivity and increased risk of diabetes [17].
Although sleep disorders such as OSA have been shown to cause disturbances in insulin sensitivity and glucose metabolism, treatment of OSA has not always improved DM2. More specifically, studies involving CPAP have shown conflicting data; some demonstrated improved insulin sensitivity while other data failed to demonstrate any improvement at all [18]. Additional data demonstrated improvement in insulin sensitivity only in patients with severe OSA. This improvement was not translated into improved glycated hemoglobin levels [19–21].
Hypertension and Sleep Disorders
Sleep disorders are associated with HTN independent of age, sex, and body habitus [22]. OSA increases the risk of developing new-onset HTN, and nearly 50% of patients with HTN have concomitant OSA [7] Similar to the relationship between OSA and DM2, increased sympathetic activity is likely the underlying mechanism between OSA and HTN. Intermittent hypoxia causes blood pressure elevations as a result of increased adrenergic activity, which leads to arteriolar hypertension that persists even after hypoxia has resolved. Both hypoxia and hypercapnia act upon chemoreceptors and cause increases in sympathetic activity [23]. One study validated this hypothesis by demonstrating that patients with sleep disorders had higher urinary metanephrines and normetanephrines compared to patients without sleep disorders [24]. The increase in sympathetic activity disrupts the physiologic decrease in blood pressure that should occur overnight which is referred to as “non-dipping” blood pressure pattern. The nocturnal HTN carries over to the daytime causing an overall increase in blood pressure as well as increase in CVD risk [25].
Cardiovascular Disease and Sleep Disorders
Sleep disorders, particularly OSA, are also closely associated with CVD. OSA has been shown to increase the risk of coronary heart disease, heart failure, stroke and cardiac arrhythmias [26], (Figure 1). Increased sympathetic activity associated with OSA directly increases the risk of thrombotic events [27] OSA can also increase the risk of cardiovascular events through changes in intrathoracic pressures, increased reactive oxygen species, coagulation defects and elevated pro-inflammatory markers (Figure 2) [26]. Hypoxia is a potent arthrogenic factor and recurrent chronic hypoxemia associated with OSA has been shown to promote artherosclerosis. Moreover, acute hypoxia may cause rupture of a vulnerable plaque and lead to an acute cardiovascular event [27]. OSA also leads to an increased an inflammatory response, which plays a role in the development of atherosclerosis. Patients with OSA were shown to have elevated inflammatory markers, such as, C-reactive protein and Interleukin-6 [28]. Treatment with nasal CPAP was proven to decrease these inflammatory markers, therefore, indirectly decreasing the risk of atherosclerosis [28].
Figure 1.
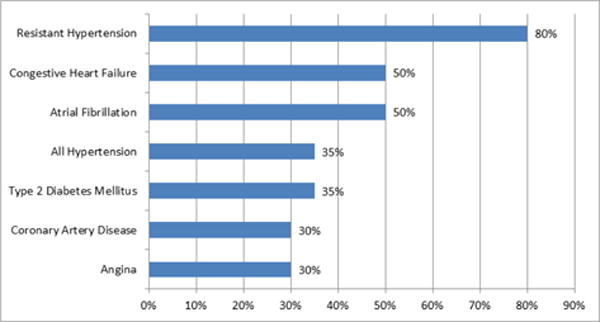
The prevalence of sleep apnea in other cardiovascular disease [9].
Figure 2.
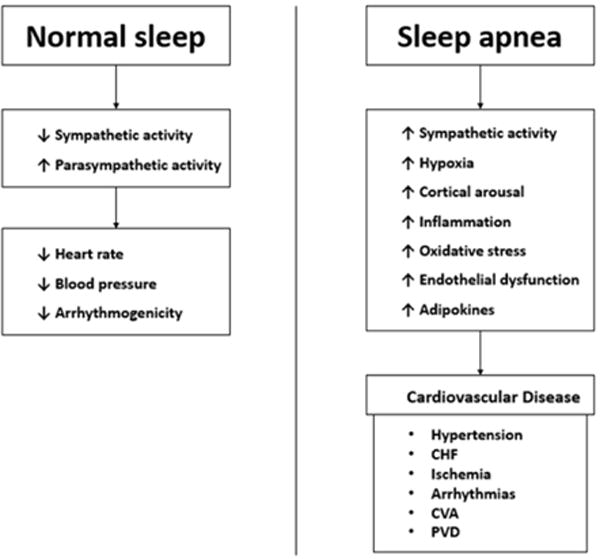
The association between obstructive sleep apnea and cardiovascular disease [9].
A large prospective study demonstrated that SDB and OSA were associated with increased cardiovascular mortality and all-cause mortality independent of confounding factors. The association was most apparent in men with severe OSA who were between ages 40–70 [29]. Although the increased association between hypertension and OSA may be partially responsible for the increases in cardiac mortality, the physiologic and hemodynamic stress may trigger fatal arrthymias [29].
Chronic Kidney Disease and Sleep Disorders
There is a growing body of evidence that sleep disorders are common in patients with CKD. While causality has not been ascertained, the associations between sleep disorders and CKD have been clearly established. Emerging evidence suggests that sleep disturbance causes changes in renal physiology; with decreased sleep, there is a decrease in angiotensin, renin and aldosterone levels leading to increased urinary excretion of sodium [30]. This may be a compensatory mechanism for the decreased nocturnal dipping of blood pressure with sleep disorders. A recent study by our group, demonstrated that sleep duration was related to CKD independent of race, psychosocial well-being and comorbidities such as DM2, CVD and HTN. This study demonstrated that patients with short sleep duration (≤ 6 hours) and long sleep duration (≥ 8 hours) had 2-fold increased odds of self-reported CKD when compared to their healthy counterparts. Although this study did not describe a causal relationship between CKD and sleep disturbance it indicates that there is an independent relationship between the two [31].
Treatment Options for Patients with Sleep Disorders
While addressing sleep disorders, it is imperative to address underlying pathophysiologic mechanisms of these diseases with lifestyle changes targeting obesity, DM2 and HTN. Lifestyle and behavioral changes include weight reduction, diet control, exercise, as well as avoidance of alcohol and tobacco. Although lifestyle changes are important, they have not always shown to be effective alone. Therefore, other modalities need to be introduced including: CPAP, oral devices and surgery.
Treatment with CPAP has become the standard of care for patients with OSA. CPAP is associated with reduction in the development of HTN and CVD which underscores the rationale for early screening and treatment of patients with OSA [32]. Men with severe OSA have significantly greater risk of fatal and non-fatal cardiovascular events, and CPAP treatment significantly reduces that risk [33]. Trials have also demonstrated that treatment with CPAP can improve many of the pathophysiologic consequences of OSA such glucose intolerance and CKD. CPAP also improves quality of life with improvement in quality of sleep, increased energy and better concentration.
Alternative treatments for OSA include: behavioral therapy, oral devices, surgery and weight loss. Oral devices include the mandibular advancement device and the tongue-retaining device. The mandibular advancement device functions through protruding the mandible forward which decreases obstruction of airflow, while the tongue-retaining device works in a similar fashion by holding the tongue in a more anterior position. Oral devices are generally used in patients with mild to moderate OSA and in patients with severe OSA who cannot tolerate or are non-compliant with CPAP. Surgery is used in patients with OSA who have an obstructive oropharynx that is potentially correctible with surgery. This is an option in patients in which CPAP, lifestyle modification and oral devices are ineffective or declined by patient [21].
In conclusion, the prevalence of sleep disorders (OSA) is increasing in the USA and worldwide. It is imperative that OSA is diagnosed and treated early as possible since sleep disorders are responsible for a variety of pathophysiologic mechanisms underlying DM2, HTN, CVD, and CKD. Disordered sleep is potentially modifiable and there should be an increased effort to improve sleep. Many people with sleep disorders go undiagnosed and untreated, particularly, in the minority populations. Further research is necessary to identify the barriers to diagnosis and treatment in these populations along with the implementation preventative strategies sooner. CPAP is currently the gold standard of treatment for OSA, although, if treatment is ineffective or compliance is an issue, alternatively, less effective therapeutic options are available.
Acknowledgments
Funding Information: This work is sponsored in part by the Brooklyn Health Disparities Center NIH grant #P20 MD006875.
References
- 1.National Center for Health Statistics. Obesity and Overweight. United States: 2015. Prevention CfDCa. [Google Scholar]
- 2.WHO. Obesity and Overweight 2015 [Google Scholar]
- 3.National Diabetes Statistics Report. Estimates of Diabetes and Its Burden in the United States. 2014:1–12. [Google Scholar]
- 4.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritscher LG, Mottin CC, Canani S, Chatkin JM. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg. 2007;17:95–99. doi: 10.1007/s11695-007-9012-7. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, et al. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13:355–362. doi: 10.4158/EP.13.4.355. [DOI] [PubMed] [Google Scholar]
- 7.Williams AJ, Houston D, Finberg S, Lam C, Kinney JL, et al. Sleep apnea syndrome and essential hypertension. Am J Cardiol. 1985;55:1019–1022. doi: 10.1016/0002-9149(85)90738-6. [DOI] [PubMed] [Google Scholar]
- 8.Pierratos A, Hanly PJ. Sleep disorders over the full range of chronic kidney disease. Blood Purif. 2011;31:146–150. doi: 10.1159/000321859. [DOI] [PubMed] [Google Scholar]
- 9.Jean-Louis CDBG, Ogedegbe G, Boutin-Foster C, Gorga J, McFarlane SI. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Rev Cardiovasc Ther. 2010;8:995–1005. doi: 10.1586/erc.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, et al. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Diabetes 2016 [Google Scholar]
- 12.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 13.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 14.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007;175:851–857. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, et al. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10:43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Ceron E, Fernandez-Navarro I, Garcia-Rio F. Effects of continuous positive airway pressure treatment on glucose metabolism in patients with obstructive sleep apnea. Sleep Med Rev. 2015;25:121–130. doi: 10.1016/j.smrv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–974. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstock TG, Wang X, Rueschman M, Ismail-Beigi F, Aylor J, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–625. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Pei JH, Chen HM. Effects of continuous positive airway pressure treatment on glycaemic control and insulin sensitivity in patients with obstructive sleep apnoea and type 2 diabetes: a meta-analysis. Arch Med Sci. 2014;10:637–642. doi: 10.5114/aoms.2014.44854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young T, Peppard P, Palta M, Hla KM, Finn L, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 23.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- 24.Elmasry A, Lindberg E, Hedner J, Janson C, Boman G. Obstructive sleep apnoea and urine catecholamines in hypertensive males: a population-based study. Eur Respir J. 2002;19:511–517. doi: 10.1183/09031936.02.00106402. [DOI] [PubMed] [Google Scholar]
- 25.Seif F, Patel SR, Walia HK, Rueschman M, Bhatt DL, Blumenthal RS, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32:267–275. doi: 10.1097/HJH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jean-Louis G, Zizi F, Brown D, Ogedegbe G, Borer J, et al. Obstructive sleep apnea and cardiovascular disease: evidence and underlying mechanisms. Minerva Pneumol. 2009;48:277–293. [PMC free article] [PubMed] [Google Scholar]
- 27.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 28.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 29.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. 2010;299:F404–F411. doi: 10.1152/ajprenal.00126.2010. [DOI] [PubMed] [Google Scholar]
- 31.Salifu I, Tedla F, Pandey A, Ayoub I, Brown C, et al. Sleep duration and chronic kidney disease: analysis of the national health interview survey. Cardiorenal Med. 2014;4:210–216. doi: 10.1159/000368205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin JM, Agusti A, Villar I, Forner M, Nieto D, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]


