Abstract
Background
Breast cancer awareness and early detection are limited in sub-Saharan Africa. Resource limitations make screening mammography or clinical breast exam (CBE) by physicians or nurses impractical in many settings. We aimed to assess feasibility and performance of CBE by laywomen in urban health clinics in Malawi.
Methods
Four laywomen were trained to deliver breast cancer educational talksand conduct CBE. After training, screening was implemented in diverse urbanhealth clinics. Eligible women were ≥30 years, with no prior breast cancer or breast surgery, and clinic attendance for reasons other than abreast concern. Wo men with abnormal CBE were referred to a study surgeon. All palpable masses confirmed by surgeon exam were pathologically sampled. Patients with abnormal screening CBE but normal surgeon exam underwentbreast ultrasound con firmation. Additionally, 50 randomly selected women with normal screening CBE underwent breast ultrasound, and 45 different women with normal CBE were randomly assigned to surgeon exam.
Results
Among 1,220 eligible women, 1,000 (82%) agreed to CBE. Lack of time (69%) was the commonest reason for refusal. Educational talk attendance was associated with higher CBE participation (83% vs 77%, p=0.012). Among 1,000 women screened, 7% had abnormal CBE. Of 45 women with normal CBE randomized to physician exam, 43 had normal exams and two had axillary lymphadenopathy not detected by CBE. Sixty of 67 women (90%) with abnormal CBE attended the referral visit. Of these, 29 (48%) had concordant abnormal physician exam. Thirty-one women (52%) had discordant normal physician exam, all of whom also had normal breast ultrasounds. Compared to physician exam, sensitivity for CBE by laywomen was 94% (CI 79-99%), specificity 58% (CI 46-70%), positive predictive value 48% (CI 35-62%), and negative predictive value 96% (CI 85-100%). Of 13 women who underwent recommended pathologic sampling of a breast lesion, two had cytologic dysplasia and all others benign results.
Conclusions and relevance
CBE uptake in Lilongwe clinics was high. CBE by laywomen compared favorably with physician exam, and follow-up was good. Our intervention can serve as a model for wider implementation. Performance in rural areas, effects on cancer stage and mortality, and cost-effectiveness require evaluation.
Keywords: Breast cancer, screening, global health, laywomen
1.1 Introduction
Breast cancer burden and mortality are high in low-and-middle-income countries (LMIC). The majority of newly diagnosed breast cancers in the United States are stage I or II, whereas most new breast cancers in LMIC are stages III or IV.1 Higher mortality in LMIC versus resource-rich settings is partly due to lack of breast cancer awareness and early detection. According to the 2003 World Health Survey, only 2% of women aged 40-69 in LMIC had received any breast cancer screening.2
Screening mammography is recommended in many high-income countries, but LMIC often lack infrastructure to implement this.3,4 International guidelines recommend alternative methods like clinical breast exam (CBE) in settings where mammography is not feasible.5-7 Even in high-resource settings, annual CBE may be as effective as mammography in reducing breast cancer mortality.8-11 CBE can also be effective and cost-effective in LMIC settings.12-15
Given health workforce constraints in LMIC, task shifting has emerged as an important strategy for service delivery, which may be valuable for CBE implementation. Task shifting involves training lower cadres of health workers to perform tasks traditionally reserved for more highly trained individuals. In several countries, CBE has been effectively task-shifted to community health workers and lay volunteers, with increased detection of early-stage cancers and high levels of agreement with physician exam.16-20 Task shifting has also been important in achieving scale-up of antiretroviral therapy (ART) for HIV-infected individuals in sub-Saharan Africa (SSA).21 Comprehensive care for HIV in SSA has increasingly integrated other health services together with ART provision, and such bundling approaches may be similarly effective for cancer screening. In SSA to date, use of laywomen to conduct CBE has only been studied in Sudan, and this approach has not been studied in combination with other health services.
In Malawi, breast cancer is the third most common cancer among women.22 Patients are typically diagnosed at late stages with long symptom durations before diagnosis.23 The Malawi Ministry of Health has prioritized early detection of breast and cervical cancer in the national strategic plan.24 However, no coordinated breast cancer screening program currently exists. We describe a pilot study training laywomen to perform CBE screening and promote breast cancer awareness among women attending various health clinics in the capital, Lilongwe. Our primary objectives were to assess feasibility and acceptability of this approach in urban settings, evaluate effectiveness of trained laywomen to conduct CBE, and describe CBE findings and follow-up among women screened. To our knowledge, this is the first breast cancer screening study in Malawi, and the first study from SSA to assess layperson-conducted CBE integrated with other health services.
1.2 Methods
We identified and recruited four laywomen as Breast Health Workers (BHWs) by engaging local staff at the research site and breast cancer advocates. BHWs underwent a four-week training program, consisting of lectures, online modules, role-playing, case discussions, CBE using simulators and patients, and oral presentation practice.25 Ministry of Health trainers taught health communication, promotion, and education skills. Breast cancer survivors shared their experiences. Surgeons taught breast cancer epidemiology, prevention, detection, and clinical care. Surgeons and research staff taught research ethics, informed consent, data collection, and professionalism. 26
The CBE practice component used a simulator with 27 different tumors and lymph nodes of varying shapes and sizes in a torso with breasts, axillae, and supraclavicular regions. It also included benign masses and models with peau d’orange. We taught BHWs to visually inspect breasts for asymmetry, lumps, skin changes, edema, nipple retraction, discharge, or axillary swellings. To palpate the breast, we taught BHWs to use the pads of the middle three fingers with overlapping dime-sized circular movements. Palpation of axillary, infraclavicular, and supraclavicular lymph nodes was also taught. They also practiced CBE under supervision with each other and on consenting patients from outpatient clinics.25
BHWs learned to deliver a standardized breast cancer educational talk using a flip chart. To assess BHW competency in delivering educational talks prior to implementation of the program, three independent Malawian evaluators unaffiliated with the study evaluated each BHW during 12 talks, using a 5-point scale for 22 discrete topics grouped into four main areas: introduction, delivery, knowledge, and interactiveness.
Malawi and U.S. IRBs were obtained prior to study commencement. Screening was implemented in five clinics. Three clinics for outpatient general medicine, sexually transmitted infections, and colposcopy were on the campus of Kamuzu Central Hospital (KCH), one of two national teaching hospitals in Malawi. KCH is a 1000-bed public tertiary care hospital in Lilongwe, serving a catchment area of five million people throughout the central region. Two clinic sites for outpatient general medicine and antenatal/family planning were at another health center in Lilongwe. Eligibility criteria for study participation included female sex, age greater than 30 years, no personal history of breast cancer or breast surgery, and clinic attendance for reasons other than a breast concern. Two BHWs worked together at each site.
BHWs began each day by giving a standardized breast cancer presentation to all patients in waiting rooms of the designated clinics. They documented the number of men and women attending the talk and any questions or comments raised. To minimize disruption to usual clinic operations, they approached patients to participate in the study only after their scheduled clinic visit. They kept a screening log to document eligibility, acceptance of CBE, and attendance of the educational presentation. If a woman was eligible and willing to participate, she was escorted to a private exam room where informed consent was obtained in the local language. The BHW collected information on patient demographics, breast cancer risk factors, and breast health history. She then performed a CBE. Any woman with an abnormal CBE or extensive breast concerns in the past 30 days by history was referred to a dedicated breast clinic at KCH staffed by study physicians. CBE abnormalities confirmed by physician exam were referred for fine needle aspiration (FNA) or core needle biopsy as appropriate. CBE abnormalities with normal physician exams were referred for breast ultrasound confirmation.
To evaluate diagnostic accuracy, we randomly assigned 50 women with normal CBE to undergo breast ultrasound at KCH, and another 45 women with normal CBE to physician exam. All patients referred for physician exam or additional diagnostic tests received reminder phone calls the day before by study staff. If a patient did not attend the scheduled visit or procedure, she was called to identify the reasons for non-attendance and to be rescheduled. Patients were given public transport reimbursements to attend follow-up visits.
Categorical baseline characteristics were analyzed using proportions and percentages, and compared between women with normal and abnormal screening CBE using chi-square and Fisher’s exact tests. The two-sided t-test was used to compare continuous variables between groups. We estimated sensitivity and specificity of BHW CBE using physician exam as the gold standard. Statistical significance was considered at an α-level of 0.05, and 95% confidence intervals (CIs) were provided where appropriate. All analyses were done using Stata SE Version 13.0 (StataCorp, College Station, TX).
1.3 Results
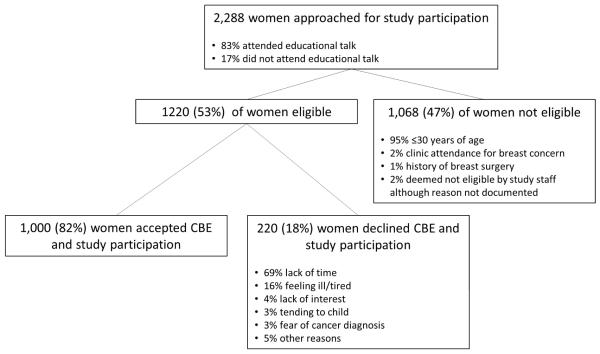
From January to April 2015, 2,860 women and 1,435 men attended 175 talks in five clinics. The talks lasted approximately 30 minutes including discussion and were given daily. A total of 2,288 women attending the clinics were screened to participate in the study, of whom 1,220 (53%) were eligible (Figure 1). The most common reasons for non-eligibility were age less than 30 years (n=1,020, 95%), clinic attendance for a breast concern (n=23, 2%), and history of breast surgery (n=12,1%). Among 1,220 eligible women, 1,000 (82%) agreed to participate in the study and undergo CBE. The most commonly stated reasons for study refusal were lack of time (69%) and feeling too ill or tired to participate (16%). Other reasons given were lack of interest (4%), tending to sick child (3%), and fear of cancer diagnosis (3%). Additional reasons were needing more time to decide, needing husband’s approval, desire for CBE but not wanting to participate in a research study, and recent CBE already performed, all of which were mentioned by less than 1% of women screened. Among eligible women, those who attended the educational talk (782/837, 83%) were significantly more likely to participate than eligible women who did not attend the talk (217/282, 77%, p=0.012), although participation was high in both groups. Additionally, participation rates among eligible women varied significantly across the five clinics ranging from 71% in the colposcopy clinic to 86% in the sexually transmitted infections clinic (p=0.001).
Figure 1.
Study recruitment.
A total of 1,000 women underwent CBE, of whom 933 (93%) had normal results and 67 (7%) abnormal results (Table 1). The mean age was 37 years with 2% reporting a family history of breast cancer and 11% being postmenopausal. Characteristics were overall similar between women with normal and abnormal CBE. More women with normal CBE reported use of contraception compared to those with abnormal CBE (57% vs 30%, p<0.001). Additionally, more women with abnormal CBE reported breast concerns in the past 30 days compared to women with normal CBE (67% vs 6%, p<0.001).
Table 1.
Characteristics of women who underwent screening clinical breast exam
| All women (n=1000) |
CBE normal (n=933) |
CBE abnormal (n=67) |
P value | |
|---|---|---|---|---|
| Mean age, years (SD) | 37.2 (8.0) | 37.2 (8.0) | 36.5 (8.1) | 0.447 |
| Family history of breast cancer, n (%) | 23 (2.3) | 18 (1.9) | 5 (7.5) | 0.015 |
| Has children, n (%) | 962 (96.2) | 902 (96.7) | 60 (89.6) | 0.003 |
| Post-menopausal, n (%) | 110 (11.0) | 99 (10.6) | 11 (16.4) | 0.142 |
| Currently breastfeeding, n (%) | 139 (13.9) | 130 (13.9) | 9 (13.4) | 0.909 |
| Currently using contraception, n (%) | 550 (55.0) | 530 (56.8) | 20 (29.9) | <0.001 |
| Injectable | 284 (28.4) | 275 (51.9) | 9 (45.0) | 0.545 |
| Oral | 67 (6.7) | 62 (11.7) | 5 (25.0) | 0.074 |
| Intrauterine device | 4 (0.4) | 3 (0.6) | 1 (5.0) | 0.022 |
| Implant | 91 (9.1) | 89 (16.8) | 2 (10.0) | 0.422 |
| Had breast concerns in past 30 days, n (%) | 103 (10.3) | 58 (6.2) | 45 (67.2) | <0.001 |
| Breast lump | 6 (0.6) | 0 (0) | 6 (13.3) | 0.004 |
| Nipple discharge | 12 (1.2) | 2 (3.4) | 10 (22.2) | 0.003 |
| Breast pain | 33 (3.3) | 9 (15.5) | 24 (53.3) | <0.001 |
| Breast Itching | 57 (5.7) | 45(77.6) | 12 (26.6) | <0.001 |
| Mean age at onset of menses, years (SD)a | 15.0 (1.8) | 15.1 (1.6) | 15.0 (1.8) | 0.93 |
| Mean age at birth of first child, years (SD)b | 22.3 (6.4) | 22.4 (6.3) | 20.4 (4.3) | 0.82 |
| Mean age at menopause, years (SD)c | 50 (6.1) | 47.1 (6.1) | 46.1 (6.2) | 0.65 |
CBE=clinical breast exam. SD=standard deviation.
870 women (812 CBE normal, 58 CBE abnormal) reported age at onset of menses.
948 women (888 CBE normal, 60 CBE abnormal) reported age at birth of their first child.
82 women (73 CBE normal, 9 CBE abnormal) reported age at menopause.
Forty-five women with normal CBE were randomly assigned to undergo physician exam. Of these women, 43 (96%) had a normal physician exam, and two had a solitary abnormal axillary lymph node not detected by CBE. In Table 2, CBE done by BHWs is compared to physician exam, yielding a sensitivity of 94% (95% CI 79-99%), specificity of 58% (95% CI 46-70%), positive predictive value of 48% (95% CI 35%-62%), and negative predictive value of 96% (95% CI 85-100%). Additionally, 50 women with normal CBE were randomly assigned to breast ultrasound, all of whom had normal findings.
Table 2.
Comparison of breast health worker clinical breast exam to physician exam
| Breast health worker exam | Physician exam normal | Physician exam abnormal |
|---|---|---|
| Normal (n=45) | 43 (95.5%) | 2 (4.4%) |
| Abnormal (n=60) | 31 (51.7%) | 29 (48.3%) |
Among 67 women with abnormal CBE, findings on exam or history leading to physician referral included the following, with some women referred for more than one reason: breast mass (n=38), lymphadenopathy (n=11), breast pain (n=11), nipple discharge (n=8), skin change (n=4), nipple/areolar change (n=4), breast asymmetry (n=1), breast swelling (n=1, and axillary mass (n=1). Sixty of 67 women referred for physician examination (90%) attended the recommended follow-up visit, of whom 29 (48%) had a concordant abnormal physician exam. Thirty-one women with abnormal CBE findings had a discordant normal physician exam, all of whom additionally had normal breast ultrasounds for confirmation.
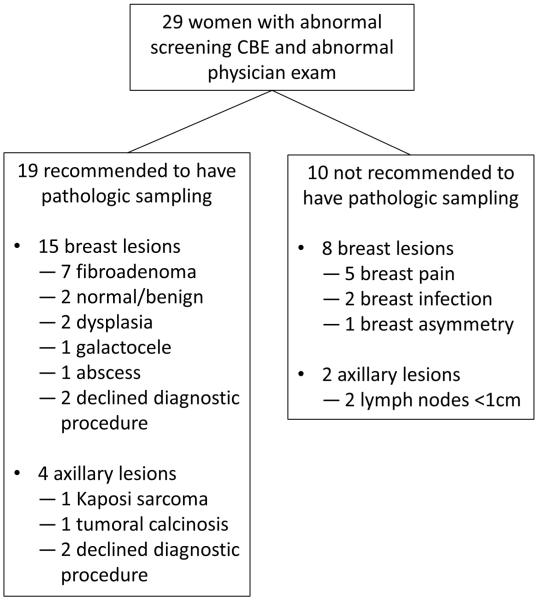
Of 29 women with abnormal CBE and concordant abnormal physician exam, 19 were recommended to undergo biopsy or FNA of their lesion, including 15 women with breast lesions, and four with axillary masses or lymph nodes (Figure 2). Of 13 breast lesions sampled, two had dysplasia, and 11 were benign. Both women with dysplasia by cytology were advised to undergo excisional biopsy, but difficult social circumstances prevented completion of these procedures. Specifically, one woman could not be contacted by phone despite daily attempts for more than three months after her FNA results were reported. The second woman with cytologic dysplasia reported domestic issues interfering with her ability to return for surgery. Additionally, two women advised to have breast lesions biopsied based on abnormal physician exam decided not to undergo any additional testing despite extensive counseling. Of four women with abnormal axillary lymph nodes advised to undergo biopsy, one was diagnosed with benign tumoral calcinosis after excisional biopsy, one was diagnosed with HIV-associated Kaposi sarcoma, one declined further work-up, and one woman moved abroad before having biopsy. Ten women with abnormal CBE and concordant abnormal physician exam did not require a subsequent diagnostic procedure, and were managed conservatively based on physician recommendations as shown in Figure 2.
Figure 2.
Follow-up of referred women with abnormal physician exam after screening clinical breast exam.
1.4 Discussion
We report a successful pilot program using trained laywomen to educate an urban population in Malawi about breast cancer and conduct CBE screening. Innovative features of our program include use of trained laywomen and integration of CBE with other health services in diverse clinical settings. Key findings were 82% uptake of CBE among eligible women, with higher uptake among women who attended educational talks. Compared to physician exam, sensitivity for CBE by BHWs was 94%, specificity 58%, positive predictive value 48%, and negative predictive value 96%. Abnormal CBE occurred in 7% of 1000 women screened, with 90% of referred women attending recommended follow-up. Two possible breast cancers based on cytology and one HIV-associated Kaposi sarcoma were ultimately detected.
We achieved high acceptance of CBE among eligible women who were not planning to undergo CBE when they arrived in clinic. The major reason for CBE refusal was lack of time, which likely reflects the study design involving recruitment after regularly scheduled visits to avoid disrupting usual clinic operations. Due to high patient volume and few clinicians in all sites studied, long wait times often left women with little time to receive other health services after planned services. Refinement of our approach could include CBE screening during clinic visits or during waiting periods before the clinical encounter, which could improve acceptance rates. Additionally, we found CBE acceptance was higher among women who attended breast cancer educational talks delivered in waiting rooms, underscoring the importance of community education efforts alongside cancer screening.
Similar to a study in Sudan, our findings suggest that laywomen can be trained to educate communities about breast cancer and perform CBE screening competently. Excellent negative predictive value in our study for CBE by BHWs compared to physician exam suggests this approach can reliably identify women who do not require further evaluation. Positive predictive value was lower, but in an acceptable range for a screening intervention which would be implemented at a population level. The 7% abnormal CBE rate with 48% positive predictive value compared to physician exam suggests that referred women would not overburden higher-level health facilities in Malawi unnecessarily. Our abnormal CBE rate was comparable to other studies from the region, as was the detection rate for potentially cancerous lesions of the breast (2 per 1000 women screened).16 Use of trained laywomen to deliver cancer-screening interventions is less costly and more easily scaled up in LMIC settings than approaches that rely on nurses and clinicians. Additionally, laywomen may be more effective at engaging community women than medical professionals.27,28
Although we recruited women attending clinics for reasons unrelated to breast health, 10% of participants reported a breast concern within the last 30 days. This finding suggests that a significant burden of unaddressed, symptomatic breast disease may exist in Malawi. Our intervention may have allowed women to express health concerns for which they were not explicitly seeking care. By contrast, in high-income countries, breast cancer screening is largely aimed at detecting subclinical disease, since women typically know to seek medical assistance for breast symptoms and have appropriate places to seek care. In our study, finding many participants who reported prevalent breast symptoms during CBE is consistent with our own prior findings of long symptom durations before breast cancer diagnosis in Malawi and extremely low breast cancer awareness among Malawian women.23 CBE scale-up in Malawi could partially address symptomatic disease and even non-breast conditions in addition to asymptomatic breast disease. This is illustrated by a patient with Kaposi sarcoma diagnosed through our program and brought into care, health impacts of CBE may be greater in settings like Malawi than high-income countries.
Our study had several strengths. We used an intensive training program customized to the learning pace of our BHWs. Second, we implemented our program in diverse urban clinics in Lilongwe. Third, we had few exclusion criteria and findings may be generalizable to other ambulatory, urban populations in SSA. Fourth, we achieved high rates of follow-up and standardized management for referred patients in a dedicated breast clinic staffed by surgeons and pathologists.
There are also several limitations to our study. First, we selected and trained four highly motivated laywomen who were paid for their work. CBE programs may not perform similarly using unpaid volunteers. Second, CBE screening was implemented in urban settings, and results may not apply to rural areas. Moreover, the intervention targeted participants who were already attending clinics for other reasons and thus were already demonstrating health-seeking behavior. It is unclear how successful the program would be in community settings. High follow-up was partially achieved through phone calls and transport reimbursements for referred women, and less active follow-up would likely result in lower rates of completed referrals. However, even with these dedicated efforts, it is notable that both women with suspicious FNAs did not complete all recommended follow-up procedures. Methods for optimizing adherence and retention must be further explored if our program is to be scaled up or replicated. Finally, our study lacked a control group and sufficient power to draw conclusions about the stage distribution of CBE-detected cancers. Larger, more definitive studies to assess clinical outcomes are needed.
In conclusion, acceptability and feasibility in Lilongwe were high for CBE screening performed by laywomen integrated with other health services. CBE conducted by BHWs compared favorably with physician exam, and high follow-up rates were achieved among women referred. Our intervention can serve as a model for wider implementation in Malawi and elsewhere in SSA, although performance in rural areas, effects on breast cancer stage distribution and mortality, and cost-effectiveness require further evaluation.
Acknowledgments
This project was supported by the UJMT Fogarty Global Health Fellows Program (grant R25TW009340). SG is supported by grants from the National Institutes of Health (K01TW009488, R21CA180815 and U54CA190152). Additional support was provided by the Medical Education Partnership Initiative (U2GPS001965) and Lineberger Comprehensive Cancer Center (P30CA016086). Clara Lee was supported by K07CA154850-01A1 We additionally acknowledge leadership of Kamuzu Central Hospital (Jonathan Ngoma), Malawi Ministry of Health (Beatrice Matanje-Mwagomba), UNC Project-Malawi (Irving Hoffman, Innocent Mofolo, Mina Hosseinipour, Francis Martinson), and Lineberger Comprehensive Cancer Center (Shelley Earp, Ned Sharpless, Lisa Carey, Blossom Damania, Dirk Dittmer) for their support of this study. We would like to thank the breast health workers for all their hard work: Emmaculate Kawawa, Diana Baluwa, Miriam Malowera, Magdalena Zgambo. We would also like to thank Bal M. Dhungel for his assistance with the pathology specimens.
National Institutes of Health Fogarty International Center (5R25TW009340) and from the State Department Fulbright Scholar Program
Footnotes
Author Contributions: LG, SG, CL, AM, CS all contributed to the design of the study. LG, SG, CS contributed to data analysis. SM, BD, GL, VM, AM contributed to data collection and study execution. All authors contributed to manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no conflicts of interest or financial matters to disclose.
References
- 1.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer epidemiology. 2009;33(5):315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the World Health Survey. PloS one. 2012;7(11):e48834. doi: 10.1371/journal.pone.0048834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panieri E. Breast-cancer awareness in low-income countries. The Lancet. Oncology. 2013;14(4):274–275. doi: 10.1016/S1470-2045(13)70020-2. [DOI] [PubMed] [Google Scholar]
- 4.Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Annals of global health. 2014;80(5):412–417. doi: 10.1016/j.aogh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113(8 Suppl):2221–2243. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 6.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008;113(8 Suppl):2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
- 7.WHO position paper on mammography screening. WHO; Switzerland: 2014. [PubMed] [Google Scholar]
- 8.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. Bmj. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher SW. ACP Journal Club. Annual mammography screening did not reduce long-term breast cancer mortality in women 40 to 59 years of age. Annals of Internal Medicine. 2014;160(10):JC7. doi: 10.7326/0003-4819-160-10-201405200-02007. [DOI] [PubMed] [Google Scholar]
- 10.Harris R. Screening is only part of the answer to breast cancer. Annals of Internal Medicine. 2014;160(12):861–863. doi: 10.7326/M14-0616. [DOI] [PubMed] [Google Scholar]
- 11.Jatoi I. Screening clinical breast examination. The Surgical clinics of North America. 2003;83(4):789–801. doi: 10.1016/S0039-6109(03)00028-8. [DOI] [PubMed] [Google Scholar]
- 12.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Tropical medicine & international health : TM & IH. 2012;17(8):1031–1043. doi: 10.1111/j.1365-3156.2012.03021.x. [DOI] [PubMed] [Google Scholar]
- 13.Boulos S, Gadallah M, Neguib S, et al. Breast screening in the emerging world: high prevalence of breast cancer in Cairo. Breast (Edinburgh, Scotland) 2005;14(5):340–346. doi: 10.1016/j.breast.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Denewer A, Hussein O, Farouk O, Elnahas W, Khater A, El-Saed A. Cost-effectiveness of clinical breast assessment-based screening in rural Egypt. World journal of surgery. 2010;34(9):2204–2210. doi: 10.1007/s00268-010-0620-3. [DOI] [PubMed] [Google Scholar]
- 15.Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol. 2007;18(7):1172–1176. doi: 10.1093/annonc/mdm105. [DOI] [PubMed] [Google Scholar]
- 16.Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. The Lancet. Oncology. 2013;14(4):363–370. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]
- 17.Hyoju SK, Agrawal CS, Pokhrel PK, Agrawal S. Transfer of clinical breast examination skills to female community health volunteers in Nepal. Asian Pacific journal of cancer prevention : APJCP. 2011;12(12):3353–3356. [PubMed] [Google Scholar]
- 18.Mittra I, Mishra GA, Singh S, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: methodology and interim results after three rounds of screening. International journal of cancer. Journal international du cancer. 2010;126(4):976–984. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 19.Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. International journal of cancer. Journal international du cancer. 2014 doi: 10.1002/ijc.29348. [DOI] [PubMed] [Google Scholar]
- 20.Ginsburg OM, Chowdhury M, Wu W, et al. An mHealth Model to Increase Clinic Attendance for Breast Symptoms in Rural Bangladesh: Can Bridging the Digital Divide Help Close the Cancer Divide? Oncologist. 2014;19(2):177–185. doi: 10.1634/theoncologist.2013-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penazzato M, Davies MA, Apollo T, Negussie E, Ford N. Task shifting for the delivery of pediatric antiretroviral treatment: a systematic review. Journal of acquired immune deficiency syndromes (1999) 2014;65(4):414–422. doi: 10.1097/QAI.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 22.GLOBOCAN Population Facts Sheets: Malawi. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- 23.Kohler REMA, Krysiak R, Liomba NG, Gopal S. Pathologically confirmed breast cancer in Malawi: a descriptive study. Malawi Med Journal. 2015 doi: 10.4314/mmj.v27i1.3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malawi Health Sector Strategic Plan: 2011-2106. Ministry of Health; Lilongwe, Malawi: [Google Scholar]
- 25.Gutnik L, Moses A, Stanley C, Tembo T, Gopal S, Lee C. From Community Laywomen to Breast Health Workers: A successful training model to implement clinical breast exam screening in Malawi; Bethune Round Table on Global Surgery; Calgary, Canada. Jun 5-7, 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutnik L, Moses A, Stanley C, Tembo T, Lee C, Gopal S. From Community Laywomen to Breast Health Workers: A Pilot Training Model to Implement Clinical Breast Exam Screening in Malawi. PloS one. 2016;11(3):e0151389. doi: 10.1371/journal.pone.0151389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider H, Hlophe H, van Rensburg D. Community health workers and the response to HIV/AIDS in South Africa: tensions and prospects. Health policy and planning. 2008;23(3):179–187. doi: 10.1093/heapol/czn006. [DOI] [PubMed] [Google Scholar]
- 28.Whalen C, Mafigiri D, McGrath J. Task shifting for tuberculosis control: A qualitative study of community-based directly observed therapy in urban Uganda. Global Public Health. 2012;7(3):270–284. doi: 10.1080/17441692.2011.552067. [DOI] [PMC free article] [PubMed] [Google Scholar]