Abstract
Introduction
For any antimicrobial approach to be successful in periodontal therapy, it is important that the antimicrobial agent targets the sub-gingival biofilm by attaining sufficient concentration at the sub-gingival site.
Aim
The purpose of the present study was to determine and compare the concentrations of ciprofloxacin present in Gingival Crevicular Fluid (GCF) and plasma after its systemic administration.
Materials and Methods
A total of 20 subjects, in the age group of 30-60 years satisfying the inclusion and exclusion criteria, were chosen from the outpatient Department of Periodontology, Government Dental College and Hospital, Hyderabad and consent was obtained. Subjects were put on oral ciprofloxacin therapy (Baycip, Bayer Corporation) of 500mg twice daily doses for five days to establish steady state tissue levels of the agent. GCF and serum samples were collected at the 72nd hour after the first dose of ciprofloxacin and were compared using unpaired t test.
Results
The mean gingival index value of the subjects was 1.8 ± 0.59 and the mean probing depth of the subjects taken in the study was 5.724 ± 0.47mm. The results of this study showed that ciprofloxacin concentrations were significantly higher (p<0.001) in GCF than in plasma.
Conclusion
Results from the present study and those from the earlier studies clearly indicate the ciprofloxacin’s ability to reach and concentrate in infected periodontal sites via GCF. This property of ciprofloxacin may be useful for eradication of periodontal pathogens, thus improving the outcome of periodontal therapy.
Keywords: Antimicrobial, Concentration, Probing depth
Introduction
There is abundant evidence to suggest that microorganisms are directly involved in the initiation and progression of various forms of periodontal disease [1,2]. It has become increasingly evident that different forms of periodontal disease in humans are associated with specific groups of microorganisms [3,4]. This observation has led to increased interest in the use of antibiotics as an adjunctive treatment in the elimination or control of the probable etiologic agents [5,6].
On many occasions, mechanical debridement, which is either non surgical or surgical, is not ideal as it is time consuming, operator and patient-dependent and difficult to master [7–9]. Some of the periodontal pathogens have the capacity to penetrate the soft tissues and the dentin. Also it is often impossible to remove all the infected structures due to anatomic factors [10–12]. Because of these facts, mechanical therapy may fail to completely eliminate the anaerobic infection within the gingival tissues or in furcations and other inaccessible areas. However, systemic antibiotic therapy is capable of eliminating pathogens not only from periodontal sites, but also from other sites in the oral cavity, due to which considerable therapeutic benefits can be gained and re-infection is preventable [13,14].
To gain maximum benefit from antibiotic therapy, an antibiotic must penetrate and reach the site of infection i.e., the periodontal pocket. To date, only a few antibiotics like Tetracycline and Fluorquinolones have been shown to penetrate from the systemic circulation into the gingival crevice [15–17]. For any antimicrobial approach to be successful in periodontal therapy, it is important that the antimicrobial agent targets the sub-gingival biofilm by attaining sufficient concentration at the sub-gingival site. It should also permit recolonization of microbiota that is compatible with periodontal health.
Studies have confirmed that the concentration of tetracycline in GCF is significantly higher than their serum levels on oral administration [16,17]. But the most significant drawback of tetracycline is that, some of the bacteria have been shown to be resistant to the drug. More recently, a similar distribution profile was reported for ciprofloxacin, a member of the fluoroquinolones family [15]. It was found that, ciprofloxacin is the only antibiotic in periodontal therapy to which all strains of Actinobacillus actinomycetemcomitans are susceptible [18,19]. It is also highly active against periodontal pathogens such as enteric rods and pseudomonas and many invasive pathogens because of its ability to penetrate cells and produce the bactericidal effects [20, 21]. We could only find, meagre literature related to ciprofloxacin concentrations among patients with periodontitis.
Aim
The present study was envisaged to determine and compare the concentrations of ciprofloxacin present in GCF and plasma after its systemic administration.
Materials and Methods
In the present quasi experimental study, a total of 20 adult patients, in the age group of 30-60 years were chosen from the outpatient Department of Periodontology, Government Dental College and Hospital, Hyderabad between the periods of May 2006 to April 2007. Prior information regarding the study was detailed to the patients and a written consent was obtained. Ethical clearance was obtained from the institutional review board.
Inclusion criteria: Subjects diagnosed with chronic periodontitis, characterized with clinical probing depths of ≥5mm and radiographic evidence of bone loss at two or more sites, with a minimum number of 20 teeth, non-smokers, systemically healthy and who did not receive any antibiotic therapy during the previous three months of the study were included.
Exclusion criteria: Patients with gross oral pathology, pregnant and lactating females, known or suspected allergy to ciprofloxacin, aggressive periodontitis, history of diabetes, anomalies of blood and immune system, a need for antibiotic prophylaxis, regular use of antacids or any other drug systemically, smokers, alcoholic patients, those taking drugs affecting bone metabolism like bisphosphonates, steroids, contraceptives, anti-inflammatory drugs and antibiotics for the last three months.
The patients were motivated and instructed to use manual toothbrush but not to use mouth rinses or irrigating solutions for the duration of the study. Subjects were put on oral ciprofloxacin therapy (Baycip, Bayer Corporation) of 500mg twice daily doses for five days to establish steady state tissue levels of the agent. Subjects were then recalled after three days following which GCF and serum samples were collected at the 72nd hour after the first dose of ciprofloxacin.
Collection of GCF and blood samples: After proper isolation and drying the area with a blast of air, supragingival plaque was removed without touching the marginal gingiva and a 1μl to 5μl calibrated volumetric micropipette (Sigma–Aldrich) was placed at the entrance of the crevice (extra-crevicular, unstimulated method). This procedure was repeated at various test sites and a volume of 1μl -3μl of GCF was collected. Micropipettes that were suspected to be contaminated with blood and saliva were discarded and the procedure was repeated. The collected GCF was immediately transferred to eppendorf tubes using the aspirating tube provided with the microcapillary pipettes. Approximately 3ml venous blood was drawn using the standard venipuncture technique. Plasma was later obtained by centrifugation. All the GCF and plasma samples were stored at -80°C until the analysis.
Measurement of ciprofloxacin levels in GCF and blood samples: A slightly modified method of Jim and colleagues was used for the High Performance Liquid Chromatography (HPLC) to determine the concentrations of ciprofloxacin in GCF and plasma samples [22]. The ciprofloxacin peaks were monitored at 280nm (excitation) - 455nm (emission). Standard graph for ciprofloxacin in mobile phase was prepared which was used to calculate the unknown concentration of ciprofloxacin in patient’s GCF samples. Similarly, standard graph for ciprofloxacin in human plasma was also prepared to calculate the unknown concentration of ciprofloxacin in patient’s plasma samples after its systemic administration.
HPLC sample preparation and determination of ciprofloxacin in GCF: GCF samples were removed from the freezer just before the analysis. Methanol-water, 50:50(v/v), was added until the final volume of the mixture for extraction was 1ml. The mixture was shaken on a vortex mixer for 5min and centrifuged at 4000rpm for 15min. Then 100 μl of the supernatant was injected into the column and the concentration of ciprofloxacin in given volume of GCF was calculated from the calibration plot for GCF analysis [Table/Fig-1]. Later concentration of ciprofloxacin in 1ml of GCF was calculated.
[Table/Fig-1]:
![[Table/Fig-1]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/df0f/4963770/2a68bb6b2f04/jcdr-10-ZC47-g001.jpg)
Standard graph obtained for estimation of ciprofloxacin in GCF samples.
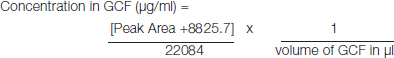 |
Determination of ciprofloxacin in plasma: Patient’s blood sample was centrifuged at 4000rpm for 15min and plasma was separated and stored at -800C until analysis. At the time of analysis, 250μl of plasma was taken and mixed with 750μl of methanol and vortexed for 3min. The mixture was centrifuged for 15min at 4000rpm and 20μl of the supernatant was injected into the column and peak area obtained. Concentration of ciprofloxacin was then calculated from the calibration graph obtained for plasma [Table/Fig-2].
[Table/Fig-2]:
![[Table/Fig-2]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/df0f/4963770/968274dc27b9/jcdr-10-ZC47-g002.jpg)
Standard graph obtained for estimation of ciprofloxacin in plasma samples.
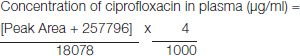 |
Statistical Analysis
All data was entered into a Microsoft Excel spreadsheet and exported for analyses using the statistical software SPSS v 17.0 (Chicago, IL, USA). The Unpaired t test was used for comparing ciprofloxacin concentration in GCF and plasma.
Results
A total of 20 patients (11 males and 9 females) within the age range of 30-60 years were selected for the study. The mean age of the subjects was 39.6±7.09 years. The mean gingival index value of the subjects was 1.8±0.59 and the mean probing depth of the subjects taken in the study was 5.724±0.47mm. Gingival Index [GI] and Probing Pocket Depth [PPD] was assessed only to check for their eligibility to participate in the study. Post intervention assessment was not carried out as the duration of study was very less which would have had insignificant effect on GI and PPD. [Table/Fig-3] shows the mean gingival index and mean probing depths values of the subjects. [Table/Fig-4] shows the concentration of ciprofloxacin in the GCF and plasma. The results of this study showed that ciprofloxacin concentrations were significantly higher (p<0.001) in GCF than in plasma. Mean GCF ciprofloxacin concentration was 2.390±0.09592 μg/ml while mean plasma ciprofloxacin concentration 0.3089±0.02678μg/ml at the 72nd hour of the ciprofloxacin systemic administration. This shows that ciprofloxacin concentration in GCF is 7-8 folds higher than that in plasma.
[Table/Fig-3]:
Mean gingival index and mean probing depth values of the subjects.
| Mean ± SD | |
|---|---|
| Gingival Index Score | 1.8 ± 0.59 |
| Probing Depth | 5.724 ± 0.47 |
[Table/Fig-4]:
Concentration of ciprofloxacin in the GCF and plasma.
| Mean ± SD | p-value | |
|---|---|---|
| Ciprofloxacin Concentration in GCF (μg/ml) | 2.39 ± 0.09592 | 0.001* |
| Ciprofloxacin Concentration in Plasma (μg/ml) | 0.3089 ± 0.02678 |
*Significant; Unpaired t test
Discussion
The present study was designed to assess and compare the levels of ciprofloxacin in GCF with those present in the serum. The results of this bioanalytical study showed that ciprofloxacin reaches 7 to 8 times higher concentrations in GCF than in plasma [Table/Fig-4]. The higher concentration of ciprofloxacin in GCF may have clinical relevance. Since ciprofloxacin’s antimicrobial activity is dose-dependent, its higher concentration and increased availability in GCF may enhance its antibacterial effects against susceptible subgingival microorganisms [23]. The Minimal Bactericidal Concentration (MBC) of most antimicrobial agents is several-fold higher than their conventional MIC value. In the present study, the mean GCF ciprofloxacin concentration attained was 2.39±0.09μg/ml, which is well in excess of ciprofloxacin’s minimum inhibitory concentration for A. actinomycetemcomitans (0.015μg/ml) and this may also correlate with the MBC value. These results are in accordance with those of the earlier studies [15,16,18,21,24].
Conway et al., have demonstrated that the mean GCF levels of this agent were four to five times than its serum level. They have also found that the total amount of ciprofloxacin perfusing inflamed periodontitis sites was greater than at healthy sites [15]. Tezel et al., reported that, the GCF concentration of ciprofloxacin was higher than in serum among both gingivitis and periodontitis patients, however its concentration was found to be significantly high in subjects with periodontitis compared to subjects with gingivitis [25]. Another study revealed that the concentrations of ciprofloxacin and doxycycline in GCF were significantly higher than in serum, but not significantly different from each other [16].
The mechanisms by which the GCF ciprofloxacin levels are higher relative to serum are unclear. Seymour et al., suggested that lymphocytes and polymorphonuclear leukocytes are the predominantly infiltrating leucocytes in the inflamed periodontium [26]. Easmon and Crane reported that ciprofloxacin levels inside human neutrophils are usually four to eight times higher than the extracellular medium [27]. Cacchillo and Walters also demonstrated that PMNs loaded with ciprofloxacin maintained therapeutic levels of the agent and killed A.actinomycetemcomitans more rapidly than did unloaded PMNs [20]. These findings indicate that PMNs may play a role in enhancing the local concentrations of the drug in GCF.
Other studies have also demonstrated that cultured gingival fibroblasts possess transporters that are capable of moving ciprofloxacin into or out of the cell to maintain equilibrium between intracellular and extracellular concentrations [27,28]. Maria Lavda et al., hypothesized that the efflux of ciprofloxacin from gingival fibroblasts could help maintain relatively high antimicrobial levels in the interstitial fluid, as antibiotic levels decrease from their peak values in the blood and tissues [16]. Much of this antibiotic content in the interstitial fluid eventually passes through the junctional epithelium and into the gingival crevice. Thus explaining the mechanism by which drugs such as tetracyclines and ciprofloxacin reach higher levels in GCF than in blood serum. The ability of ciprofloxacin to get concentrated in other bodily fluids such as urine, prostatic fluid, bronchial secretions and saliva has also been reported by some studies [29,30].
To the best of our literature search, the present study is the first study to assess the concentration of ciprofloxacin in GCF among Indian subjects. This study validates the findings of few previous studies. Aside from tetracycline and ciprofloxacin, no other antimicrobial agents are known to distribute at significantly higher levels in GCF than in serum [15]. As there are very few drugs which can reach to a significant concentration in GCF, ciprofloxacin can be effective systemic medicine with or without scaling and root planning in management of periodontitis.
Limitation
However before generalizing the study findings, limitations have to be considered. In the present study the sample size was not calculated scientifically and there is no comparison group to prove its true efficacy. Till now, scientific evidence comparing the antimicrobial efficacy of doxycycline and ciprofloxacin against the periodontal pathogens is lacking. There is a need to prove this fact for the transfer of this finding from bench to bedside.
Conclusion
In conclusion, the results from the present study and those from the earlier studies clearly indicate the ciprofloxacin’s ability to reach and concentrate in infected periodontal sites via GCF. This property of ciprofloxacin may be useful for eradication of periodontal pathogens, thus improving the outcome of periodontal therapy.
Financial or Other Competing Interests
None.
References
- [1].Barbosa FCB, Mayer MPA, Saba-Chujfi E, Cai S. Subgingival occurrence and antimicrobial susceptibility of enteric rods and pseudomonads from Brazilian periodontitis patients. Oral MicrobiolImmunol. 2001;16:306–10. doi: 10.1034/j.1399-302x.2001.016005306.x. [DOI] [PubMed] [Google Scholar]
- [2].Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal disease. J Periodontol. 1988;15:316–23. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- [3].Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- [4].Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14(4):727–52. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Slots J, Ting M. Systemic antibiotics in the treatment of periodontal disease. J Periodontology. 2002;28:106–76. doi: 10.1034/j.1600-0757.2002.280106.x. [DOI] [PubMed] [Google Scholar]
- [6].Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodont Res. 2002;37:389–98. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- [7].Chen C, Slots J. The current status and future prospects of altering the pathogenic microflora of periodontal disease. Curr Opin Periodontol. 1993:71–77. [PubMed] [Google Scholar]
- [8].Cobb CM. Non-surgical pocket therapy. Mechanical Ann Periodontal. 1996;1:443–90. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- [9].Drisko CH. Nonsurgical periodontal therapy. Periodontol. 2000. 2001;25:77–88. doi: 10.1034/j.1600-0757.2001.22250106.x. [DOI] [PubMed] [Google Scholar]
- [10].Muller HP, Lange DE, Muller RF. Actinobacillus actinomycetemcomitans recovery from extracrevicular locations of the mouth. Oral Microbiol Immunol. 1993;8(6):344–48. doi: 10.1111/j.1399-302x.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- [11].Rams TE, Feik D, Young V, Hammond BF, Slots J. Enterococci in human periodontitis. Oral Microbial Immunol. 1990;7:249–52. doi: 10.1111/j.1399-302x.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- [12].Saglie FR, Smith CT, Newman MG. The presence of bacteria within the oral epithelium in periodontal disease. II immunohistochemical identification of bacteria. J Periodontol. 1986;57:492–500. doi: 10.1902/jop.1986.57.8.492. [DOI] [PubMed] [Google Scholar]
- [13].Van Rensberg CE, Joone G, Anderson R. Interactions of oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, perfloxacin and flerfloxacin in the intraphagocytic eradication of Staphylococcus aureus. J Med Microbiol. 1990;3:15–17. doi: 10.1099/00222615-32-1-15. [DOI] [PubMed] [Google Scholar]
- [14].Walker CB, Karpinia K, Baehni P. Chemotherapeutics: antibiotics and other antimicrobials. Periodontol 2000. 2004;36:146–65. doi: 10.1111/j.1600-0757.2004.03677.x. [DOI] [PubMed] [Google Scholar]
- [15].Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol. 2000;71:1448–52. doi: 10.1902/jop.2000.71.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lavda M, Clausnitzer E, Walters JD. Distribution of systemic ciprofloxacin and doxycycline to gingiva and gingival crevicular fluid. J Periodontol. 2004;75(12):1663–67. doi: 10.1902/jop.2004.75.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pahkla ER, Koppel T, Naaber P, Saag M, Loivukene K. The efficacy of non-surgical and systemic antibiotic treatment on smoking and non-smoking periodontitis patients. Stomatologija, Baltic Dental and Maxillofacial Journal. 2006;8:116–21. [PubMed] [Google Scholar]
- [18].Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand J Dent Res. 1993;101:299–303. doi: 10.1111/j.1600-0722.1993.tb01124.x. [DOI] [PubMed] [Google Scholar]
- [19].Soleymani Shayesteh Y, Khorsand A, Salary MH, Mehrizy H. Comparison of systemic ciprofloxacin in elimination of A.actinomycetemcomitans from active sites with combination of metronidazole and amoxicillin in patients with aggressive periodontitis: a randomized double blind controlled trial. Journal of Dentistry of Tehran University of Medical Sciences. 2004;1(2):24–28. [Google Scholar]
- [20].Cacchillo DA, Walters JD. Effect of ciprofloxacin on killing of Actinobacillus actinomycetemcomitans by polymorphonuclear leukocytes. Antimicrob Agents Chemother. 2002;46:1980–84. doi: 10.1128/AAC.46.6.1980-1984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Muller HP, Holerrieth S, Burkhardtr U, Hofler U. In-vitro antimicrobial susceptibility of oral strains of Actinobacillus actinomycetemcomitans to seven antibiotics. J Clin Periodontol. 2002;2:736–42. doi: 10.1034/j.1600-051x.2002.290810.x. [DOI] [PubMed] [Google Scholar]
- [22].Slots J, Feik D, Rams TE. In-vitro antimicrobial sensitivity of enteric rods and pseudomonas from advanced adult periodontitis. Oral Microbial Immunol. 1990;5:298–301. doi: 10.1111/j.1399-302x.1990.tb00428.x. [DOI] [PubMed] [Google Scholar]
- [23].Gould IM, Milne K, Jason C. Concentration-dependent bacterial killing, adaptive resistance and post-antibiotic effect of ciprofloxacin alone and in combination with gentamicin. Drugs Exp Clin Res. 1990;16(12):621–28. [PubMed] [Google Scholar]
- [24].Tözüm TF, Yildirim A, Cağlayan F, Dinçel A, Bozkurt A. Serum and gingival crevicular fluid levels of ciprofloxacin in patients with periodontitis. J Am Dent Assoc. 2004;135(12):1728–32. doi: 10.14219/jada.archive.2004.0127. [DOI] [PubMed] [Google Scholar]
- [25].Tezel A, Yucel O, Orbak R. The gingival crevicular fluid ciprofloxacin level in subjects with gingivitis and periodontitis, and its effects on clinical parameters. J Periodontol Res. 2005;40:395–400. doi: 10.1111/j.1600-0765.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- [26].Seymour GJ, Powell RN, Davies WI. The immunopathogenesis of progressive chronic inflammatory periodontal disease. J Oral Pathol. 1979;8(5):249–46. doi: 10.1111/j.1600-0714.1979.tb01826.x. [DOI] [PubMed] [Google Scholar]
- [27].Easmon CS, Crane JP. Uptake of ciprofloxacin by human neutrophils. J Periodontol Res. 1985;1:67–73. doi: 10.1093/jac/16.1.67. [DOI] [PubMed] [Google Scholar]
- [28].Yang Q, Nakkula RJ, Walters JD. Accumulation of ciprofloxacin and minocycline by cultured human gingival fibroblasts. J Dent Res. 2002;81:836–40. doi: 10.1177/154405910208101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jacobs F, Marchal M, de Francquen P, Kains JP, Gangji D, Thys JP. Penetration of ciprofloxacin into human pleural fluid. Antimicrob Agents Chemother. 1990;34(5):934–36. doi: 10.1128/aac.34.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Keren G, Alhalel A, Bartov E, Kitzes-Cohen R, Rubinstein E, Segev S, et al. The intravitreal penetration of orally administered ciprofloxacin in humans. Invest Ophthalmol Vis Sci. 1991;32(8):2388–92. [PubMed] [Google Scholar]


