Salmonella enterica serovar Typhi (S. Typhi) causes typhoid fever, a systemic disease of humans that is estimated to cause more than 200,000 annual deaths (Butler, 2011; Crump and Mintz, 2010; Parry et al., 2002). Unlike other Salmonella enterica serovars, which can infect a broad range of animals, S. Typhi can only infect humans, which has hampered the development of a convenient animal model for the study of typhoid fever. Recently, it was reported in Cell (Mathur et al., 2012) that mice lacking Toll-like receptor 11 (TLR11) could be lethally infected with S. Typhi after oral or systemic inoculation. It was also postulated that TLR11-mediated recognition of Salmonella flagellin prevents S. Typhi infection in wild-type (C57BL/6) mice and that the lack of functional TLR11 renders humans susceptible to the bacterial infection (Mathur et al., 2012). It was therefore proposed that TLR11-deficient mice could serve as a convenient animal model for typhoid fever (Mathur et al., 2012). However, we report here that infection studies conducted in 4 different laboratories have found that TLR11-deficient mice do not show enhanced susceptibility to S. Typhi regardless the route of inoculation. We also observed no binding of flagellin to TLR11 and found no differences in the response of wild type and TLR11-deficient mice to the administration of bacterial flagellin, which are inconsistent with the proposed role of this Toll receptor in the detection of this bacterial protein (Mathur et al., 2012).
We infected TLR11−/− and wild-type isogenic control mice either orally or intraperitoneally with different strains of S. Typhi. In addition to the strain used in the original study (Ty2) (Mathur et al., 2012), we used other virulent S. Typhi strains including ISP2825 (Galán and Curtiss III, 1991), and A8/14353363984-6 and 3290A481, two clinical isolates obtained from the Yale Clinical Microbiology laboratory. Mice were obtained from Dr. Sankar Ghosh and their genotype verified by standard genotyping techniques (see supplementary experimental procedures). None of the mice showed any signs of disease even when inoculated with dozes up to 100-fold higher than those used by Mathur et al (Mathur et al., 2012) (Fig. 1 and Supplementary Materials). Consistent with this finding, no S. Typhi colony forming units (c. f. u.) were recovered from the spleens of animals (WT or TLR11−/−) orally or intraperitonelly inoculated with S. Typhi (Fig. 1 A–G). Mice orally or intravenously inoculated with S. Typhi Ty2 showed no differences in CFU detected in fresh fecal pellets (Fig. 1H), spleens (Fig. 1I), or livers (Fig. 1J). Survival studies independently conducted at 4 different geographical sites demonstrated that mice defective in TLR11 receptor do not show enhanced susceptibility to S. Typhi. Taken together these results indicate that TLR11 −/− mice cannot serve as a model for the study of typhoid fever and S. Typhi pathogenesis as originally proposed (Mathur et al., 2012). Unlike the previous report (Mathur et al., 2012), we found no evidence of flagellin binding to TLR11. We injected WT and TLR11−/− mice with recombinant Salmonella flagellin and measured serum levels of IL-12 and IL-6 two hours post-injection. We found no differences in the levels of these cytokines in WT and TLR11−/− mice (Fig. S1). Similarly, we found no differences in the levels of IL-12, IL-6, and TNF produced by sort-purified WT and TLR11−/− lamina propria mouse macrophages or splenic dendritic cells in response to flagellin (Fig. S1). These results are inconsistent with the proposed recognitions of flagellin by TLR11 (Mathur et al., 2012).
Figure 1. TLR11 −/− mice do not support S. Typhi replication.
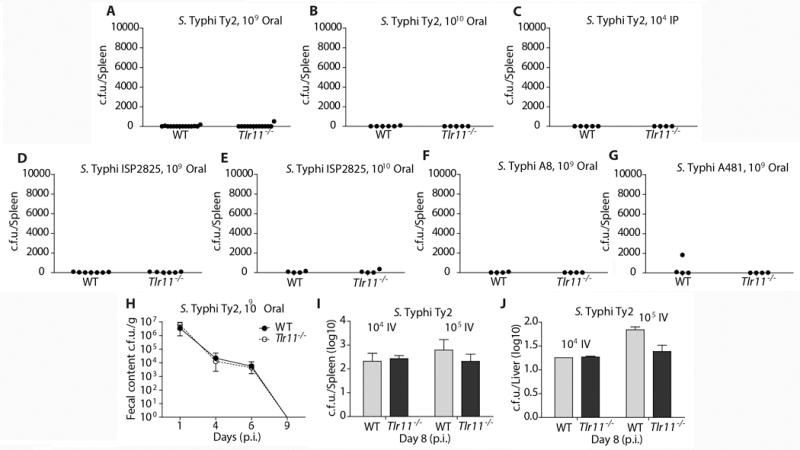
(A–G) Groups of sex- and age (6 to 10 weeks)-matched control and TLR11−/− mice were infected with the indicated S. Typhi strains. Infection routes and doses are indicated in the figure panels. Shown are c. f. u. 24 days after infection obtained from spleens of animals infected with S. Typhi Ty2 (A–C), ISP2825 (D and E), A8/14353363984-6 (F) and 3290A481 (G). (H) Groups (n = 5) of male and female mice aged 9 to 14 weeks were inoculated via oral gavage with approximately 1×1010 CFU of S. Typhi Ty2 in a volume of 0.2 ml. Fresh fecal pellets were collected at indicated time points. (I and J) Mice infected intravenously infectd with the indicated dozes of S. Typhi Ty2 and the c. f. u. in spleens (I) and livers (J) enumerated 8 days after infection. See supplemental experimental procedures for information on each laboratory’s experimental contributions.
We cannot offer an explanation for the variance between our collective studies and those reported by Mathur et al. The differences in the results obtained are drastic in that TLR11 −/− mice not only did not succumb to S. Typhi after oral or systemic infection, but the animas cleared the infection. The strain of S. Typhi used in this study was the same as that used in the study of Mathur et al (Mathur et al., 2012) and same observations were made with several other S. Typhi clinical isolates. Furthermore the infection protocols were similar (see Supplement Experimental Procedures).
All laboratories used the TLR11 −/− mice that were originally generated at Yale University (Zhang et al., 2004). Differences in animal facilities are unlikely to account for the vastly different results obtained since animal husbandry is largely consistent across different animal facilities and in one case, experiments were conducted in the same animal facility (Yale University) as that used to conduct the previous study (Mathur et al., 2012).
We are also uncertain why we did not find evidence of TLR11-mediated flagellin-sensing, and note that the Ghosh lab had previously reported that flagellin was not able to stimulate TLR11 (Zhang et al., 2004), a variance that was not discussed by Mathur et al.
In conclusion, 5 independent laboratories at 4 different institutions were unable to reproduce the reported increased susceptibility of TLR11-deficient mice to S. Typhi, and therefore these animals cannot serve as a model to study typhoid fever. The conclusion that species-specific expression of TLR11 and its binding to flagellin determines S. Typhi susceptibility is also questioned by our studies. Urgent efforts to develop a convenient animal model for typhoid fever must therefore continue.
Supplementary Material
Acknowledgments
Work in the Galán laboratory was supported by National Institute of Allergy and Infectious Diseases (NIAID) Grant AI079022. J.S. was supported in part by a fellowship from the Northeast Biodefense Center U54-AI057158. Work in the McSorley laboratory was supported by AI055743 and AI056712. Work in the Pier lab was supported by NIAID Grant AI057159, a component of Award Number U54 AI057159. Work in the Grishin laboratory was supported by the National Institute of General Medical Sciences Grant GM094575 and the Welch Foundation Grant I-1505. Work in the Bäumler laboratory was supported by NIAID award AI044170. Work in the Yarovinsky laboratory is supported by NIAID Grant AI085263, and the Burroughs Wellcome Foundation.
References
- Butler T. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin Microbiol Infect. 2011;17:959–963. doi: 10.1111/j.1469-0691.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- Crump J, Mintz E. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JE, Curtiss R., III Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella typhi are deficient for entry into mammalian cells. Infect Immun. 1991;59:2901–2908. doi: 10.1128/iai.59.9.2901-2908.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R, Oh H, Zhang D, Park S, Seo J, Koblansky A, Hayden M, Ghosh S. A mouse model of Salmonella typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C, Hien TT, Dougan G, White N, Farrar J. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden M, Greenblatt M, Bussey C, Flavell R, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


