Abstract
Objective
This longitudinal study examined whether changes in neuropsychological functioning were associated with the trajectory of ADHD-related symptoms and impairment between preschool and school-age.
Method
The sample consisted of 3- and 4-year-old children (N=138) who were identified as being “at-risk” for ADHD based on parent and teacher reports. Neuropsychological functioning was measured annually using the NEPSY at four points of time (Mean ages: 4.19, 5.36, 6.35 and 7.35 years). ADHD symptoms and impairment were assessed using semi-annual parent and teacher reports on the ADHD-RS-IV and the Children’s Problems Checklist over 10 points of time (Mean age at baseline and final assessment = 4.19 and 8.81 years, respectively). Hierarchical linear modeling was used to assess the trajectories of change in neuropsychological functioning and ADHD severity as well as the association of change in neuropsychological functioning with change in ADHD severity over time.
Results
Baseline neuropsychological functioning was not significantly associated with the slope of change in ADHD severity. However, the magnitude of change in neuropsychological functioning was linearly associated with the trajectory of ADHD symptom severity and impairment such that individuals with greater neuropsychological growth over time had a greater diminution of ADHD severity and impairment. Family socioeconomic status at baseline was significantly associated with initial ADHD severity and impairment but not with change over time.
Conclusion
Interventions that enhance neuropsychological functioning from an early age may be beneficial to attenuating long-term ADHD severity and impairment.
Keywords: ADHD, neuropsychology, preschool, longitudinal study, developmental trajectory
Attention-deficit/Hyperactivity Disorder (ADHD) is defined by developmentally inappropriate levels of inattention and hyperactivity/impulsivity that typically emerge during the preschool years and often persist into adulthood, causing significant functional disability throughout the lifespan (1). Longitudinal studies of inattentive and hyperactive/impulsive preschool children indicate both stability and variability of these behaviors over time (2, 3). As compared to controls, a much greater portion of these “high risk” preschool children subsequently meet criteria for ADHD. Nevertheless, approximately half of 3 – 4 year-old children identified by parent ratings of behavioral problems no longer had difficulties by 6 years of age (4).
Several factors have been associated with variability in the trajectory of ADHD, including age at initial assessment (5, 6), the presence of comorbid disruptive behavior disorders (5), and objectively measured behavioral inhibition deficits (7). Yet, these findings provide limited insight into the processes and mechanisms by which the course of ADHD is altered across development. From a developmental psychopathology perspective (8, 9), ADHD is not the result of a fixed deficit, but rather it is a clinical manifestation of neurodevelopmental vulnerability whose trajectory is mediated by changes in brain structure and function in response to an array of interacting genetic and environmental factors throughout development.
Consistent with this view, longitudinal studies (10, 11) have found that children with ADHD follow a sequential pattern of cortical development similar to their non-ADHD peers, but development was delayed by as much as 2 – 3 years throughout most of the cortex. Further, changes in cortical thickening were associated with clinical outcome such that ADHD children with poorer outcome had “fixed” thinning of the left medial prefrontal cortex and those with better outcomes had right parietal normalization, suggestive of compensatory cortical change (10). Additionally, cortical thinning in the dorsolateral prefrontal cortex, anterior cingulate, and inferior parietal lobe that continues into adulthood has been linked to the persistence of ADHD symptoms (12). Similarly, based on differences in cortical thickness between those with and without persisting symptomatology, a 33-year follow-up of children with ADHD concluded that diagnostic remission may result from compensatory maturation of the brain (13).
Consistent with these structural neuroimaging studies, preliminary functional magnetic resonance imaging data indicate that brain activation during inhibition parallels the degree of symptom persistence in adolescents with childhood ADHD, with remitters more closely resembling their never-ADHD peers relative to persisters (14). Although not consistently observed (15), neuropsychological test performance of adolescents who had ADHD in childhood has also been shown to vary as a function of ADHD persistence; only those who continued to meet criteria for ADHD at follow-up differed significantly from controls on measures of working memory, inhibitory control and sustained attention (16–18). Thus, more optimal cortical development and neurocognitive functioning appears to be associated with a diminution of ADHD symptoms from childhood through adulthood (10,12–14,16–21).
Yet, elucidating the relations between neurocognitive development and ADHD trajectories has been hampered by the fact that existing studies include only two or three assessment points, one in middle childhood and the other(s) in adolescence or early adulthood. This limits the ability to carefully examine developmental trajectories between preschool and early childhood when ADHD is first emerging and trajectories are most variable (2, 3). Determining relations between early neurocognitive development and the emergence of ADHD would not only have considerable heuristic value, but could have a substantial impact on the development of early interventions (9, 22). Further limiting is the fact that most existing studies have focused on the presence or absence of a categorical ADHD diagnosis at follow-up. Given that ADHD symptoms present on a continuum (23), a dimensional approach (24) with multiple time-points would provide greater sensitivity to detecting factors associated with change in symptoms and impairment.
The primary aim of this longitudinal study is to employ a dimensional approach with multiple assessment points to test two hypotheses that might account for the varied trajectories among preschool children characterized by inattention and hyperactivity/impulsivity. The first hypothesis posits that poorer outcomes are associated with early neurocognitive dysfunction and that those with more optimal behavioral trajectories are phenocopies without the early neural dysfunction that underlies ADHD.
Our second hypothesis posits that the often-observed decline of symptoms with age is accounted for by the degree to which the later development of higher cortical circuitry and functions are able to compensate through ‘top down’ regulatory control (19). Thus, irrespective of baseline neuropsychological functioning, those with greater overall neurocognitive growth over time will show a trajectory characterized by a diminution of symptoms and greater clinical improvement. In contrast, those with less robust cognitive growth over time will show a persisting or worsening symptom pattern over development.
This study focused on a sample of 3- and 4-year-old children who were characterized as “at-risk” for ADHD based upon elevated symptom ratings from parents and/or teachers. Parent and teacher ratings of ADHD symptoms and ADHD-related impairment were acquired every six months for four consecutive follow-up years. In addition, neuropsychological functioning was evaluated annually. Using hierarchical linear modeling (HLM) individual trajectories were identified based upon behavioral ratings. Subsequently, the degree to which neuropsychological functioning at baseline (hypothesis 1), and change in neuropsychological functioning from baseline (hypothesis 2), accounted for trajectory variability across children was determined.
Method
Participants
Participants were 138 children recruited through screenings at preschools and referrals from preschools and local pediatric and mental health providers in the New York metropolitan area. The sample was ethnically and racially diverse (see Table 1). For inclusion, children had to be rated by parents and/or teachers as having at least six separate symptoms of hyperactivity-impulsivity, and/or inattention, as indicated by a rating of “Often” or “Very often”, on the Attention Deficit/Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV; 25). Thus children did not need to meet diagnostic criteria for ADHD, but rather exhibited varying levels of symptom severity and were symptomatic in at least one setting at the time of recruitment. Children were excluded if they had a Full Scale IQ of less than 80 (as measured by the WPPSI-III), a neurological or pervasive developmental disorder, were taking systemic medication for a chronic medical condition (including ADHD), or if the parent or child did not speak English.
TABLE 1.
Descriptive characteristics of the sample at baseline
| Variable | Mean | Standard Deviation | Range |
|---|---|---|---|
| Age at baseline (years) | 4.19 | 0.48 | 3.00–4.92 |
| Full Scale IQ | 102.14 | 12.80 | 80–138 |
| ADHD-RS-IV (Parent) | 27.68 | 10.96 | 0–48 |
| ADHD-RS-IV (Teacher) | 30.42 | 13.26 | 0–54 |
| CPC (Parent) | 3.77 | 3.56 | 0–15 |
| CPC (Teacher) | 3.94 | 4.77 | 0–18 |
| Family SES | 60.22 | 18.27 | 20–97 |
|
| |||
| N | % | ||
|
| |||
| Boys | 104 | 75.4 | - |
| White | 82 | 59.4 | - |
| Black | 22 | 15.9 | - |
| Asian | 8 | 5.8 | - |
| Mixed/ Other | 26 | 19.8 | - |
| Hispanic | 48 | 34.8 | - |
ADHD-RS-IV: ADHD Rating Scale-IV; CPC: Children’s Problem Checklist, SES: Socio-Economic Status
No child was taking systemic medications at the time of recruitment. At the age of 8 years, 34 children were taking ADHD medications, including stimulants (N=19), non-stimulants (N=4), or a combination of stimulants and non-stimulants (N=6); an additional 5 children were taking an ADHD medication along with either an antidepressant or atypical antipsychotic medication. Parents were asked to rate the behavior of the child while not on medication, and to withhold stimulant medications at every follow-up assessment.
Procedure
Parents and teachers rated children’s ADHD symptoms and impairment when they were 3–4 years-old (Baseline, BL) and then at four annual follow-ups (F1, F2, F3 and F4) and five 6-month follow-ups (F0.5, F1.5, F2.5, F3.5, F4.5) (mean age at F4.5=8.81, SD=0.51 years). Children were tested in the laboratory to assess neuropsychological functioning using the NEPSY at BL and then at three subsequent annual follow-ups (F1, F2, and F3; mean age at F3=7.35, SD=0.51 years). This study was approved by the University’s Institutional Review Board. After a complete description of the study, written informed consent was obtained from parents.
Measures
ADHD-RS-IV (25)
This rating scale, based on the 18 symptoms specified in the DSM-IV, was completed by parents and teachers. Symptoms are rated on a 4-point scale ranging from 0 (Never or rarely) to 3 (Very often). This scale has good psychometric properties for both school-aged (25) and preschool (26) children. The average correlation between parent and teacher reports across all points of time in the present sample was .31. Cronbach’s alpha ranged from .93–.96 and .91–.96 for teacher and parent reports, respectively.
Children’s Problem Checklist (CPC; 27)
Parent and teacher reports using this brief, psychometrically-sound measure of impairment in young children were used to characterize impairment due to ADHD over time. Items (See appendix 1) are rated on a 4-point scale consisting of no, mild, moderate or severe problems. The mean correlation between parent and teacher report was .17. Cronbach’s alpha ranged from .74–.85 for teacher and .64–.81 for parent reports.
NEPSY (28)
The NEPSY assesses neuropsychological functioning in five domains: Attention/Executive, Language, Sensorimotor, Visuospatial, and Memory. It was administered by well-trained graduate students. In the normative sample test-retest reliability for the five domains ranged from .70–.91 for 3–4-year-olds; and from .79–.87 for 5–12-year-olds (28). In our sample, Cronbach’s alphas of the five domains at BL, F1, F2, and F3 were .66, .74, .71, and .66, respectively.
Nakao-Treas Socioeconomic Prestige Index (29) was used to measure SES at BL. High scores on this index are indicative of higher SES. Both mothers’ and fathers’ scores on the index were separately coded and the higher of the two scores was adopted to indicate family SES.
Missing Data
The mean (SD) number of time-points for data on ADHD symptoms and impairment included in the longitudinal analysis was 7.55 (2.78). Participants at F1 and F4, but not at other points of time, had higher SES relative to those who did not participate (p<.05). There were no significant differences in gender, race and ethnicity between those who participated and those who did not at any time point.
Data Analysis
HLM (30) was used to assess individual intercept (initial levels) and slope (change) of trajectories of children over time while accounting for the lack of independence between repeated observations of each child. Restricted maximum likelihood estimation was used to estimate the random effects models. Some variables in the model (ADHD at F1.5, F2 and F4.5) had kurtosis values above 2.00. An examination of the Level 2 residuals indicated some divergence from normality in the tails of the distribution of the Mahalanobis distance. Therefore robust standard errors are reported (31).
While assessment of ADHD symptoms and impairment was carried out approximately every six months, neuropsychological functioning was assessed annually. Factor analysis showed a single factor underlying ADHD severity and impairment at each time-point. Therefore, an overall mean of parent and teacher reports on the ADHD-RS-IV and CPC was used to indicate child ADHD symptoms and impairment. Similarly, the mean of the five domain scores at each time-point was used to indicate neuropsychological functioning. Family SES was included as a covariate.
The first model was designed to investigate trajectories of change in ADHD severity and impairment across 10 points of time. Age was centered at 8 years. For instance, a child who was assessed at age 8.25 years was given a value of .25. This model showed the average level of ADHD symptoms at age 8, the average rate of change and the amount of variability in the average level of symptoms and the rate of change. This model is represented by the equations:
π0j and π1j are the intercept and slope respectively for participant j, and eij is the Level 1 regression residual for each participant. In the Level 2 equations, γ00 and γ10 indicate the average intercept and average slope respectively. The Level 2 error terms r0j and r1j, signify differences between the individual and the sample on the intercept and slope respectively.
Model 2 similarly investigated the trajectory of change in NEPSY scores. Child age centered at 4 years was included as a Level 1 variable. For instance, a child who was assessed at age 4.25 was given a value of .25 for Time of assessment. To obtain a value for the empirical Bayes slope of change in neuropsychological functioning, the same model was run. This value of the slope was saved for later use as a predictor of change in ADHD severity (see Model 4 in results below).
To test hypothesis 1 (Model 3), scores on neuropsychological functioning at BL were used to predict change in the severity of ADHD symptoms and impairment after controlling for BL family SES. The Level 1 model included the continuous variable ‘Age’ centered at 8 years. The level-2 model included BL neuropsychological functioning, and family SES. This model is represented by the following equations:
Model 4 was identical to Model 3 in all aspects except that the slope of NEPSY (calculated in Model 2) was entered instead of BL NEPSY. As in the above model, the Level 1 model included the continuous variable of ‘Age’ centered at age 8 years. The level-2 model included the slope of change in NEPSY and family SES.
Results
Model 1: Change in ADHD symptoms and impairment
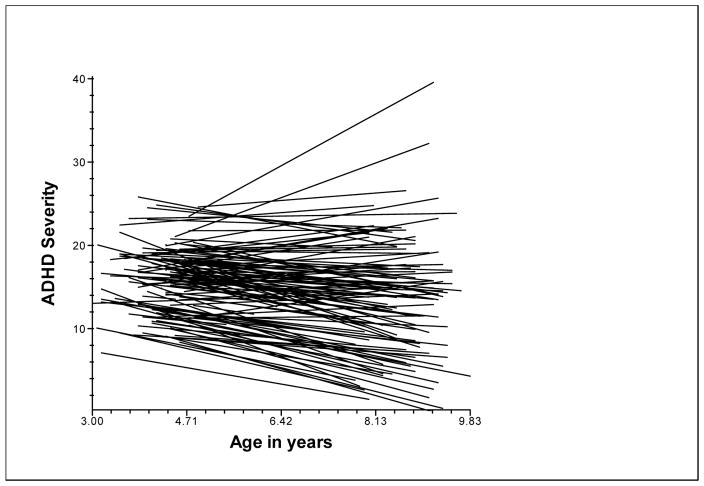
This model provided the average level of ADHD severity at age 8 years, the average rate of change, and the variability in the intercept and rate of change. The estimate of mean ADHD symptoms and impairment at age 8 was 13.40 (DF=138; SE=0.62; T ratio=21.46; p<.001). The average slope or rate of change in ADHD symptoms and impairment at 8 years was −0.55 units (DF=138; SE=0.16; T ratio= −3.45; p=.001). There was significant variation around the intercept: π2 (133) = 1044.46; p<.001; and the slope: π2 (133) = 297.87; p<.001. This indicates that the children varied in their ADHD severity at age 8 and in their rate of change in ADHD severity. Therefore both these parameters were modeled to understand the developmental trajectory of children. Figure 1 shows the variability in the trajectories of ADHD symptoms and impairment.
Figure 1.
Trajectories of Change in ADHD severity and impairment
Note: ADHD Severity refers to the mean of parent and teacher ratings of symptoms and impairment
Model 2: Change in Neuropsychological functioning
The average predicted value of neuropsychological functioning at Age 4 was 94.64 (SE=.73; df=137; T ratio=128.77; p<.001) and this value increased on average by 3.11 units (SE=.31; df=137; T ratio= 10.00; p<.001) with a unit increase in time (approximately every year). Moreover, there was significant variation around the intercept: π2 =262.02; df=123; p<.001; and the slope: π2=172.92; df=123; p=.002. This indicates that children varied in their initial values and in their rate of change in neuropsychological functioning. Overall, scores on neuropsychological functioning improved over time, though they improved less for some children than for others.
Model 3: BL Neuropsychological functioning predicting Change in ADHD severity
BL level of neuropsychological functioning was used to predict change in the severity of ADHD symptoms and impairment (Hypothesis 1) holding constant family SES. BL neuropsychological functioning was not significantly associated with the intercept or change in ADHD severity. Higher BL SES was associated with lower initial values of ADHD severity (Table 2).
TABLE 2.
Association of BL neuropsychological functioning with change in ADHD symptom severity and impairment
| Fixed Effect | Coefficient | SE | T-ratio | p-value |
|---|---|---|---|---|
| Level 1 intercept | ||||
| Intercept | 20.26 | 6.43 | 3.15 | .002 |
| SES | −0.10 | 0.03 | −3.04 | .003 |
| BL NEPSY | −0.01 | 0.07 | −0.14 | .89 |
| Level 1 linear slope | ||||
| Intercept | −1.40 | 1.34 | −1.04 | .30 |
| SES | −0.01 | 0.01 | −0.87 | .39 |
| BL NEPSY | −0.01 | 0.01 | −0.90 | .37 |
| Random Effect | Variance | DF | Chi-square | P-value |
|---|---|---|---|---|
| Level 1 intercept | 39.69 | 130 | 963.28 | <.001 |
| Level 1 linear slope | 1.31 | 130 | 289.94 | <.001 |
Model 4: Change in Neuropsychological functioning predicting Change in ADHD severity
To test Hypothesis 2, the Bayesian estimated slope of change in NEPSY was used as a Level 2 variable to predict change in the severity of ADHD symptoms and impairment after controlling for family SES. Overall, an improvement in neuropsychological functioning was significantly associated with a decrease in ADHD severity over time (see Table 3). The predicted fall in intercept of ADHD severity at age 8, for a unit increase in neuropsychological functioning was 1.90 units. A unit change in slope of neuropsychological functioning was associated with a fall of .28 units in ADHD severity. Higher SES was associated with lower intercept of ADHD severity. There were no substantial differences in coefficients when the models 3 and 4 were re-run without family SES. Further, the coefficients with and without robust standard errors were similar.
TABLE 3.
Association of change in neuropsychological functioning with change in ADHD symptom severity and impairment
| Fixed Effect | Coefficient | SE | T-ratio | p-value |
|---|---|---|---|---|
| Level 1 intercept | ||||
| Intercept | 23.80 | 2.35 | 10.14 | <.001 |
| SES | −0.07 | 0.03 | −2.36 | .02 |
| Change in NEPSY | −1.90 | 0.42 | −4.49 | <.001 |
| Level 1 linear slope | ||||
| Intercept | 0.50 | 0.62 | 0.81 | .42 |
| SES | −.00 | 0.01 | −0.32 | .75 |
| Change in NEPSY | −0.28 | 0.11 | −2.44 | .02 |
| Random Effect | Variance | DF | Chi-square | P-value |
|---|---|---|---|---|
| Level 1 intercept | 34.57 | 130 | 861.56 | <.001 |
| Level 1 linear slope | 1.61 | 130 | 279.40 | <.001 |
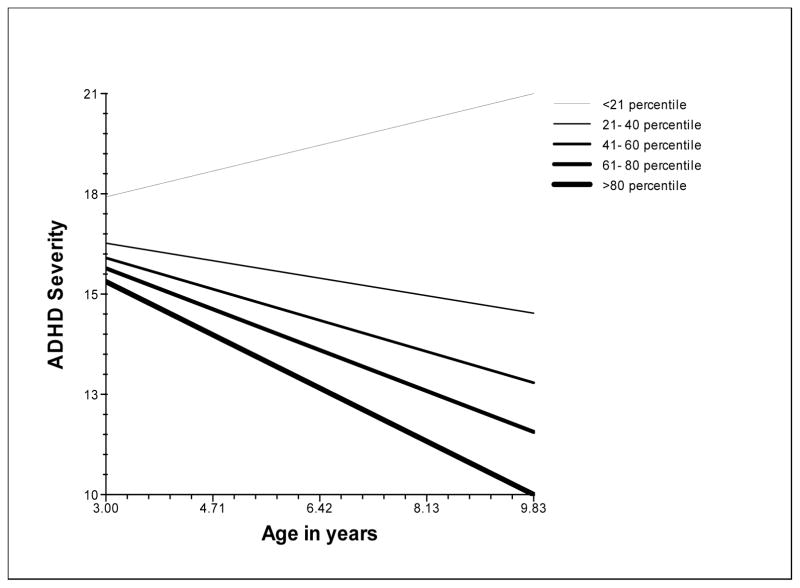
Figure 2 shows the association between trajectories of ADHD severity and change in neuropsychological functioning. Children who showed the least improvement in neuropsychological functioning relative to their peers (<21th percentile of change) showed an escalation of their ADHD severity. The greatest improvement in neuropsychological functioning (>80th percentile) was associated with the greatest fall in ADHD severity.
Figure 2.
Change in ADHD severity at different levels of change in neuropsychological functioning
Note: ADHD Severity refers to the mean of parent and teacher ratings of symptoms and impairment
As noted earlier, several children received medication treatment during the follow-up period. Model 4 was re-run including medication treatment at Age 8 (N/Y) as a covariate. The fixed effect of medication treatment at age 8 was marginally associated with the intercept (B=2.65; SE=1.53; df=91; t ratio=1.73, p=.09) but not with the slope of change in ADHD at that age (B=−.45; SE=.41; df=91; t ratio=1.09, p=.28). The slope of change in neuropsychological functioning continued to be a significant predictor of the change in ADHD severity and impairment (p=.005).
Discussion
This is the first study to examine the longitudinal association between overall neuropsychological functioning and trajectories of children’s ADHD symptoms and impairment during the transition from preschool through school-age. The results indicate that poorer neuropsychological functioning at baseline was not significantly associated with the intercept of ADHD severity at age 8 years or with the rate of change in ADHD severity (Hypothesis 1). However, greater improvement in neuropsychological functioning was linked to an attenuation of ADHD severity, while lesser neuropsychological growth was associated with an escalation in symptoms and impairment (Hypothesis 2). These findings suggest that early neuropsychological status may not be etiologically-linked to the subsequent trajectory of ADHD severity. Rather, change in neuropsychological functioning, irrespective of level, seems to track the ADHD severity over development.
These findings are consistent with neuroimaging data that suggest that ADHD is characterized by delays or deficits in cortical brain development (10, 11) and its remission may be marked by a normalization of cortical structures (21, 32). Further, these longitudinal findings provide a more detailed and downward extension of data derived from adolescents and young adults who were diagnosed with ADHD in middle childhood (16–18). Those studies found that relative to “ADHD persisters,” individuals who had a diminution of symptoms over time performed better on an array of neurocognitive measures linked to executive functions. However, those studies did not include baseline neurocognitive assessments, and as such, it was impossible to know whether clinical improvement was associated with change in neuropsychological functioning.
The present findings are consistent with a developmental psychopathology model in which brain function (as assessed using neuropsychological tests) alters the course of ADHD during early childhood, and provide preliminary support for the notion that a diminution of ADHD symptoms and impairment across the lifespan is the result of cortical development (19). However, these data demonstrate an association, not causation. It is possible that children who become less symptomatic over time perform better on tests because their behavior is less dysregulated during the testing session or because decreased severity allows children to better attend which in turn facilitates cognitive growth.
This study used prospective longitudinal data from multiple sources, enhancing the validity of our findings and reducing the likelihood of reporter-bias influencing the results. This study also included an impairment measure, which is required for ADHD diagnosis but is not often included in studies assessing change in ADHD severity over time. The use of a dimensional approach to measuring ADHD may have enabled us to more effectively test variations in symptom severity over time. Moreover, the use of HLM enabled the study to account for individual variability in the initial levels and change in ADHD severity. The association of change in neuropsychological functioning with change in ADHD severity was evident even after the developmental stage of the child (age), family SES, and medication treatment were factored in as covariates.
It is important to note that although all the children in this study had greater than 6 symptoms of ADHD as per parent or teacher report at BL, some would not have met the DSM-IV cross-situationality and/or impairment criteria required for a diagnosis of ADHD at the time of recruitment. Inclusion of these children and the exclusion of asymptomatic children may have enhanced the generalizability of this study to a wider population of inattentive and hyperactive/impulsive preschool children.
Despite the significant association between neuropsychological functioning and ADHD severity, there was a great deal of unexplained variance. This suggests that other factors, perhaps parenting relationships, child temperament, and stability of households need to be investigated for their influence on the trajectories of ADHD.
This study employed a global measure of neuropsychological functioning rather than more specific measures of cognitive functioning for three reasons. First, ADHD is a heterogeneous disorder in which children show deficits across multiple domains of executive and non-executive function (33, 34). Second, neuropsychological functioning as measured on the NESPY is best conceptualized as single factor rather than individual sub-tests which tap into distinct functions or unique domains (35). Third, neuroimaging data indicate delays in cortical development in ADHD throughout much of the neocortex, not just in one specific region (10). Further research may try to disentangle distinct neuropsychological domains that are most closely linked to ADHD symptom reduction.
Considering the high economic cost of ADHD (36, 37) and the individual difficulties in terms of behavioral, social and academic problems for both preschool- (38) and school-aged children (39), an understanding of factors that lower the severity of ADHD may have strong public health implications. While the data from this study cannot shed light on the possible environmental and/or genetic factors that influenced both changes in neuropsychological functioning and ADHD severity, our findings suggest that interventions that enhance cognitive functioning in at-risk children (20, 22) may have the potential to prospectively diminish ADHD severity and impairment.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Mental Health grant R01MH068286 (PI Jeffrey M. Halperin).
Footnotes
All authors report no financial relationships with commercial interests.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, D.C: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- 2.Campbell SB. Behavior problems in preschool children: Clinical and developmental issues. New York: The Guilford Press; 2002. [Google Scholar]
- 3.Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62(8):896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 4.Campbell S. Parent-referred problem three-year-olds: Developmental changes in symptoms. J Child Psychol Psychiatry. 1987;28(6):835–845. doi: 10.1111/j.1469-7610.1987.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 5.Tandon M, Si XM, Luby J. Preschool Onset Attention-Deficit/Hyperactivity Disorder: Course and predictors of stability over 24 Months. Journal of Child and Adolescent Psychopharmacology. 2011;21(4):321–330. doi: 10.1089/cap.2010.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauermeister JJ, Bird HR, Shrout PE, Chavez L, Ramirez R, Canino G. Short-Term Persistence of DSM-IV ADHD Diagnoses: Influence of context, age, and gender. J Am Acad Child Adolesc Psychiatry. 2011;50(6):554–562. doi: 10.1016/j.jaac.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Stauffenberg C, Campbell SB. Predicting the early developmental course of symptoms of Attention Deficit Hyperactivity Disorder. Journal of Applied Developmental Psychology. 2007;28(5–6):536–552. doi: 10.1016/j.appdev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: Potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51(4):368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SB, Halperin JM, Sonuga-Barke ESJ. A developmental perspective on attention deficit/hyperactivity disorder (ADHD) In: Lewis M, Rudolph K, editors. Handbook of Developmental Psychopathology. Springer; In press. [Google Scholar]
- 10.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 11.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport J. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with Attention-Deficit/Hyperactivity disorder. Cerebral Cortex. 2007;17(6):1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 13.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Lerch JP, He J, Zijdenbos A, Kelly C, Milham MP, Castellanos FX. Brain gray matter deficits at 33-Year follow-up in adults With Attention-Deficit/Hyperactivity Disorder established in childhood. Archives of General Psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry. 2005;44(1):47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Petty CR, Ball SW, Fried R, Doyle AE, Cohen D, Henderson C, Faraone SV. Are cognitive deficits in attention deficit/hyperactivity disorder related to the course of the disorder? A prospective controlled follow-up study of grown up boys with persistent and remitting course. Psychiatry Research. 2009;170(2–3):177–182. doi: 10.1016/j.psychres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: Profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49(9):958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedard ACV, Trampush JW, Newcorn JH, Halperin JM. Perceptual and motor inhibition in adolescents/young adults with childhood-diagnosed ADHD. Neuropsychology. 2010;24(4):424–434. doi: 10.1037/a0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: Attention, inhibition, response perseveration, and the impact of comorbidity. Developmental Neuropsychology. 2005;27(1):107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- 19.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 20.Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: Can we alter the developmental trajectory of ADHD? Neuroscience and Biobehavioral Reviews. 2011;35(3):621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halperin JM, Bedard ACV, Curchack-Lichtin J. Preventive interventions for ADHD: A neurodevelopmental perspective. Neurotherapeutics. 2012;9(3):531–541. doi: 10.1007/s13311-012-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TCEM, Boomsma DI. Maternal ratings of attention problems in ADHD: Evidence for the Existence of a Continuum. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16:S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DuPaul GJ, Power T, Anastopoulos A, Reid R. ADHD rating scales-IV: checklists, norms and clinical interpretation. New York, NY: Guilford Press; 2012. [Google Scholar]
- 26.McGoey KE, DuPaul GJ, Haley E, Shelton TL. Parent and teacher ratings of Attention-Deficit/Hyperactivity disorder in preschool: The ADHD rating Scale-IV preschool version. Journal of Psychopathology and Behavioral Assessment. 2007;29(4):269–276. [Google Scholar]
- 27.Healey DM, Miller CJ, Castelli KL, Marks DJ, Halperin JM. The impact of impairment criteria on rates of ADHD diagnoses in preschoolers. Journal of Abnormal Child Psychology. 2008;36(5):771–778. doi: 10.1007/s10802-007-9209-1. [DOI] [PubMed] [Google Scholar]
- 28.Korkman M, Kirk U, Kemp S. NEPSY: A developmental neuropsychological assessment. San Antonio, Tx: The Psychological Corporation; 1998. [Google Scholar]
- 29.Nakao K, Treas J. Updating occupational prestige and socioeconomic scores - How the new measures measure up. Sociological Methodology. 1994;24:1–72. [Google Scholar]
- 30.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 31.Maas CJM, Hox JJ. The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Computational Statistics & Data Analysis. 2004;46(3):427–440. [Google Scholar]
- 32.Shaw P, Gogtay N, Rapoport J. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping. 2010;31(6):917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 34.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Stinnett TA, Oehler-Stinnett J, Fuqua DR, Palmer LS. Examination of the underlying structure of the NEPSY: A developmental neuropsychological assessment. Journal of Psychoeducational Assessment. 2002;20(1):66–82. [Google Scholar]
- 36.Marks DJ, Mlodnicka A, Bernstein M, Chacko A, Rose S, Halperin JM. Profiles of service utilization and the resultant economic impact in preschoolers with Attention Deficit/Hyperactivity Disorder. Journal of Pediatric Psychology. 2009;34(6):681–689. doi: 10.1093/jpepsy/jsn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, Swensen AR. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: Excess costs of persons with ADHD and their family members in 2000. Current Medical Research and Opinion. 2005;21(2):195–205. doi: 10.1185/030079904X20303. [DOI] [PubMed] [Google Scholar]
- 38.DuPaul GJ, McGoey KE, Eckert TL, VanBrakle J. Preschool children with attention-deficit/hyperactivity disorder: Impairments in behavioral, social, and school functioning. J Am Acad Child Adolesc Psychiatry. 2001;40(5):508–515. doi: 10.1097/00004583-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Gaub M, Carlson CL. Behavioral characteristics of DSM-IV ADHD subtypes in a school-based population. Journal of Abnormal Child Psychology. 1997;25(2):103–111. doi: 10.1023/a:1025775311259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.