Abstract
Background
Short bowel syndrome (SBS) is a life-threatening condition with few solutions. Tissue engineered intestine (TEI) is a potential treatment, but donor intestine is a limiting factor. Expanded epithelial surrogates termed enteroids may serve as a potential donor source.
Materials and Methods
To produce TEI from enteroids, crypts were harvested from mice and enteroid cultures established. Enteroids were seeded onto polymer scaffolds using Matrigel or culture medium, and implanted in immunosuppressed mice for 4 weeks. Histology was analyzed using Periodic Acid Schiff (PAS) staining and immunofluorescence (IF). Neomucosa was quantified using ImageJ software. To determine if TEI could be produced from enteroids established from small intestinal biopsies, 2×2mm pieces of jejunum were processed for enteroid culture, enteroids were expanded and seeded onto scaffolds, and scaffolds implanted for 4 weeks.
Results
Enteroids in Matrigel produced TEI in 15/15 scaffolds, whereas enteroids in medium produced TEI in 9/15 scaffolds. Use of Matrigel led to more neomucosal surface area compared to media (10,520±2,905 μm vs. 450±127 μm, p<0.05). Histologic examination confirmed the presence of crypts and blunted villi, normal intestinal epithelial lineages, intestinal subepithelial myofibroblasts, and smooth muscle cells. Crypts obtained from biopsies produced an average of 192±71 enteroids. A single passage produced 685±58 enteroids, which was adequate for scaffold seeding. TEI was produced in 8/9 scaffolds seeded with expanded enteroids.
Conclusions
Enteroids can be obtained from minimal starting material, expanded ex vivo, and implanted to produce TEI. This method shows promise as a solution to the limited donor intestine available for TEI production in patients with SBS.
Keywords: Tissue engineering, Short bowel syndrome, Intestine, Enteroids, Epithelial culture, intestinal stem cells
1. Introduction
Short bowel syndrome (SBS) is a vexing clinical condition with no ideal solution. Patients with SBS have typically lost over 50-75% of their normal small bowel length. This vastly decreases the functional absorptive area of the intestine, often resulting in weight loss, dehydration, and vitamin deficiencies. The management of patients with SBS is primarily designed to mitigate these complications. Repletion of nutritional and fluid deficiencies is achieved with parenteral nutrition (PN). Although lifesaving, PN is associated with substantial risks including venous thrombosis, central line associated blood stream infections, cholestasis, and liver failure.[1, 2] Surgical therapies such as the Bianchi procedure and serial transverse enteroplasty have been developed to lengthen the remaining small bowel. Although successful in select cases, not all patients are candidates and the procedures often fail.[3, 4] The only current clinical approach that replaces lost intestine is heterotopic small bowel transplantation. This modality can be successful in select cases; however, the 5-year survival remains low at 55% and is associated with the difficulties and complications of long term immunosuppression.[5] A proposed solution to the shortcomings of current SBS treatment is the development of tissue engineered intestine (TEI).[6-8]
Ideally, TEI would utilize the patient's native tissue to grow a completely functional and immunologically compatible length of intestine to replace the intestine that has been lost. Currently, the most commonly used animal model of TEI utilizes organoid units containing intestinal crypts and their mesenchymal niche that are seeded onto bioabsorbable scaffolds and implanted into the peritoneal cavity of a host animal. Utilizing these general methods, TEI has successfully been produced in rats, mice, swine, and dogs.[9-12] Additionally, human TEI has been produced by implanting human organoid units into immunosuppressed mice.[13] The TEI created utilizing these models has been shown to contain the epithelial, mesenchymal, neural, and smooth muscle components critical to functioning intestine.[10, 12-22]
Despite the quality of the TEI currently produced, limitations regarding a healthy tissue source for TEI production hinder translational application. In the described methods, a substantial quantity of healthy intestine must be digested to obtain an adequate number of organoid units to produce TEI. Moreover, the TEI produced is relatively small and unpredictable in size, ranging from a few millimeters to 2 cm in diameter. In order to replace the long lengths of intestine needed for SBS patients, even greater quantities of starting material would likely be needed. However, patients with SBS have little healthy intestine to spare for the production of TEI.
Sato et al.,[23, 24] have previously demonstrated that intestinal stem cell (ISC) containing crypts can be harvested and established in a 3-D culture system to produce intestinal epithelial surrogates known as enteroids. Enteroids contain crypt and villus domains with all of the terminally differentiated epithelial lineages. They can be passaged for greater than 18 months while maintaining their phenotype. They have also been shown to expand in vitro in a logarithmic fashion and maintain their expansion capacity even after cryopreservation.[25] These unique characteristics make enteroid culture a potential clinically applicable tissue reservoir for the production of TEI in patients with SBS.
Therefore, we hypothesized that TEI can be grown from minimal starting material comparable to that which a SBS patient could provide, when utilizing ex vivo enteroid culture and expansion. To test this hypothesis, we first established a reproducible seeding and implantation protocol to reliably produce TEI from enteroids. We then investigated the possibility that crypts could be obtained from minimal starting material to produce TEI.
2. Materials and Methods
2.1 Animal Use
All animal procedures were performed with the approval of our Institutional Animal Care and Use Committee (Protocol #AR12-0001). All enteroid cultures were established from male (n=13) and female (n=10) LGR5-EGFP transgenic mice bred in house. The colony was established from breeders obtained from The Jackson Laboratory (Bar Harbor, ME). All seeded scaffolds were implanted into male (n=38) or female (n=44) Non-Obese Diabetic/Severe Combined Immunodeficiency (NOD/SCID) mice bred in house. This colony was established from breeders obtained from Charles River Laboratories (Wilmington, MA).
2.2 Crypt Isolation and Enteroid Culture
Donor intestine for enteroid culture was obtained from LGR5-EGFP transgenic mice in which LGR5+ ISC located in the crypts express green fluorescent protein, allowing them to be tracked throughout all steps of experimentation. After euthanasia, a laparotomy was performed and the proximal half of the small intestine starting two centimeters distal to the pylorus was removed. We utilized the proximal intestine due to previous in vitro studies showing the crypts from the proximal intestine were more efficient at producing enteroids.[25] The lumen was opened longitudinally and enteric contents rinsed away with phosphate buffered saline (PBS) (GE Life Sciences, Marlborough, MA). Villi were scraped away using a glass coverslip and the remaining tissue was minced and washed to remove debris. Once clean, the tissue was incubated in 2 mM ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, Waltham, MA) in PBS for 30 min at 4°C. After incubation, tis sue fragments were allowed to settle and the EDTA was discarded. The tissue was resuspended in ice-cold PBS and agitated by trituration for 30 sec to release fragments of mucosa including crypts. The remaining tissue was allowed to settle and the supernatant containing the mucosal fragments was filtered through a 70 μm sieve (BioDesign Inc. of New York, Carmel, NY). The sieve removed the larger mucosal fragments including remaining villi and allowed the crypts to flow through. The process of resuspension, trituration, and filtration was repeated until mucosal fragments were no longer identified in the supernatant. The flow-through from each fractionation and filtration were combined and pelleted at 300 g for five min. at 4°C. Pelleted crypts were then re suspended in basic crypt medium consisting of Advanced DMEM/F12 supplemented with 2 mM GlutaMAX, 10 mM HEPES, and 100 U/mL penicillin/100 μg/mL streptomycin (all from Thermo Fisher Scientific, Waltham, MA), and pelleted at 100-150 g for 2 min. to remove single cells in the supernatant. This process was repeated three times. Prior to the final centrifugation, crypt density was determined.
After crypt isolation was completed, pelleted crypts were resuspended in growth factor reduced Matrigel (Sigma, St. Louis, MO) at a density of 100-200 crypts/50 μL of Matrigel. The cold Matrigel-crypt suspension was then plated onto pre-warmed 24-well culture plates with 50 μL of Matrigel per well. Plates were placed in an incubator at 37°C for 15 min to allow the Matrigel to completely solidify, and then each well received 500 μl of complete enteroid culture medium consisting of basic crypt medium supplemented with 1 mM N-Acetylcysteine (Sigma, St. Louis, MO), 1× N2 supplement (Thermo Fisher Scientific, Waltham, MA), 1× B27 supplement (Thermo Fisher Scientific, Waltham, MA), 50 ng/mL HB-EGF (R&D Systems, Minneapolis, MN), 100 ng/mL Noggin (R&D Systems, Minneapolis, MN), and 500 ng/mL R-spondin (Sino Biological Inc., Beijing, China). Media was changed every 3-4 days during culture.
2.3 Scaffold Fabrication, Seeding, and Implantation
Polyglycolic acid (PGA) Biofelt (2 mm thick; 60 mg/cm3 density) (Biomedical Structures, Warwick, RI) was utilized for scaffold production. An 8 mm × 30 mm section of PGA Biofelt was wrapped around a 3 mm diameter mandrel to produce 3 cm long × 3 mm internal diameter (ID) scaffolds. Once on the mandrel, a 5% polylactic acid (PLLA) (Sigma, St. Louis, MO) in chloroform solution was sprayed onto the exterior using an atomizer. Once the PLLA coating was dry, scaffolds were soaked in 100% ethanol for 30 min and then washed three times with PBS. Scaffolds were then collagen coated by soaking them in a 0.4 mg/mL type I bovine collagen solution (Advanced BioMatrix, Carlsbad, CA). Scaffolds were then removed from the mandrels, cut to the desired implantation length of 5 mm, and sterilized with ethylene oxide gas.
All enteroids were seeded onto 5 mm long × 3 mm ID PGA tubular scaffolds. Enteroids at day 10-14 of culture were released from the Matrigel by aspirating through a p1000 pipette tip and resuspended in basic crypt media. Enteroids were centrifuged at 200 g for 2 min. and supernatants discarded. This process of resuspension and centrifugation was repeated twice to wash away any dead cells. Before the final centrifugation, the number of enteroids in solution was quantified using phase contrast microscopy. Pelleted enteroids were resuspended in either growth factor reduced Matrigel or complete enteroid culture media. Sterile PGA scaffolds were pre-warmed to 37°C and the enteroids were seeded on to the scaffolds using 50 μL of seeding medium to a seeding density corresponding to the desired treatment group. Seeded scaffolds were maintained at 37°C until implantation into imm unocompromised NOD/SCID mice. Mice were anesthetized using isoflurane anesthesia and a midline laparotomy performed. Seeded scaffolds were placed into the peritoneal cavity, the midline laparotomy was closed in layers, and scaffolds were allowed to incubate for 4 weeks.
2.4 Histology and Immunofluorescence (IF)
For enteroids, beads of Matrigel were fixed for two hours in 10% formalin and paraffin embedded. Progressive 5 μm sections were taken and stained with hematoxylin and eosin (H&E) to determine their morphology. For TEI, explanted scaffolds were fixed overnight in 10% formalin. Each specimen was bisected perpendicular to the axis of the lumen, and paraffin embedded with the cut surface facing down. Progressive 5 μm sections were taken at 300 μm intervals through the length of the implant. Sections were stained with H&E to determine the presence of TEI, and to assess the architecture and quantify of neomucosa present. An implant was considered positive for TEI if any section was identified that contained simple columnar epithelium consistent with intestinal epithelium. To determine the amount of neomucosa produced, a representative slide from each implant was selected for further analysis. Using ImageJ software, the basilar surface of all identifiable simple columnar epithelium was outlined and the length quantified.
To identify the components present in TEI, a combination of Periodic Acid-Schiff (PAS) staining and IF was used. PAS staining was used to identify goblet cells as noted by the characteristic bright pink staining of mucin filled vacuoles. IF was used to identify LGR5+ ISC, Paneth cells, enterochromaffin cells, enterocytes, intestinal subepithelial myofibroblasts (ISEMFs), and smooth muscle cells. IF was also used to identify ISEMFs and smooth muscle cells in cultured enteroids. We did not study the epithelial components of enteroids as these have been previously identified.[24] Antigen retrieval was performed by boiling the slides in 10 mM Na-Citrate buffer (pH 6.0) in a pressure cooker (120°C; 15 psi) for 30 min. Slides were incubated at 4°C overnight with the following prima ry antibodies: green fluorescent protein (1:100; Abcam, Cambridge, MA), lysozyme (1:500; Abcam), chromogranin A (1:200; Abcam), villin (1:500; Thermo Fisher Scientific, Waltham, MA), smooth muscle alpha actin (1:500; Thermo Fisher Scientific), vimentin (1:500; Millipore, Billerica, MA), and desmin (1:500; Abcam, Cambridge, MA). After incubation in primary antibodies, Alexa Fluor 568 and Alexa Fluor 488 secondary antibodies (Life Technologies, Carlsbad, CA) were used for labeling, and the slides mounted using Vectashield with DAPI (Vector Laboratories, Burlingame, CA). Images of enteroids were acquired using differential interference contrast (DIC) microscopy (Leica DMI 4000B, Buffalo Grove, IL). Light microscopy images of tissue were obtained using an Olympus SZX7 microscope or an Olympus BX51 microscope (Center Valley, PA). Fluorescent images were obtained using a Zeiss 710 confocal microscope (Peabody, MA).
2.5 Experimental Design
To investigate the potential use of enteroids as a tissue source for TEI production we needed to: 1) identify a reliable enteroid seeding medium for TEI growth, 2) determine the optimal enteroid seeding density for TEI growth, and 3) establish that TEI could be produced from minimal starting material.
2.5.1 Enteroid Seeding Medium
To determine a reliable enteroid seeding medium for TEI growth, enteroids were seeded onto PGA scaffolds using either growth factor reduced Matrigel or complete enteroid culture media. The protocol for establishing enteroid culture (section 2.2) was performed ten separate times using ten different mice to obtain enough enteroids for completion of the experiments. Fifteen scaffolds were seeded and implanted in both the Matrigel and complete enteroid culture media arms as described in section 2.3. All implants were evaluated histologically for the presence of TEI and the quantity of neomucosa produced four weeks after implantation.
2.5.2 Enteroid Seeding Density
To determine the optimal seeding density for TEI growth, enteroids were seeded onto PGA scaffolds at 10, 20, and 30 enteroids/mm3 of scaffold material. The protocol for establishing enteroid culture (section 2.2) was performed ten separate times using ten different mice to obtain enough enteroids for completion of the experiments. Enteroids were seeded at the desired seeding density with Matrigel, and implanted in NOD/SCID mice using the methods described in section 2.3. Fifteen scaffolds were seeded at 10 enteroids/mm3, fifteen scaffolds at 20 enteroids/mm3, and thirteen scaffolds at 30 enteroids/mm3. All implants were evaluated for the presence of TEI and the quantity of neomucosa produced four weeks after implantation. These specimens were additionally analyzed using PAS staining and IF to identify TEI components.
2.5.3 Enteroid Expansion
To determine if enteroids represent a clinically viable tissue reservoir for TEI production, enteroid culture was established from minimal starting material, comparable in size to an endoscopic biopsy, expanded ex vivo for scaffold seeding, and implanted to grow TEI. Nine 2 × 2 mm pieces of proximal mouse jejunum were obtained from three different LGR5-EGFP mice. Each piece of tissue was processed individually to establish enteroid culture using the methods described in section 2.2. Enteroids were passaged for expansion to obtain enough material to seed scaffolds for implantation. Passaging was performed every 7-14 days by aspirating the enteroids through a 30 gauge needle to break them into small enteroid fragments. The fragments were then resuspended in fresh Matrigel and re-plated in 24-well culture plates. Enteroids were quantified before and after passage using DIC microscopy (Leica DMI 4000B) to establish passage efficiency. Once enough enteroids were obtained for seeding, scaffolds were seeded and implanted for four weeks using the methods described in section 2.3. All implants were evaluated for the presence of TEI.
2.6 Statistical Analyses
To compare the length of neomucosa produced from the two different seeding mediums, the Mann-Whitney test was used for analysis. P values less than 0.05 were considered significant. To compare the length of neomucosa produced from the three different seeding mediums, the Kruskal-Wallis test was used for analysis. P values less than 0.05 were considered significant. Pairwise comparisons between every two seeding densities were performed using the Mann-Whitney test. Bonferroni correction was used for multiple comparisons and p values less than 0.017 were considered significant. Gender of the donor or host animals was not considered in analysis. All analyses were performed using GraphPad Prism 6 (La Jolla, CA).
3. Results
3.1 Enteroid Seeding Medium
Seeding of crypts in Matrigel produced TEI in 15/15 (100%) attempts, whereas seeding with complete enteroid culture medium produced TEI in only 9/15 (60%) attempts (Figure 1A). Regarding the quantity of neomucosa produced, when seeded with Matrigel, implants grew an average of 10,520 ± 2,905 μm of neomucosa which was significantly greater than the average of 450 ± 127 μm when seeded with complete enteroid culture media (p ≤ 0.05) (Figure 1B). The architecture of scaffolds seeded with Matrigel ranged from a simple columnar epithelium to crypt domains with overlying blunted villi (Figure 2). Figure 2 also demonstrates how neomucosal length was outlined for the quantification shown in Figure 1B.
Figure 1. Effects of enteroid seeding substrate on the production of TEI.
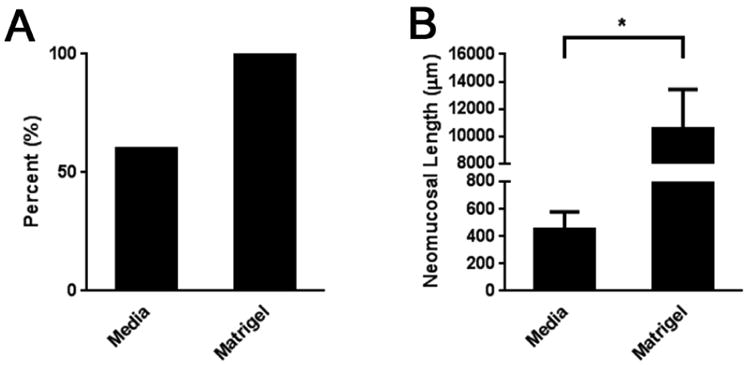
A) Percent positivity of TEI. B) Neomucosal length of TEI. Enteroids were seeded onto PGA scaffolds after 11-14 days in culture using either complete enteroid culture medium or Matrigel. Scaffolds were then implanted into the peritoneal cavity of NOD/SCID mice four weeks after which they were explanted and analyzed using H&E staining. Positivity was defined as the presence of simple columnar epithelium consistent with intestinal mucosa. Neomucosal length was quantified on a representative section using ImageJ software by tracing the basal surface of all simple columnar epithelium. n = 15 for both media and Matrigel groups. *p ≤ 0.05.
Figure 2. Architecture of TEI produced from enteroid seeding.
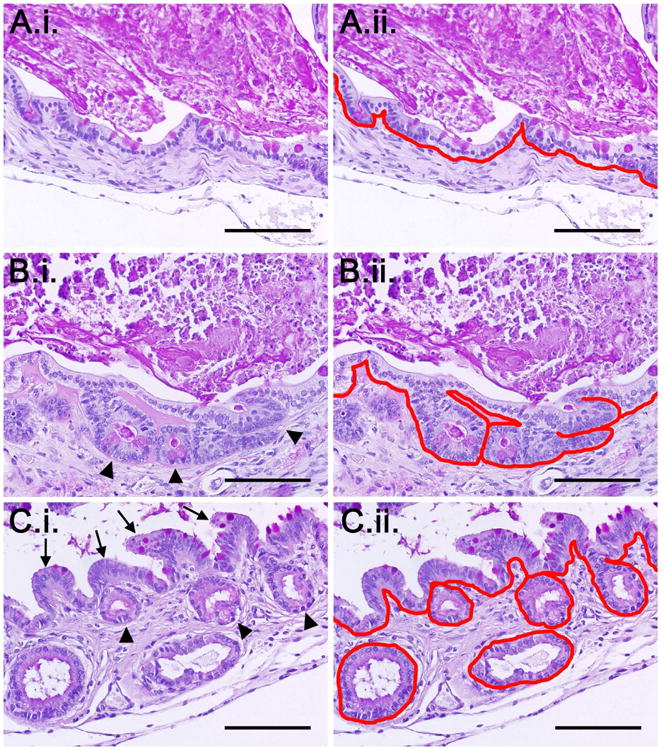
Shown are representative images demonstrating: A.i) simple columnar epithelium, B.i) flat simple columnar epithelium with underlying crypt domains (arrowheads), and C.i) crypt domains (arrowheads) with overlying blunted villi (arrows). A.ii-C.ii) Respective images of the varying types of neomucosa with the basement membrane traced in red to demonstrate how the neomucosa was quantified. All images are stained PAS. The bright pink staining in the lumen represents retained cellular debris and mucin. Scale bars = 100 μm.
3.2 Enteroid Seeding Density
Seeding enteroids at 20 enteroids/mm3 of scaffold material produced TEI in 15/15 (100%) attempts compared to 14/15 (93.3%) attempts at 10 enteroids/mm3 and 8/13 (61.5%) attempts at 30 enteroids/mm3 (Figure 3A). Regarding the quantity of neomucosa produced, when seeded at 20 enteroids/mm3 implants grew an average of 14,896 ± 2,974 μm of neomucosa which was significantly greater than the average of 5,970 ± 1,753 μm or the average of 2,461 ± 943 μm grown in the 10 and 30 enteroids/mm3 groups, respectively (p ≤ 0.01) (Figure 3B). There were no significant differences between the amount of neomucosa produced in the 10 or 30 enteroids/mm3 seeding groups. The histologic architecture in this experiment is shown in Figure 2. Seeding at 20 enteroids/mm3 of scaffold material offered the highest likelihood of producing TEI and produced the greatest quantity of neomucosa.
Figure 3. Effect of enteroid seeding density on the production of TEI.
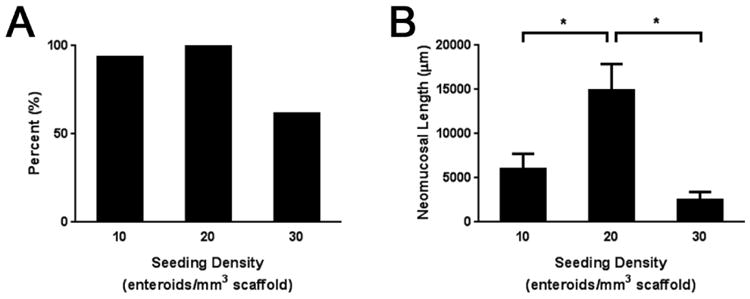
A) Percent positivity of TEI. B) Neomucosal length of TEI. Enteroids were seeded to PGA scaffolds after 11-14 days in culture at 10, 20, or 30 enteroids/mm3 of scaffold. The scaffolds were then implanted into the peritoneal cavity of NOD/SCID mice four weeks after which they were explanted and analyzed using H&E staining. Positivity was defined as the presence of simple columnar epithelium consistent with intestinal mucosa. Neomucosal length was quantified on a representative section using ImageJ software by tracing the basal surface of all simple columnar epithelium. n = 15 for 10 and 20 enteroids/mm3; n = 13 for 30 enteroids/mm3. *p ≤ 0.01.
3.3 Enteroid Expansion from Minimal Starting Material
Crypts were successfully obtained from each of the nine fragments of LGR5-EGFP intestine and grown into enteroids (Figure 4). Each fragment initially produced an average of 192 ± 71 enteroids. The nine colonies of established enteroids were passaged after 14 days of culture, yielding an average of 685 ± 58 enteroids. This provided an approximate passage ratio of 1 : 3.5. Only a single passage was necessary to obtain enough crypts to seed a single PGA scaffold in each of the nine attempts. TEI was produced from expanded enteroids in 8/9 (88.9%) attempts. The architecture observed in these nine specimens was consistent with that demonstrated in Figure 2.
Figure 4. LGR5+ enteroids.
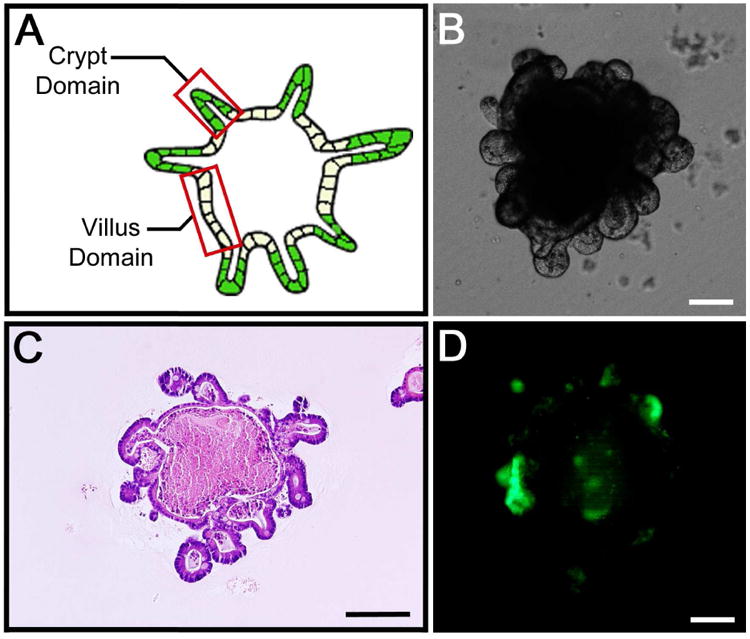
A) Illustration of a typical enteroid demonstrating the crypt domains as represented by the peripherally oriented buds and the villus domains as represented by the epithelium between the crypt domains. B) DIC microscopy showing a typical enteroid after 11 days in culture. Note the peripherally oriented buds corresponding to the crypt domains. C) H&E staining of a typical enteroid after 14 days in culture with peripherally oriented buds consistent with crypt domains. Note luminal contents consistent with cellular debris and mucin. D) Fluorescence microscopy confirming that the peripheral buds of the enteroids represent crypt domains that contain LGR+EGFP ISC (green). Scale bars = 100 μm.
3.4 Components of Enteroids and TEI
For TEI, a combination of PAS staining and IF identified LGR5+ ISC as well as all of the terminally differentiated epithelial cell lineages including goblet cells, Paneth cells, enterocytes, and enterochromaffin cells (Figure 5). LGR5+ ISC were demonstrated by staining for GFP, which identifies the LGR5+ ISC and confirms that the TEI produced is derived from the LGR5-EGFP donor enteroids that were seeded onto the scaffolds. IF also identified the presence of ISEMFs and smooth muscle cells in TEI but not in cultured enteroids (Figure 6).
Figure 5. TEI contains ISCs and differentiated intestinal epithelial cells.
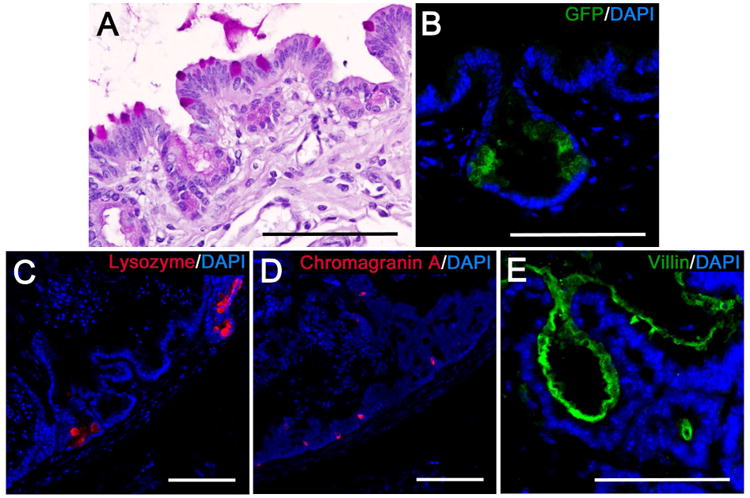
Shown are representative images demonstrating: A) PAS staining to identify goblet cells as noted by mucin filled vacuoles that stain bright pink, B) IF labeling for green fluorescent protein (GFP) (green) to identify LGR5+ ISC in crypt domains. C) IF labeling of lysozyme (red) to identify Paneth cells, D) IF labeling of chromagranin A to identify enterochromaffin cells (red), E) IF labeling of villin in the brush border to identify enterocytes (green). For all IF images, nuclei labeled with DAPI are represented in blue. Scale bars = 100 μm.
Figure 6. TEI contains ISEMFs and smooth muscle cells.
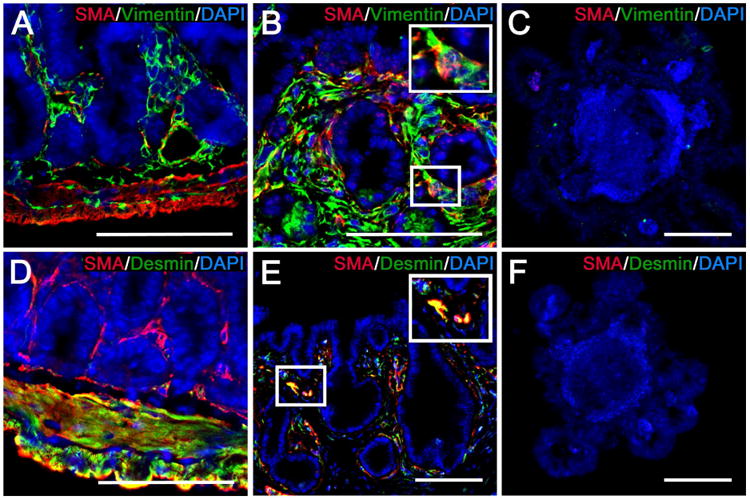
Shown are representative images from native intestine (A, D), TEI (B, E), and enteroids (C, F) that have been subjected to IF labeling for the identification of ISEMFs and smooth muscle cells. A-C) ISEMFs are identified by co-localization of labeling for smooth muscle alpha actin (SMA) (red) and vimentin (green). D-F) smooth muscle cells are identified by co-localization of labeling for SMA (red) and desmin (green). A) Native intestine shows strong SMA and vimentin co-localization in the submucosal layers only, indicative of ISEMFs. There is some vimentin staining in the muscularis that occurs in the mesenchymal stroma between the longitudinal and circular layers. B) TEI demonstrates the same SMA and vimentin co-localization surrounding the crypt domains. C) SMA and vimentin are not evident in enteroids. D) Native intestine shows strong SMA and desmin co-localization in the muscularis, indicative of smooth muscle cells. There is SMA labeling in the submucosa without desmin co-localization, consistent with ISEMFs. E) TEI demonstrates similar SMA and desmin co-localization surrounding all areas of the epithelium, however, it appears scattered and less organized than that observed in native intestine. F) SMA and desmin are not evident in enteroids. Scale bars = 100 μm.
4. Discussion
The ability to produce TEI has significantly advanced in recent years. Intestinal organoid units obtained from healthy intestine have been shown to produce a complex intestinal tissue containing the major components of normal intestine. Organoids obtained from rodents, dogs, swine, and humans have been used to produce TEI.[9-12] Moreover, short segments of TEI produced by these methods have been anastomosed as interposition intestinal grafts without detrimental effects, and with some evidence that the TEI produced may help to reverse the effects of SBS when anastomosed in line in an animal model of SBS.[26] However, there remain multiple challenges to translational application, including an adequate source of intestinal tissue for TEI production. Organoid units for TEI production are obtained by the digestion of healthy intestinal tissue. To avoid the need for immunosuppression, autologous organoid units need to be obtained. However, SBS patients have limited expendable healthy intestine. A reliable and expandable donor tissue reservoir needs to be identified. Given the ability for long term maintenance and logarithmic expansion in vitro, we investigated the use of enteroid culture as a potential tissue reservoir for the production of TEI.
On examination of the seeding substrate, TEI from enteroids seeded in complete enteroid culture medium only was inconsistently produced, and when it was present the quantity of neomucosa was limited. However, when seeded using Matrigel, TEI was always present with substantially increased neomucosa. Others have noted similar findings when seeding organoid units in Matrigel for the production of TEI.[27] The advantages provided by Matrigel are unclear, but there are several potential benefits. Matrigel is a sterile extract of the Englebreth-Holm-Swarm mouse sarcoma that contains numerous proteins and basement membrane components including laminins, type IV collagen, and growth factors.[28] The growth factors present are numerous and include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), nerve growth factor (NGF), and basic fibroblast growth factor (bFGF). Matrigel has been shown to enhance differentiation in numerous cell lines in vitro and to promote angiogenesis and adipogenesis.[28, 29] Although we utilized growth factor reduced Matrigel, which has been processed to remove the bulk of these proteins, they are not depleted.[30, 31] Potential benefits of Matrigel in the production of TEI include: 1) enhanced cell attachment through the viscous nature of the seeding substrate as well as basement membrane proteins including type IV collagen and laminins; 2) the differentiation and proliferative effects on the epithelium from the contained growth factors; and 3) the angiogenic properties of Matrigel potentially providing enhanced blood supply. Further investigation is necessary to determine Matrigel's beneficial effects.
We have demonstrated significant effects on TEI production with variable enteroid seeding densities. Seeding with 20 enteroids/mm3 of scaffold was the optimal seeding density found. In standard cell culture, it is well known that low seeding density significantly decreases cell survival in many cell lines, especially in primary cell cultures. This may be due to decreased cell-derived signaling at low cell densities.[32] It is possible that this same effect occurs when enteroids are seeded at low densities in vivo. The general effect of inadequate seeding density has been observed in other areas of tissue engineering. In models of bone, cartilage, and adipose tissue engineering, seeding densities that are too low produce less tissue, and increasing the concentration of starting material leads to an increase in the amount of engineered tissue produced.[33-35] On the other hand, seeding densities that are too high have also been shown to hinder proliferation, decrease differentiation, and alter protein expression, resulting in less desirable tissue properties.[36, 37] These studies underscore the importance of identifying optimal seeding densities. To our knowledge, we are the first to demonstrate the importance of optimizing seeding density in any model of TEI. Further studies are needed to better understand how variable seeding densities alter TEI production and phenotype.
Others have previously shown that enteroids cultured in vitro can be utilized to grow neomucosa in vivo [38]. However, the architecture was limited to relatively small, isolated cysts of simple columnar epithelium. Additionally, they were unable to grow any neomucosa unless they were seeded with enteroids co-cultured with ISEMFs. In the data presented here, TEI could be produced in every attempt when paired with an ideal seeding medium and optimal seeding density. Moreover, the organization of the neomucosa more closely recapitulated crypt-villus architecture, and contained all of the expected intestinal epithelial cell lineages. This was achieved in the absence of ISEMF co-culture. The TEI produced also contained ISEMFs and smooth muscle cells, even though these cell types were not identified in the enteroids seeded onto the scaffolds. We speculate that this is may be due to the location of scaffold implantation. In the models that required the use of co-cultured ISEMFs, scaffolds were implanted in subcutaneous pockets.[38-41] We utilized the peritoneal cavity as an incubator for the production of TEI. It is possible that an intraperitoneal location provides local paracrine signaling effects to the implants by way of proximity to the surrounding viscera, which contain a robust mesenchymal niche in the form of peri-crypt ISEMFs. The presence of ISEMFs and smooth muscle cells in the TEI, despite their absence in the seeded enteroids, suggests that either: 1) the intraperitoneal location allows their recruitment into the TEI, or 2) trans-differentiation occurs from an epithelial lineage into the other cell types. The mechanism by which this occurs is not known and further investigation into the source of the identified ISEMFs and smooth muscle cells through lineage tracing will be performed in the future.
With a reliable method for TEI production from enteroids established, we then investigated their potential as a clinically applicable tissue reservoir. We demonstrated that a single small piece of intestine comparable in size to an endoscopic biopsy had the ability to establish enteroid cultures. The number of enteroids obtained from a single biopsy was only enough to grow in a single 50 μL drop of Matrigel. Despite having very few enteroids to work with initially, passaging and expansion were successful. Only one passage was necessary to reach the number of enteroids needed for scaffold seeding in mice. Coupling the findings we have demonstrated here with the fact that enteroids can be maintained for many months and expanded logarithmically, enteroid expansion can be easily scaled up to the size and length of scaffold required for human applications. Figure 7 illustrates how the methods established here may ultimately apply to patients with SBS.
Figure 7. The potential use of enteroid culture in human tissue engineered intestine.
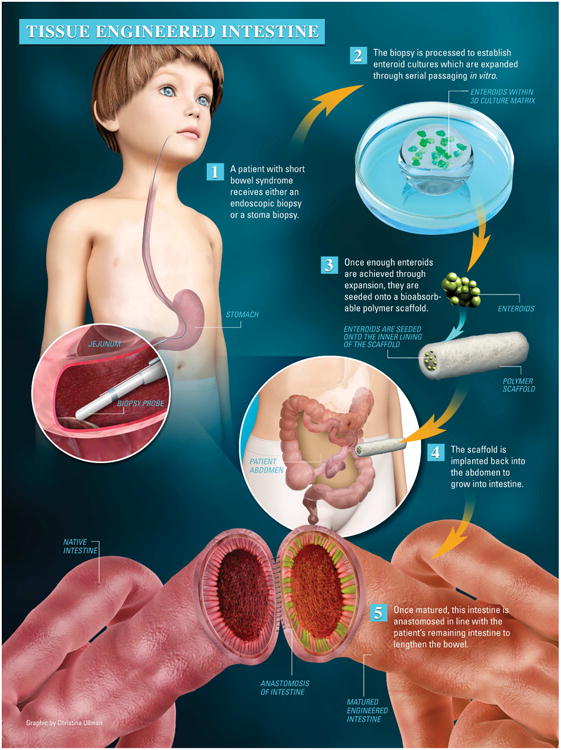
1) A patient with SBS undergoes either an endoscopic or stoma biopsy. 2) The biopsy is processed to establish enteroid cultures which can be expanded as needed for the length of intestine required. 3) The appropriate number of enteroids are seeded onto a bioabsorbable scaffold of appropriate length and, 4) implanted back into the peritoneal cavity of the same SBS patient. 5) Once matured, the TEI is anastomosed with the patient's remaining native bowel to lengthen and reverse the effects of SBS.
Our study demonstrates that enteroids are a potentially viable tissue source for TEI production. Although this method shows great potential, it is not without limitation. The growth of TEI from enteroids was not adequate when seeded in simple enteroid growth medium and required the use of Matrigel as a seeding substrate. Due to its origin from a mouse tumor, it is unlikely that Matrigel will be approved for clinical use. Studies investigating type I collagen as an alternative for intestinal epithelial growth have been met with some success.[39, 40] However, optimal alternatives to Matrigel are yet to be determined. There are also limitations with the complexity of the TEI we have produced. Although all of the necessary epithelial components as well as ISEMFs and smooth muscle cells are present, the architecture and organization of the TEI is not optimal. There is crypt-villus architecture, however, the villi are blunted and do not reach the lengths that are typical of native intestine. Although it appears that ISEMFs and smooth muscle cells are being recruited into the TEI, they are disorganized and do not recapitulate the laminated nature of native intestine. There is already some evidence showing that co-seeding with other cell lineages improves the quantity of the epithelium and the overall quality of TEI in other models.[38, 41, 42] Further efforts are needed to establish methods for organized co-seeding of enteroids with other cell types such as ISEMFs, smooth muscle cells, and neural stem cells in order to augment the architecture and organization of the TEI produced. Although we have demonstrated the presence of cell lineages consistent with native intestine, we still need to investigate the function of the TEI produced. Further investigation of functional components including the presence of disaccharidases and ion and nutrient transporters is necessary to determine whether this TEI has absorptive function. Lastly, we have begun evaluating different polymer scaffolds and architectures to further help direct differentiation and neomucosa formation.[22, 43]
5. Conclusions
In conclusion, we have established a reliable method of TEI production utilizing ex vivo enteroid cultures. We have demonstrated the importance of an optimal seeding density to maximize tissue production. With these methods established, we were able to show proof of concept that TEI can be produced from the limited starting material that a patient with SBS can provide. We conclude that ex vivo enteroid cultures represent a potential tissue source for the production of TEI. Further studies of enteroid cultures are underway to maximize the translational capabilities of this technique in the production of TEI for clinical use.
Acknowledgments
We thank Han Yin, M.S. in the Battelle Center for Mathematical Medicine at Nationwide Children's Hospital for his assistance in statistical analyses. Funding was from The Research Institute at Nationwide Children's Hospital (GEB) and from NIH R43DK107168 (JJ, GEB).
Disclosures Statement: Jed Johnson is a founder and employee of Nanofiber Solutions, Inc. Nanofiber Solutions is a biotechnology company that designs and manufactures nanofiber scaffolds for tissue engineering applications. Although we have utilized Nanofiber Solutions scaffolds in other research, no Nanofiber Solutions scaffolds were utilized in the work presented here. The other authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Neither funding source held any role in the study design, data collection, analysis, or interpretation, writing of the report, or the decision to submit for publication.
Footnotes
Author Contributions: Barrett Cromeens was involved with all aspects of design, experimentation, data analysis, and writing of the manuscript. Yanchun Liu, Johnathan Stathopoulos, and Yijie Wang were involved with experimentation, data analysis, and manuscript writing. Jed Johnson was involved with the experimental design, data analysis, and manuscript writing. Gail Besner was involved with all the previously mentioned domains.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barrett P. Cromeens, Email: Barrett.Cromeens@nationwidechildrens.org.
Yanchun Liu, Email: Yanchun.Liu@nationwidechildrens.org.
Johnathan Stathopoulos, Email: Johnathan.Stathopoulos@uscmed.sc.edu.
Yijie Wang, Email: Yijie.Wang@nationwidechildrens.org.
Jed Johnson, Email: Jed.Johnson@nanofibersolutions.com.
References
- 1.O'Keefe SJ. Nutritional Issues in the Short Bowel Syndrome - Total Parenteral Nutrition, Enteral Nutrition and the Role of Transplantation. Nestle Nutr Inst Workshop Ser. 2015;82:75–90. doi: 10.1159/000382005. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti A, Basso MS, Castro M, Calce A, Pietrobattista A, Gambarara M. Prevalence of life-threatening complications in pediatric patients affected by intestinal failure. Transplant Proc. 2007;39:1632–3. doi: 10.1016/j.transproceed.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 3.Dore M, Junco PT, Andres AM, Sanchez-Galan A, Amesty MV, Ramos E, et al. Surgical Rehabilitation Techniques in Children with Poor Prognosis Short Bowel Syndrome. Eur J Pediatr Surg. 2015 doi: 10.1055/s-0035-1567805. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Ching YA, Gura K, Modi B, Jaksic T. Pediatric intestinal failure: nutrition, pharmacologic, and surgical approaches. Nutr Clin Pract. 2007;22:653–63. doi: 10.1177/0115426507022006653. [DOI] [PubMed] [Google Scholar]
- 5.Lao OB, Healey PJ, Perkins JD, Horslen S, Reyes JD, Goldin AB. Outcomes in children after intestinal transplant. Pediatrics. 2010;125:e550–8. doi: 10.1542/peds.2009-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant CN, Grikscheit TC. Tissue engineering: a promising therapeutic approach to necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:112–6. doi: 10.1053/j.sempedsurg.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Guner YS, Chokshi N, Petrosyan M, Upperman JS, Ford HR, Grikscheit TC. Necrotizing enterocolitis--bench to bedside: novel and emerging strategies. Semin Pediatr Surg. 2008;17:255–65. doi: 10.1053/j.sempedsurg.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Spurrier RG, Grikscheit TC. Tissue engineering the small intestine. Clin Gastroenterol Hepatol. 2013;11:354–8. doi: 10.1016/j.cgh.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Choi RS, Vacanti JP. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant Proc. 1997;29:848–51. doi: 10.1016/s0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 10.Agopian VG, Chen DC, Avansino JR, Stelzner M. Intestinal stem cell organoid transplantation generates neomucosa in dogs. J Gastrointest Surg. 2009;13:971–82. doi: 10.1007/s11605-009-0806-x. [DOI] [PubMed] [Google Scholar]
- 11.Barthel ER, Speer AL, Levin DE, Sala FG, Hou X, Torashima Y, et al. Tissue engineering of the intestine in a murine model. J Vis Exp. 2012:e4279. doi: 10.3791/4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–12. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Torashima Y, et al. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg. 2013;48:129–37. doi: 10.1016/j.jpedsurg.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng Part A. 2011;17:1841–50. doi: 10.1089/ten.tea.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi RS, Riegler M, Pothoulakis C, Kim BS, Mooney D, Vacanti M, et al. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J Pediatr Surg. 1998;33:991–6. doi: 10.1016/s0022-3468(98)90520-6. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 16.Duxbury MS, Grikscheit TC, Gardner-Thorpe J, Rocha FG, Ito H, Perez A, et al. Lymphangiogenesis in tissue-engineered small intestine. Transplantation. 2004;77:1162–6. doi: 10.1097/01.tp.0000121506.34924.3c. [DOI] [PubMed] [Google Scholar]
- 17.Grant CN, Mojica SG, Sala FG, Hill JR, Levin DE, Speer AL, et al. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am J Physiol Gastrointest Liver Physiol. 2015;308:G664–77. doi: 10.1152/ajpgi.00111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez A, Grikscheit TC, Blumberg RS, Ashley SW, Vacanti JP, Whang EE. Tissue-engineered small intestine: ontogeny of the immune system. Transplantation. 2002;74:619–23. doi: 10.1097/00007890-200209150-00006. [DOI] [PubMed] [Google Scholar]
- 19.Tavakkolizadeh A, Berger UV, Stephen AE, Kim BS, Mooney D, Hediger MA, et al. Tissue-engineered neomucosa: morphology, enterocyte dynamics, and SGLT1 expression topography. Transplantation. 2003;75:181–5. doi: 10.1097/01.TP.0000044101.03656.9F. [DOI] [PubMed] [Google Scholar]
- 20.Kaihara S, Kim S, Benvenuto M, Kim BS, Mooney DJ, Tanaka K, et al. End-to-end anastomosis between tissue-engineered intestine and native small bowel. Tissue Eng. 1999;5:339–46. doi: 10.1089/ten.1999.5.339. [DOI] [PubMed] [Google Scholar]
- 21.Kim SS, Kaihara S, Benvenuto MS, Choi RS, Kim BS, Mooney DJ, et al. Effects of anastomosis of tissue-engineered neointestine to native small bowel. J Surg Res. 1999;87:6–13. doi: 10.1006/jsre.1999.5743. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Rager T, Johnson J, Enmark J, Besner GE. Enriched Intestinal Stem Cell Seeding Improves the Architecture of Tissue-Engineered Intestine. Tissue Eng Part C Methods. 2015;21:816–24. doi: 10.1089/ten.TEC.2014.0389. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Clevers H. Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol. 2013;945:319–28. doi: 10.1007/978-1-62703-125-7_19. [DOI] [PubMed] [Google Scholar]
- 24.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 25.Fuller MK, Faulk DM, Sundaram N, Shroyer NF, Henning SJ, Helmrath MA. Intestinal crypts reproducibly expand in culture. J Surg Res. 2012;178:48–54. doi: 10.1016/j.jss.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240:748–54. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wulkersdorfer B, Kao KK, Agopian VG, Dunn JC, Wu BM, Stelzner M. Growth factors adsorbed on polyglycolic acid mesh augment growth of bioengineered intestinal neomucosa. J Surg Res. 2011;169:169–78. doi: 10.1016/j.jss.2009.11.719. [DOI] [PubMed] [Google Scholar]
- 28.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Polykandriotis E, Arkudas A, Horch RE, Kneser U. To matrigel or not to matrigel. Am J Pathol. 2008;172:1441. doi: 10.2353/ajpath.2008.071215. author reply -2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 31.Laschke MW, Rucker M, Jensen G, Carvalho C, Mulhaupt R, Gellrich NC, et al. Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J Biomed Mater Res A. 2008;85:397–407. doi: 10.1002/jbm.a.31503. [DOI] [PubMed] [Google Scholar]
- 32.Freshney RI. Culture of animal cells : a manual of basic technique and specialized applications. Hoboken, N.J.: Wiley-Blackwell; 2010. [Google Scholar]
- 33.Leotot J, Lebouvier A, Hernigou P, Bierling P, Rouard H, Chevallier N. Bone-Forming Capacity and Biodistribution of Bone Marrow-Derived Stromal Cells Directly Loaded Into Scaffolds: A Novel and Easy Approach for Clinical Application of Bone Regeneration. Cell Transplant. 2015;24:1945–55. doi: 10.3727/096368914X685276. [DOI] [PubMed] [Google Scholar]
- 34.Bean AC, Tuan RS. Fiber diameter and seeding density influence chondrogenic differentiation of mesenchymal stem cells seeded on electrospun poly(epsilon-caprolactone) scaffolds. Biomed Mater. 2015;10:015018. doi: 10.1088/1748-6041/10/1/015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown CF, Yan J, Han TT, Marecak DM, Amsden BG, Flynn LE. Effect of decellularized adipose tissue particle size and cell density on adipose-derived stem cell proliferation and adipogenic differentiation in composite methacrylated chondroitin sulphate hydrogels. Biomed Mater. 2015;10:045010. doi: 10.1088/1748-6041/10/4/045010. [DOI] [PubMed] [Google Scholar]
- 36.Lode A, Bernhardt A, Gelinsky M. Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: influence of seeding density on colonization, proliferation and osteogenic differentiation. J Tissue Eng Regen Med. 2008;2:400–7. doi: 10.1002/term.110. [DOI] [PubMed] [Google Scholar]
- 37.Hadidi P, Yeh TC, Hu JC, Athanasiou KA. Critical seeding density improves the properties and translatability of self-assembling anatomically shaped knee menisci. Acta Biomater. 2015;11:173–82. doi: 10.1016/j.actbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, et al. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One. 2014;9:e84651. doi: 10.1371/journal.pone.0084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One. 2014;9:e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, et al. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng Part C Methods. 2013;19:961–9. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lahar N, Lei NY, Wang J, Jabaji Z, Tung SC, Joshi V, et al. Intestinal subepithelial myofibroblasts support in vitro and in vivo growth of human small intestinal epithelium. PLoS One. 2011;6:e26898. doi: 10.1371/journal.pone.0026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2015 doi: 10.2217/rme.15.70. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boomer L, Liu Y, Mahler N, Johnson J, Zak K, Nelson T, et al. Scaffolding for challenging environments: materials selection for tissue engineered intestine. J Biomed Mater Res A. 2014;102:3795–802. doi: 10.1002/jbm.a.35047. [DOI] [PubMed] [Google Scholar]


