Abstract
The purpose of this study was to evaluate the effects of lutein, zeaxanthin and meso-zeaxanthin on macular pigment optical density (MPOD) in randomized controlled trials (RCTs) among patients with age-related macular degeneration (AMD) and healthy subjects. Medline, Embase, Web of Science and Cochrane Library databases was searched through May 2016. Meta-analysis was conducted to obtain adjusted weighted mean differences (WMD) for intervention-versus-placebo group about the change of MPOD between baseline and terminal point. Pearson correlation analysis was used to determine the relationship between the changes in MPOD and blood xanthophyll carotenoids or baseline MPOD levels. Twenty RCTs involving 938 AMD patients and 826 healthy subjects were identified. Xanthophyll carotenoids supplementation was associated with significant increase in MPOD in AMD patients (WMD, 0.07; 95% CI, 0.03 to 0.11) and healthy subjects (WMD, 0.09; 95% CI, 0.05 to 0.14). Stratified analysis showed a greater increase in MPOD among trials supplemented and combined with meso-zeaxanthin. Additionally, the changes in MPOD were related with baseline MPOD levels (rAMD = −0.43, p = 0.06; rhealthy subjects = −0.71, p < 0.001) and blood xanthophyll carotenoids concentration (rAMD = 0.40, p = 0.07; rhealthy subjects = 0.33, p = 0.05). This meta-analysis revealed that lutein, zeaxanthin and meso-zeaxanthin supplementation improved MPOD both in AMD patients and healthy subjects with a dose-response relationship.
Keywords: lutein, zeaxanthin, meso-zeaxanthin, macular pigment optical density
1. Introduction
The macula is a specialized part in the posterior pole of retina, since it mediates central vision, provides the sharpest visual acuity and facilitates the best color discrimination [1]. As the major functional component in the macular region, macular pigment (MP) was uniquely concentrated in the inner and central layers and mainly composed of xanthophyll carotenoids, including lutein, zeaxanthin and meso-zeaxanthin [2,3,4,5,6,7,8]. The concentration of these carotenoids in the macular region is about 1000 times greater than that in the blood [8]. The exquisite degree of biological selectivity in the retina indicated that these carotenoids played a pivotal role in maintaining the normal morphology and function of the macula [9]. Furthermore, lutein, zeaxanthin and meso-zeaxanthin are believed to play a major role in protecting retina and retinal pigment epithelium from light-initiated oxidative damage by scavenging reactive oxygen species and filtering blue light, which was involved in the putative pathogenesis of many age-related eye diseases [10,11,12,13,14,15]. Thus, elevated MP affords protection against the development of many retinal diseases, especially for age-related macular degeneration (AMD); contrarily, low MP enhanced the risk of these diseases [4,6,12,13].
Data from epidemiologic studies suggested that dietary lutein and zeaxanthin intake were inversely associated with the risk of AMD [16,17,18]. In addition, our previous studies also found that supplementation with these macular carotenoids partially reversed the loss of visual function in patients with early AMD by elevating macular pigment optical density (MPOD), suggesting a causative role of MPOD for the maintenance of normal visual function [19]. Although some intervention studies have showed that lutein, zeaxanthin and meso-zeaxanthin supplementation resulted in significant morphologic changes in macular pigment, the response was variable among different studies and even a few studies failed to find such an increase in MPOD [20,21,22]. Populations with specific genetic backgrounds or nutritional status may potentially affect the transport and deposition processes of these carotenoids from blood to macula during supplementation [13,17]. The efficacy of supplementation for the different study populations and supplement dose remained uncertain. Furthermore, total zeaxanthin increases with decreasing eccentricity in the macula, and tends to be the dominant carotenoid at the central fovea [23]. These specific distribution patterns suggest that zeaxanthin may play a crucial role in the center of the retina. In addition, It was hypothesized that meso-zeaxanthin, a geometrical isomer of zeaxanthin, was able to protect against age-related eye damage by the special antioxidant properties and light filtering properties [5,24,25]. However, whether zeaxanthin and meso-zeaxanthin should be added in combination with lutein remained to be confirmed. Besides, MPOD depends on the stimuli that are used for its measurement [19,21]. Thus, the influence of different methods used in included studies should be explored.
Therefore, we performed a meta-analysis of randomized controlled trials (RCTs) to determine the effect of lutein, zeaxanthin and meso-zeaxanthin supplementation on MPOD in AMD patients and healthy subjects.
2. Materials and Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26].
2.1. Data Sources and Search Strategy
A comprehensive search was performed to identify all relevant articles in Medline, Embase, Web of Science, and Cochrane Library database up to May 2016, using the search terms lutein, zeaxanthin, meso-zeaxanthin, xanthophyll or carotenoids in conjunction with each of the following words: macular pigment optical density, macular pigment density, macular pigment, MPOD and MP, as well as combinations of these terms. References from retrieved articles were also reviewed for pertinent studies. No language restriction was applied for searching and study inclusion. Experts in the field were content in terms of additional information or potential unpublished studies in the case of missing data.
2.2. Study Selection
The titles and abstracts of potentially eligible studies were identified by the search strategy. Then, the full text articles were reviewed to determine whether they met the inclusion criteria. Studies were included in the meta-analysis if they fulfilled the following criteria: (1) eligible studies were limited to randomized controlled trials (RCTs); (2) subjects were randomized to receive lutein, zeaxanthin or/and meso-zeaxanthin supplement or placebo; (3) the outcome of interest was MPOD; (4) studies reported the change of MPOD between baseline and at the end of study in the intervention and placebo group. When studies were conducted in healthy subjects, these subjects should be free of retinal disease. If multiple articles were published from the same study, only the most updated data was selected for analysis. Three investigators (Rong Liu, Jun Hui Du and Tao Liu) independently reviewed all identified publications for inclusion using predetermined criteria, with discrepancies resolved by consensus.
2.3. Data Extraction and Study Quality Assessment
For each included study, study characteristics and demographics was recorded as follows: first author, publication year, sample size, population characteristics (age, sex and country), interventions (dose of lutein/zeaxanthin/meso-zeaxanthin and duration of follow-up), change in the mean with standard deviation (SD) for MPOD, numbers enrolled and lost to follow-up. This needs to be clear in the manuscript. When several means and standard deviations were present in a single study, the data was pooled by combining groups into a single group according to the Cochrane recommendation. Where final SDs were not available from trials, they were calculated from confidence intervals (CI) or standard errors reported in study. If the information of blood lutein and zeaxanthin concentration was showed in studies, it was also extracted for further relevant analysis.
Methodological quality of each study was evaluated by the Jadad score, a 5-point study quality assessment instrument. This scale consists of three aspects: the method of randomization, the adequacy of blinding, and the description of withdrawals and dropouts. Studies that scored three or more were considered to be categorized as high quality. Data extraction and quality assessment was conducted independently and in duplicate by three investigators (Rong Liu, Jun Hui Du and Tao Liu), and any disagreement was adjudicated by a fourth author (Le Ma).
2.4. Statistical Analysis
The weighted mean differences (WMD) and corresponding 95% CIs were used as the primary summary measure of the effect of lutein/zeaxanthin/meso-zeaxanthin supplement on MPOD. Statistical heterogeneity among studies was evaluated by Q tests and the degree of heterogeneity was assessed by I2 statistics. WMD for MPOD were pooled using inverse-variance weighting with the fixed effects or random-effects models. To explore the potential sources of between-study heterogeneity, meta-regression analyses were conducted stratified by health status (AMD patients vs. healthy participants), dose of lutein, zeaxanthin or meso-zeaxanthin supplementation (>10 mg vs. ≤10 mg), duration of intervention (≥12 month vs. <12 month), mean age of subjects (>70 years vs. ≤70 years), zeaxanthin (with zeaxanthin vs. without zeaxanthin), meso-zeaxanthin (with meso-zeaxanthin vs. without meso-zeaxanthin ), other antioxidants use (with other antioxidants vs. without other antioxidants) and geographic area (Europe vs. Asia vs. North America), measurement method of MPOD (objective (fundus autofluorescence, spectral fundus reflectance and VISUCAM NM/FA) vs. psychophysical (heterochromatic flicker photometry and macular assessment profile)) [27]. In pooling dose-response analysis, the relationship between the dose of lutein/zeaxanthin/meso-zeaxanthin supplement and the change in MPOD in each study was examined by linear regression model. The association between the increase in MPOD and blood xanthophyll carotenoids concentration was investigated using Pearson correlation analysis. Sensitivity analyses to examine the influence of each individual study were performed by iteratively excluding each study from this meta-analysis and comparing the point estimates without and with one study at a time. Publication bias was assessed by the Egger regression asymmetry test and the Begg adjusted rank correlation test [28,29]. All statistical analyses were conducted by Stata software, version 10.0 (Stata Corp, College Station, TX, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Literature Search
A total of 2456 potentially relevant publications were retrieved during our initial search. After duplicate publications detection and abstract review, full-text versions of the remaining 133 articles were then retrieved for detailed evaluation. Of these, 114 retrieved trials were not eligible due to duplicate publications, lack of a control group, outcomes not suitable for the meta-analysis, means or SDs of pretest and posttest data not included in the publication and not provided by the authors on request. Finally, the remaining 20 articles were eligible for inclusion in our analysis [17,20,21,22,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
3.2. Study Characteristics
The characteristics of the included studies are presented in Table 1. In these trials, 12 were performed in Europe, 6 in USA and 2 in China. The number of participants in each study ranged from 19 to 172, comprising a total of 1764. Most studies included both men and women, except for 2 in which only men or women were selected. 8 trials supplemented with lutein vs. placebo, 2 treated with zeaxanthin vs. placebo, 8 intervened by combining lutein and zeaxanthin vs. placebo, and 8 had multiple arms (lutein, zeaxanthin or/and meso-zeaxanthin combined with other antioxidants, vs. placebo). The dosage of lutein, zeaxanthin or/and meso-zeaxanthin in the intervention groups among trials varied from 0 mg/day to 20 mg/day. The duration of intervention and follow-up ranged from 8 weeks to 2 years. MPOD was measured by the objective methods in 7 studies, and psychophysical methods in 13 trials. All included studies had a Jadad score of 3 or more, indicating generally high methodological quality.
Table 1.
Characteristics of the eligible randomized clinical trials.
| Authors (Year) | Study Participants | Trial Duration | No. of Groups | Lnterventions | Measurement Method for MPOD | Follow-Up Rates (%) | Quality Score * |
|---|---|---|---|---|---|---|---|
| Trieschmann et al. (2007) [20] | 130 AMD patients aged (71.4 ± 7.6) years in Germany | 6 months | 2 | 12 mg lutein and 1 mg zeaxanthin combined with other antioxidants; placebo | Fundus autofluorescence | 94.6 | 3 |
| Richer et al. (2007) [21] | 90 AMD patients aged (74.1 ± 7.5) years in the USA | 12 months | 3 | 10 mg lutein; 10 mg lutein combined with other antioxidants; placebo | HFP | 84.4 | 5 |
| Weigert et al. (2011) [30] | 126 AMD patients aged (71.6 ± 8.6) years in Austria | 6 months | 2 | 20 mg lutein daily in months 1 to 3 and 10 mg lutein daily in months 4 to 6; placebo | Spectral fundus reflectance | 87.3 | 3 |
| Arnold C et al. (2013) [31] | 20 AMD patients aged (66.0 ± 8.0) years in Germany | 10 weeks | 2 | 10 mg lutein plus 3 mg zeaxanthin; placebo | VISUCAM NM/FA | 100.0 | 5 |
| García-Layana et al. (2013) [32] | 44 AMD patients aged (68.5 ± 8.5) years in Spain | 12 months | 2 | 12 mg lutein plus 0.6 mg zeaxanthin combined with other antioxidants; placebo | HFP | NR | 3 |
| Dawczynski et al. (2013) [33] | 172 AMD patients aged (70.0 ± 10.0) years in Germany | 12 months | 3 | 10 mg lutein, 1 mg zeaxanthin combined with other antioxidants; 20 mg lutein, 2 mg zeaxanthin combined with other antioxidants; placebo | VISUCAM NM/FA | 84.3 | 3 |
| Murray et al. (2013) [34] | 72 AMD patients aged (70.5 ± 8.7) years in UK | 12 months | 2 | 10 mg lutein daily; placebo | HFP | 86.9 | 5 |
| Arnold C et al. (2013) [35] | 172 AMD patients aged (69.0 ± 10.0) years in Germany | 12 months | 3 | 10 mg lutein plus 1 mg zeaxanthin combined with other antioxidants; 20 mg lutein plus 2 mg zeaxanthin combined with other antioxidants; placebo | VISUCAM NM/FA | 84.3 | 5 |
| Huang et al. (2015) [36] | 112 AMD patients aged (69.1 ± 7.4) years in China | 24 months | 4 | 10 mg lutein; 20 mg lutein; 10 mg lutein plus 10 mg zeaxanthin; placebo | Fundus autofluorescence | 96.4 | 5 |
| Kvansakul et al. (2005) [37] | 92 healthy men in UK | 12 months | 4 | 10 mg lutein; 10 mg zeaxanthin; 10 mg lutein plus 10 mg zeaxanthin in months 1 to 6 and 20 mg lutein; 20 mg zeaxanthin; 10 mg lutein plus 10 mg zeaxanthin in months 7 to 12; placebo | MAP | 79.3 | 4 |
| Bone et al. (2007) [38] | 19 healthy subjects in the USA | 120 days | 2 | 14.9 mg of meso-zeaxanthin, 5.5 mg of lutein, and 1.4 mg of zeaxanthin; placebo | HFP | NR | 3 |
| Johnson et al. (2008) [39] | 57 healthy women in the USA | 4 months | 3 | 12 mg lutein plus 0.5 mg zeaxanthin;12 mg lutein plus 800 mg DHA; placebo | HFP | 86.0 | 4 |
| Bone et al. (2010) [40] | 100 healthy subjects in the USA | 140 days | 4 | 5 mg lutein; 10 mg lutein; 20 mg lutein; placebo | HFP | 87.0 | 4 |
| Connolly et al. (2011) [17] | 44 healthy subjects in Ireland | 6 months | 2 | 10.6 mg meso-zeaxanthin, 5.9 mg lutein, and 1.2 mg zeaxanthin; placebo | HFP | 79.5 | 5 |
| Nolan et al. (2011) [41] | 121 healthy subjects in Ireland | 12 months | 2 | 12 mg lutein, 1 mg zeaxanthin combined with other antioxidants; placebo | HFP | 62.8 | 4 |
| Landrum et al. (2012) [42] | 30 healthy subjects in the USA | 24 weeks | 3 | 20 mg lutein diacetate; 20 mg lutein; placebo | HFP | NR | 3 |
| Loughman et al. (2012) [22] | 36 healthy subjects in Ireland | 6 months | 3 | 20 mg lutein plus 2 mg zeaxanthin; 10 mg meso-zeaxanthin, 10 mg lutein plus 2 mg zeaxanthin; placebo | HFP | 88.9 | 5 |
| Yao et al. (2013) [43] | 120 healthy subjects in China | 12 months | 2 | 20 mg lutein; placebo | HFP | 82.5 | 4 |
| Bovier et al. (2015) [44] | 102 healthy subjects in the USA | 4 months | 3 | 20 mg zeaxanthin; 8 mg lutein plus 26 mg zeaxanthin combined with other antioxidants; placebo | HFP | 67.6 | 4 |
| Nolan et al. (2016) [45] | 105 healthy subjects in Ireland | 12 months | 2 | 10 mg lutein, 2 mg zeaxanthin, and 10 mg meso-zeaxanthin; placebo | Autofluorescence | 80.0 | 5 |
Abbreviations: AMD, age-related macular degeneration; HFP, heterochromatic flicker photometry; MPOD, macular pigment optical density; NR, not report. * Study quality was judged based on the Jadad scale.
3.3. The Effect of Lutein, Zeaxanthin or/and Meso-zeaxanthin Supplementation on MPOD in Patients with AMD
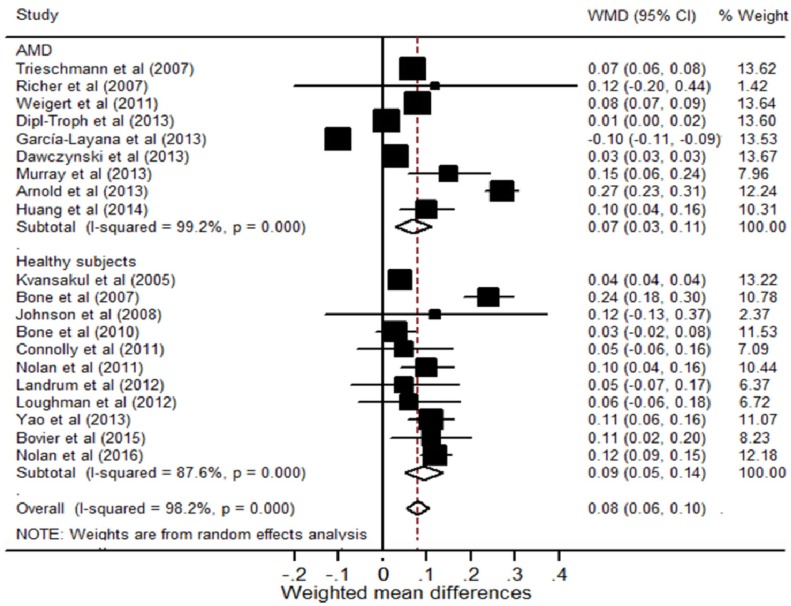
Nine RCTs evaluated the efficacy of these carotenoids supplement on the changes in MPOD for AMD patients (Figure 1). The I2 test for heterogeneity was 99.2% (p < 0.001); and the results from random-effects models suggested that combing trials produced a MPOD increase by 0.07 ODU (95% CI, 0.03 to 0.11) in favor of supplementation vs. placebo. In the stratified analysis, a longer supplementation time had a marginally greater effect in comparison with the shorter time (0.17 vs. 0.05; between-group difference, 0.12; p = 0.05; Table 2). Trials measured MPOD with objective methods showed a larger increase in MPOD compared with those by psychophysical methods, although the difference did not reach statistical significance (0.09 vs. 0.05; between-group difference, 0.04; p = 0.37). The dose-response meta-analysis estimate showed a 0.005 ODU improvement in MPOD for a 1 mg/day increase in these carotenoids supplement. In sensitivity analysis, exclusion of any single trial from the analysis did not alter the overall findings of the effect of supplementation on MPOD. No evidence of publication bias was detected in this study by either Begg (p = 0.68) or Egger test (p = 0.83).
Figure 1.
Forest plot showing the efficacy of lutein, zeaxanthin and meso-zeaxanthin supplementation on macular pigment optical density for patients with AMD and healthy subjects. Error bars indicate 95% CIs of the WMDs. The sizes of the squares correspond to the study weight in the random-effects meta-analysis. Diamonds represent the meta-analysis summary effect estimate. AMD, age-related macular degeneration; CI, confidence interval; WMD, weighted mean differences.
Table 2.
Stratified analysis for the lutein or/and zeaxanthin or/and meso-zeaxanthin supplements effect on macular pigment optical density (MPOD) across the assessed randomized controlled trials (RCTs).
| Subgroup | AMD Patients | Healthy Populations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | WMD | 95% CI | Pz | Ph | N | WMD | 95% CI | Pz | Ph | |
| Dose of supplement | ||||||||||
| >10 mg | 10 | 0.07 | 0.04, 0.12 | <0.001 | 0.93 | 15 | 0.12 | 0.09, 0.15 | <0.001 | 0.01 |
| ≤10 mg | 4 | 0.09 | −0.07, 0.19 | 0.40 | 4 | 0.05 | 0.03, 0.07 | 0.02 | ||
| Duration of intervention | ||||||||||
| ≥12 months | 11 | 0.17 | 0.09, 0.24 | <0.001 | 0.05 | 6 | 0.07 | 0.04, 0.10 | <0.001 | 0.83 |
| <12 months | 3 | 0.05 | 0.01, 0.09 | <0.001 | 13 | 0.08 | 0.03, 0.13 | <0.001 | ||
| Mean age | ||||||||||
| >70 years | 7 | 0.06 | 0.03, 0.09 | <0.001 | 0.85 | |||||
| ≤70 years | 7 | 0.11 | 0.02, 0.19 | <0.001 | ||||||
| Zeaxanthin | ||||||||||
| With | 9 | 0.07 | 0.04, 0.11 | <0.001 | 0.60 | 11 | 0.09 | 0.06, 0.13 | <0.001 | 0.21 |
| Without | 5 | 0.08 | 0.07, 0.09 | 0.41 | 8 | 0.08 | 0.03, 0.08 | 0.03 | ||
| Meso-zeaxanthin | ||||||||||
| With | 4 | 0.13 | 0.05, 0.22 | 0.001 | 0.02 | |||||
| Without | 15 | 0.06 | 0.03, 0.08 | <0.001 | ||||||
| Other antioxidants | ||||||||||
| With | 7 | 0.08 | 0.04, 0.13 | <0.001 | 0.97 | 3 | 0.10 | 0.05, 0.15 | 0.99 | 0.55 |
| Without | 7 | 0.08 | 0.04, 0.13 | <0.001 | 16 | 0.07 | 0.05, 0.10 | <0.001 | ||
| Geographic area | ||||||||||
| Europe | 9 | 0.08 | 0.04, 0.11 | <0.001 | 0.80 | 8 | 0.06 | 0.03, 0.09 | <0.001 | 0.50 |
| Asia | 3 | 0.10 | 0.05, 0.15 | 0.27 | 1 | 0.11 | 0.06, 0.16 | - | ||
| USA | 2 | 0.12 | −0.15, 0.38 | 0.97 | 10 | 0.09 | 0.02, 0.15 | <0.001 | ||
| Methods | ||||||||||
| Objective | 10 | 0.09 | 0.07, 0.12 | <0.001 | 0.37 | |||||
| Psychophysical | 4 | 0.05 | −0.15, 0.24 | <0.001 | ||||||
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; MPOD, macular pigment optical density; Ph, P for between-study heterogeneity; Pz, P for Z test; RCTs: randomized controlled trials; WMD, weighted mean differences.
3.4. The Effect of Lutein, Zeaxanthin or/and Meso-zeaxanthin Supplementation on MPOD in Healthy Subjects
The changes in MPOD with these carotenoids supplement for healthy subjects were assessed in 11 RCTs (Figure 1). When all these studies were pooled into the meta-analysis, the intervention group evidently exhibited an augmentation in MPOD by 0.09 ODU compared with placebo (95% CI, 0.05 to 0.14). For subgroup analysis, trials that intervened exceeding 10 mg macular carotenoids per day produced a higher WMD of 0.12 (95% CI, 0.09 to 0.15) than a WMD of 0.05 (95% CI, 0.03 to 0.07) in trials that only supplemented with less than 10 mg (between-group difference, 0.07; p = 0.01). Moreover, a greater increase in MPOD was observed in trials supplemented combined with meso-zeaxanthin in comparison with those without meso-zeaxanthin (WMD, 0.13 vs. 0.07; between-group difference, 0.06; p = 0.02; Table 2). Additionally, participants receiving additional zeaxanthin supplement did not have a more response in MPOD compared with those who taking only lutein supplement. In the dose-response meta-analysis, each additional 1 mg of these carotenoids supplementation was associated with a 0.004 ODU increase in MPOD. The sensitivity analysis by excluding each of the studies also did not appreciably influence the pooled WMD. No publication bias was found for Begg’s rank correlation test (p = 0.54) or Egger’s linear regression test (p = 0.05).
3.5. The Relationship between Baseline MPOD Levels and the Change in MPOD
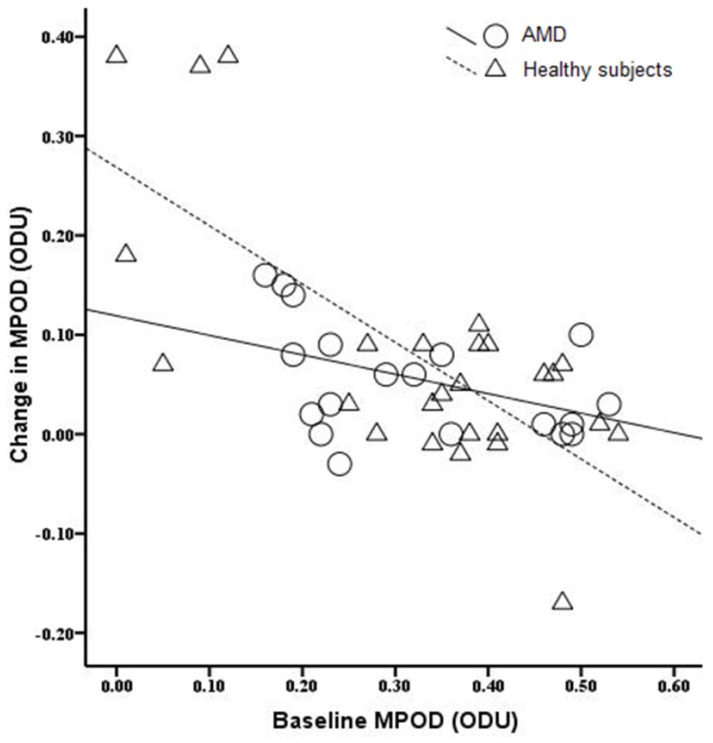
Correlation analysis was used to investigate the association between baseline MPOD levels and the change in MPOD during treatment (Figure 2). For healthy subjects, the changes in MPOD during supplementation were significantly related with baseline levels (r = −0.71, p < 0.001). Moreover, the increase in MPOD for AMD patients also marginally exhibited a negative correlation with baseline MPOD (r = −0.43, p = 0.06).
Figure 2.
Scatterplot showing the relationship between baseline MPOD levels and the change in MPOD from baseline. MPOD, macular pigment optical density; ODU, optical density unit.
3.6. The Relationship between Blood Xanthophyll Carotenoids Concentration and the Change in MPOD
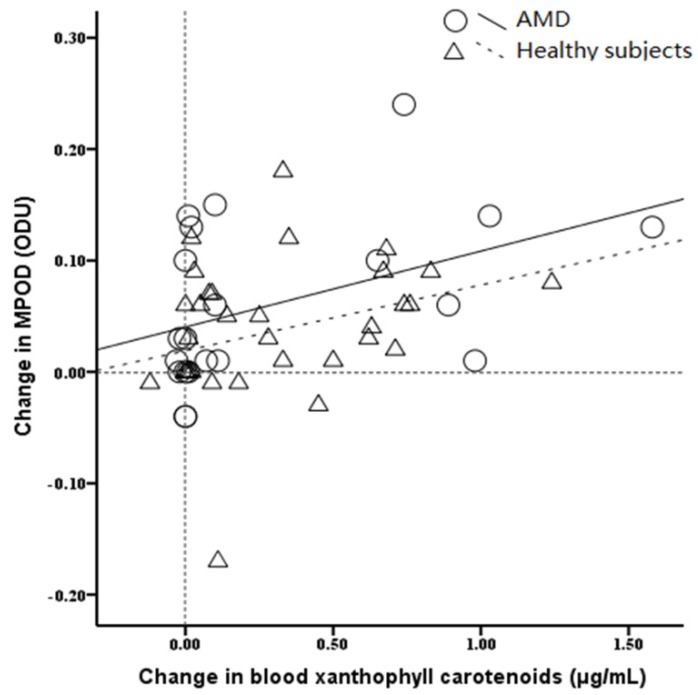
We subsequently evaluated the relationship between the change in serum carotenoids concentration and the change in MPOD (Figure 3). The results showed that MPOD was improved with the postintervention increase in blood concentrations both in AMD patients (r = 0.40, p = 0.07) and the healthy populations (r = 0.33, p = 0.05).
Figure 3.
Scatterplot showing the relationship between blood xanthophyll carotenoids concentration and the change in MPOD during supplementation. MPOD, macular pigment optical density; ODU, optical density unit.
4. Discussion
In the current study, we evaluated the effects of lutein, zeaxanthin and meso-zeaxanthin supplementation on MOPD based on the data from the RCTs. Our results showed that the carotenoids supplementation significantly increased the level of MPOD and the inclusion of meso-zeaxanthin resulted in a greater increase in macular pigment compared to supplements lacking this central carotenoid. The increment in MPOD was positively correlated with changes in blood xanthophyll carotenoids concentration. Furthermore, supplementation with these carotenoids for longer than 12 months, a higher dose and the three carotenoids in combination were more effective on MPOD augmentation.
Previous studies have found that the decrease in MP was related with the functional abnormalities of the macula, which eventually led to some age-related degenerative eye diseases [46,47]. Neuringer et al. reported that monkeys fed with the xanthophyll-free diets were found to have no detectable MP in the retina and adipose tissue [47]. As the main constituents of the yellow pigment, lutein, zeaxanthin and meso-zeaxanthin are uniquely concentrated in the macula [12,48,49]. It is hypothesized that these carotenoids could protect the photoreceptor outer segments and the retinal pigment epithelium by screening these susceptible retinal structures from actinic blue light and quenching reactive oxygen species [50]. Barker et al. demonstrated that lutein and zeaxanthin supplementation of xanthophyll-free monkeys and the resulting accumulation of MP provided significant foveal protection against short-wavelength photochemical damage [11]. Their results were in agreement with those reported by Thomson et al., in which quails supplemented with 6-month xanthophyll carotenoids significantly decreased number of dying photoreceptors in retina [51]. Moreover, these carotenoids have also been suggested to offer protection to reduce the lipofuscin accumulation and enhance in lysosomal stability and viability [52]. Thus, lutein, zeaxanthin and meso-zeaxanthin may have a possible specific function in the maintenance of human retinal structures [7,17,48].
Some reports revealed that the donor eyes with AMD showed a drastic decline of MP levels as compared to eyes without AMD [53]. According to previous studies, a lower MPOD appeared to be associated with an increased risk of progression to AMD [54,55]. Our previous intervention study has demonstrated a significant benefit of lutein and zeaxanthin supplementation on the increase of MPOD for patients with early AMD [19]. Consistent with these findings, the results of the present study showed that supplementation with these carotenoids significantly increased the level of MPOD not only in AMD patients but also in healthy subjects. Moreover, the change in MPOD was accompanied by the improvement of these xanthophyll carotenoids statuses. These suggested that supplementation with lutein, zeaxanthin and meso-zeaxanthin lead to the improvements in MPOD as a consequence of maintaining the normal morphology of retina by elevating blood levels [54]. In addition, our results also showed that participants receiving with higher doses supplement were associated with a greater increase in MPOD, especially for the healthy subjects. Previous studies suggested that a consumption of lutein and zeaxanthin above 6-14 mg daily was considered to reduce the risk of eye diseases such as AMD as well as in alleviating the symptoms if present [56,57]. However, epidemiological studies indicated that the combined daily dietary intake of these carotenoids was only approximately 2 mg per day in western countries [58]. Therefore, the additional consumption of these carotenoids supplements should be warranted.
Although zeaxanthin is deposited throughout the human retina, it is preferentially accumulated at the fovea region of macula [59]. Such a specific distribution pattern of these carotenoids within the human macula indicated that combined zeaxanthin and lutein might result in greater improvements in MPOD than lutein alone; however, absence of significantly greater response was noted with combination treatment in the present study. This finding may be partly attributed to the fact that zeaxanthin deposition at the fovea during supplementation may be limited [60,61]. Due to the high chemical similarity of lutein and zeaxanthin, tissue-specific xanthophyll binding proteins may mediate lutein and zeaxanthin capture by competition for the same absorption mediator [61]. Once these protein receptors are saturated, they could not capture more macular xanthophylls, which may limit the amount of zeaxanthin being additionally accumulated [62]. Meanwhile, the relatively higher levels of zeaxanthin naturally present at the central fovea may also limit deposition of zeaxanthin in this area [63]. This hypothesis was also supported by our results that a significant negative association was detected between the changes in MPOD and the baseline levels. Thus, the populations with lower MP may benefit more from the additionally supplementation of xanthophyll carotenoids. Furthermore, meso-zeaxanthin is a different molecular to lutein and zeaxanthin which resides directly over the central of the macula. Although trace amount of meso-zeaxanthin existed in some kind of fish, it could not be found in raw fruits and vegetables, or detected in blood serum [64]. It has the ability to protect against chronic and cumulative eye damage through its capacity to filter the most energetic and potentially damaging wavelengths of visible light and to neutralize free radicals produced by oxidative stress [65]. It has been shown that 1:1:1 mixture of lutein, zeaxanthin and meso-zeaxanthin could quench singlet oxygen more efficiently than any of the three individually. The reason could be explained that three carotenoids may form specific aggregates, which could enhance their ability to quench singlet oxygen [7,17]. Loughman et al. reported the observed change in MPOD was not statistically significant among subjects receiving lutein and zeaxanthin supplementation for 6 months, as the supplement did not contain meso-zeaxanthin [22]. The results of this meta-analysis also indicated that having meso-zeaxanthin in the supplement offers a greater increase in MPOD than supplements lacking this carotenoid, which was in accordance with previous study. In addition, Thurnham and Xu demonstrated that meso-zeaxanthin supplementation caused no noticeable toxicological effects on rats [5,25]. Therefore, additional meso-zeaxanthin supplementation should be encouraged.
Several potential limitations should be taken into account. First, these included studies selected different methods for MPOD measurement. Although the results of the stratified analysis revealed that this factor did not significantly alter the effect of lutein, zeaxanthin or/and meso-zeaxanthin supplementation on MPOD, the potential influence from this factor could not be ruled out completely. As the stimuli that are used for MPOD measurement, such as peak wavelength, width of the measuring and reference lights, stimulus size, varied across studies, our results might also be affected by these potential confounding factors. Second, majority of the studies intervened less than 2 years, and it is unclear whether a higher dosing strategy over time may be associated with greater benefit. Fortunately, the Central Retinal Enrichment Supplementation Trials (CREST) will illustrate the role of longer-term nutritional supplementation in maintaining the levels of xanthophyll carotenoids in blood and macula, and clarify the effects of lutein, zeaxanthin and meso-zeaxanthin on visual function in normal subjects and in subjects with early AMD [66]. Third, the relatively small sample sizes of the included RCTs in this meta-analysis would reduce the statistical power to assess the association between supplementation with the macular carotenoids and MPOD. However, all of the included studies were considered of high quality, which might enhance the reliability of results. Fourth, other variables, like glare disability and dietary supplementation with carotenoid rich foods, are not included in present study. Thus, further research is needed to study the association between different responses and dietary supplementation with carotenoids-rich foods. Finally, although no significant publication bias was detected, the potential bias could not be ruled out.
5. Conclusions
The present meta-analysis demonstrated significant benefits of lutein, zeaxanthin and meso-zeaxanthin supplementation on MPOD augmentation both in AMD patients and healthy subjects with a dose-response relationship. Moreover, such improvement was positively associated with the increase in blood xanthophyll carotenoids level. As most of the studies involved less than 12 months of follow-up, which limits the evaluation of extended effect of these carotenoids, further larger-scale and longer-term RCTs are required to examine the effects of xanthophyll carotenoids on protecting the morphological integrity of the retina and preventing the progression of AMD.
Acknowledgments
This study was partially supported by grants from the National Natural Science Foundation of China (NSFC-81202198, NSFC-81473059); the Natural Science Foundation of Shaanxi Province of China (2013JQ4008); New-star Plan of Science and Technology of Shaanxi Province (2015LJXX-07); the China Postdoctoral Science Special Foundation (2015T81036); the Fundamental Research Funds for the Central Universities (qngz2016004); and the China Postdoctoral Science Foundation Funded Project (2014M560790).
Author Contributions
L.M., R.L. and X.H.L. designed and conducted the study; R.L., J.H.D. and X.H.L. collected the data; R.L., J.H.D., S.S.W., X.H.L. and T.L. analyzed the data; L.M., R.L., J.H.D., S.S.W., X.H.L. and T.L. prepared the manuscript; L.M., R.L., J.H.D. and S.S.W. critically revised the manuscript; and L.M., R.L., J.H.D., S.S.W., X.H.L. and T.L. gave final approval of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Trevino R., Kynn M.G. Macular function surveillance revisited. Optometry. 2008;79:397–403. doi: 10.1016/j.optm.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Koo E., Neuringer M., SanGiovanni J.P. Macular xanthophylls lipoprotein-related genes and age-related macular degeneration. Am. J. Clin. Nutr. 2014;100:336S–346S. doi: 10.3945/ajcn.113.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone R.A., Landrum J.T., Fernandez L., Tarsis S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Invest. Ophthalmol. Vis. Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 4.Meagher K.A., Thurnham D.I., Beatty S., Howard A.N., Connolly E., Cummins W., Nolan J.M. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br. J. Nutr. 2013;110:289–300. doi: 10.1017/S0007114512004837. [DOI] [PubMed] [Google Scholar]
- 5.Li B., Ahmed F., Bernstein P.S. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch. Biochem. Biophys. 2010;504:56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurnham D.I., Nolan J.M., Howard A.N., Beatty S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015;253:1231–1243. doi: 10.1007/s00417-014-2811-3. [DOI] [PubMed] [Google Scholar]
- 7.Connolly E.E., Beatty S., Thurnham D.I., Loughman J., Howard A.N., Stack J., Nolan J.M. Augmentation of macular pigment following supplementation with all three macular carotenoids: An exploratory study. Curr. Eye Res. 2010;35:335–351. doi: 10.3109/02713680903521951. [DOI] [PubMed] [Google Scholar]
- 8.Bone R.A., Landrum J.T., Dixon Z., Chen Y., Llerena C.M. Lutein and Zeaxanthin in the Eyes, Serum and Diet of Human Subjects. Exp. Eye Res. 2000;71:239–245. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett H., Howells O., Eperjesi F. The role of macular pigment assessment in clinical practice: A review. Clin. Exp. Optom. 2010;93:300–308. doi: 10.1111/j.1444-0938.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabour-Pickett S., Beatty S., Connolly E., Loughman J., Stack J., Howard A., Klein R., Klein B.E., Meuer S.M., Myers C., et al. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina. 2014;34:1757–1766. doi: 10.1097/IAE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 11.Barker F.M., Snodderly D.M., Johnson E.J., Schalch W., Koepcke W., Gerss J., Neuringer M. Nutritional manipulation of primate retinas, V: Effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest. Ophthalmol. Vis. Sci. 2011;52:3934–3942. doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan J.M., Stack J., O’Donovan O., Loane E., Beatty S. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp. Eye Res. 2007;84:61–74. doi: 10.1016/j.exer.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Nolan J.M., Akkali M.C., Loughman J., Howard A.N., Beatty S. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp. Eye Res. 2012;101:9–15. doi: 10.1016/j.exer.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Hammond B.R., Johnson E.J., Russell R.M., Krinsky N.I., Yeum K.J., Edwards R.B., Snodderly D.M. Dietary modification of human macular pigment density. Invest. Ophthalmol. Vis. Sci. 1997;38:1795–1801. [PubMed] [Google Scholar]
- 15.Berrow E.J., Bartlett H.E., Eperjesi F., Gibson J.M. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy-a randomised controlled trial. Br. J. Nutr. 2013;109:2008–2014. doi: 10.1017/S0007114512004187. [DOI] [PubMed] [Google Scholar]
- 16.Ho L., van Leeuwen R., Witteman J.C., van Duijn C.M., Uitterlinden A.G., Hofman A., de Jong P.T., Vingerling J.R., Klaver C.C. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and ω-3 fatty acids: The Rotterdam study. Arch. Ophthalmol. 2011;129:758–766. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 17.Connolly E.E., Beatty S., Loughman J., Howard A.N., Louw M.S., Nolan J.M. Supplementation with all three macular carotenoids: Response, stability, and safety. Invest. Ophthalmol. Vis. Sci. 2011;52:9207–9217. doi: 10.1167/iovs.11-8025. [DOI] [PubMed] [Google Scholar]
- 18.Joachim N., Mitchell P., Rochtchina E., Tan A.G., Wang J.J. Incidence and progression of reticular drusen in age-related macular degeneration: Findings from an older Australian cohort. Ophthalmology. 2014;121:917–925. doi: 10.1016/j.ophtha.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Ma L., Yan S.F., Huang Y.M., Lu X.R., Qian F., Pang H.L., Xu X.R., Zou Z.Y., Dong P.C., Xiao X., et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012;119:2290–2297. doi: 10.1016/j.ophtha.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Trieschmann M., Beatty S., Nolan J.M., Hense H.W., Heimes B., Austermann U., Fobker M., Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA study. Exp. Eye Res. 2007;84:718–728. doi: 10.1016/j.exer.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Richer S., Devenport J., Lang J.C. LAST II: Differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry. 2007;78:213–219. doi: 10.1016/j.optm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Loughman J., Nolan J.M., Howard A.N., Connolly E., Meagher K., Beatty S. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Invest. Ophthalmol. Vis. Sci. 2012;53:7871–7880. doi: 10.1167/iovs.12-10690. [DOI] [PubMed] [Google Scholar]
- 23.Yonova-Doing E., Hysi P.G., Venturini C., Williams K.M., Nag A., Beatty S., Liew S.H., Gilbert C.E., Hammond C.J. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp. Eye Res. 2013;115:172–177. doi: 10.1016/j.exer.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trieschmann M., van Kuijk F.J., Alexander R., Hermans P., Luthert P., Bird A.C., Pauleikhoff D. Macular pigment in the human retina: Histological evaluation of localization and distribution. Eye (Lond.) 2008;22:132–137. doi: 10.1038/sj.eye.6702780. [DOI] [PubMed] [Google Scholar]
- 25.Thurnham D.I., Howard A.N. Studies on meso-zeaxanthin for potential toxicity and mutagenicity. Food Chem. Toxicol. 2013;59:455–463. doi: 10.1016/j.fct.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Howells O., Eperjesi F., Bartlett H. Measuring macular pigment optical density in vivo: A review of techniques. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011;249:315–347. doi: 10.1007/s00417-010-1577-5. [DOI] [PubMed] [Google Scholar]
- 28.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 30.Weigert G., Kaya S., Pemp B., Sacu S., Lasta M., Werkmeister R.M., Dragostinoff N., Simader C., Garhöfer G., Schmidt-Erfurth U., et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2011;52:8174–8178. doi: 10.1167/iovs.11-7522. [DOI] [PubMed] [Google Scholar]
- 31.Arnold C., Jentsch S., Dawczynski J., Böhm V. Age-related macular degeneration: Effects of a short-term intervention with an oleaginous kale extract-a pilot study. Nutrition. 2013;29:1412–1417. doi: 10.1016/j.nut.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 32.García-Layana A., Recalde S., Alamán A.S., Robredo P.F. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients. 2013;5:543–551. doi: 10.3390/nu5020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawczynski J., Jentsch S., Schweitzer D., Hammer M., Lang G.E., Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013;251:2711–2723. doi: 10.1007/s00417-013-2376-6. [DOI] [PubMed] [Google Scholar]
- 34.Murray I.J., Makridaki M., van der Veen R.L., Carden D., Parry N.R., Berendschot T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Invest. Ophthalmol. Vis. Sci. 2013;54:1781–1788. doi: 10.1167/iovs.12-10715. [DOI] [PubMed] [Google Scholar]
- 35.Arnold C., Winter L., Fröhlich K., Jentsch S., Dawczynski J., Jahreis G., Böhm V. Macular xanthophylls and ω-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: A randomized trial. JAMA Ophthalmol. 2013;131:564–572. doi: 10.1001/jamaophthalmol.2013.2851. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y.M., Dou H.L., Huang F.F., Xu X.R., Zou Z.Y., Lu X.R., Lin X.M. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: A randomised, double-blind, placebo-controlled trial. Br. J. Ophthalmol. 2015;99:371–375. doi: 10.1136/bjophthalmol-2014-305503. [DOI] [PubMed] [Google Scholar]
- 37.Kvansakul J., Rodriguez-Carmona M., Edgar D.F., Barker F.M., Köpcke W., Schalch W., Barbur J.L. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic. Physiol. Opt. 2006;26:362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 38.Bone R.A., Landrum J.T., Cao Y., Howard A.N., Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr. Metab. (Lond.) 2007;4:12. doi: 10.1186/1743-7075-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson E.J., Chung H.Y., Caldarella S.M., Snodderly D.M. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am. J. Clin. Nutr. 2008;87:1521–1529. doi: 10.1093/ajcn/87.5.1521. [DOI] [PubMed] [Google Scholar]
- 40.Bone R.A., Landrum J.T. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch. Biochem. Biophys. 2010;504:50–55. doi: 10.1016/j.abb.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolan J.M., Loughman J., Akkali M.C., Stack J., Scanlon G., Davison P., Beatty S. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vis. Res. 2011;51:459–469. doi: 10.1016/j.visres.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Landrum J., Bone R., Mendez V., Valenciaga A., Babino D. Comparison of dietary supplementation with lutein diacetate and lutein: A pilot study of the effects on serum and macular pigment. Acta Biochim. Pol. 2012;59:167–169. [PubMed] [Google Scholar]
- 43.Yao Y., Qiu Q.H., Wu X.W., Cai Z.Y., Xu S., Liang X.Q. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition. 2013;29:958–964. doi: 10.1016/j.nut.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Bovier E.R., Hammond B.R. A randomized placebo-controlled study on the effects of lutein and zeaxanthin on visual processing speed in young healthy subjects. Arch. Biochem. Biophys. 2015;572:54–57. doi: 10.1016/j.abb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Nolan J.M., Power R., Stringham J., Dennison J., Stack J., Kelly D., Moran R., Akuffo K., Corcoran L., Beatty S. Enrichment of Macular Pigment Enhances Contrast Sensitivity in Subjects Free of Retinal Disease: Central Retinal Enrichment Supplementation Trials—Report 1. Invest. Ophthalmol. Vis. Sci. 2016;57:3429–3439. doi: 10.1167/iovs.16-19520. [DOI] [PubMed] [Google Scholar]
- 46.Beatty S., Murray I.J., Henson D.B., Carden D., Koh H.H., Boulton M.E. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest. Ophthalmol. Vis. Sci. 2001;42:439–446. [PubMed] [Google Scholar]
- 47.Neuringer M., Sandstrom M.M., Johnson E.J., Snodderly D.M. Nutritional manipulation of primate retinas, I: Effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 48.Hammond B.R., Fletcher L.M., Roos F., Wittwer J., Schalch W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Invest. Ophthalmol. Vis. Sci. 2014;55:8583–8589. doi: 10.1167/iovs.14-15573. [DOI] [PubMed] [Google Scholar]
- 49.Lien E.L., Hammond B.R. Nutritional influences on visual development and function. Prog. Retin. Eye Res. 2011;30:188–203. doi: 10.1016/j.preteyeres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Kumar N., Mrejen S., Fung A.T., Marsiglia M., Loh B.K., Spaide R.F. Retinal pigment epithelial cell loss assessed by fundus autofluorescence imaging in neovascular age-related macular degeneration. Ophthalmology. 2013;120:334–341. doi: 10.1016/j.ophtha.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 51.Thomson L.R., Toyoda Y., Delori F.C., Garnett K.M., Wong Z.Y., Nichols C.R., Cheng K.M., Craft N.E., Dorey C.K. Long term dietary supplementation with zeaxanthin reduces photoreceptor death in light-damaged Japanese quail. Exp. Eye Res. 2002;75:529–542. doi: 10.1006/exer.2002.2050. [DOI] [PubMed] [Google Scholar]
- 52.Yu C.C., Nandrot E.F., Dun Y., Finnemann S.C. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking αvβ5 integrin. Free Radic. Biol. Med. 2012;52:660–670. doi: 10.1016/j.freeradbiomed.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bone R.A., Landrum J.T., Mayne S.T., Gomez C.M., Tibor S.E., Twaroska E.E. Macular pigment in donor eyes with and without AMD: A case-control study. Invest. Ophthalmol. Vis. Sci. 2001;42:235–240. [PubMed] [Google Scholar]
- 54.Biesalski H.K., Aggett P.J., Anton R., Bernstein P.S., Blumberg J., Heaney R.P., Henry J., Nolan J.M., Richardson D.P., van Ommen B., et al. 26th Hohenheim Consensus Conference, September 11, 2010 Scientific substantiation of health claims: Evidence-based nutrition. Nutrition. 2011;27:S1–S20. doi: 10.1016/j.nut.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Puell M.C., Palomo-Alvarez C., Barrio A.R., Gómez-Sanz F.J., Pérez-Carrasco M.J. Relationship between macular pigment and visual acuity in eyes with early age-related macular degeneration. Acta. Ophthalmol. 2013;91:e298–e303. doi: 10.1111/aos.12067. [DOI] [PubMed] [Google Scholar]
- 56.Johnson E.J., Maras J.E., Rasmussen H.M., Tucker K.L. Intake of lutein and zeaxanthin differ with age, sex and ethnicity. J. Am. Diet. Assoc. 2010;110:1357–1362. doi: 10.1016/j.jada.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Trumbo P.R., Ellwood K.C. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: An evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Am. J. Clin. Nutr. 2006;84:971–974. doi: 10.1093/ajcn/84.5.971. [DOI] [PubMed] [Google Scholar]
- 58.Granado F., Blázquez S., Olmedilla B. Changes in carotenoid intake from fruit and vegetables in the Spanish population over the period 1964–2004. Public Health Nutr. 2007;10:1018–1023. doi: 10.1017/S1368980007662314. [DOI] [PubMed] [Google Scholar]
- 59.Wisniewska A., Subczynski W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006;41:1257–1265. doi: 10.1016/j.freeradbiomed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Vachali P.P., Besch B.M., Gonzalez-Fernandez F., Bernstein P.S. Carotenoids as possible interphotoreceptor retinoid-binding protein (IRBP) ligands: A surface plasmon resonance (SPR) based study. Arch. Biochem. Biophys. 2013;539:181–186. doi: 10.1016/j.abb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhosale P., Li B., Sharifzadeh M., Gellermann W., Frederick J.M., Tsuchida K., Bernstein P.S. Purification and partial characterization of a lutein-binding protein from human retina. Biochemistry. 2009;48:4798–4807. doi: 10.1021/bi9004478. [DOI] [PubMed] [Google Scholar]
- 62.Schalch W., Cohn W., Barker F.M., Köpcke W., Mellerio J., Bird A.C., Robson A.G., Fitzke F.F., van Kuijk F.J. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin-the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 2007;458:128–135. doi: 10.1016/j.abb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 63.Bhosale P., Bernstein P.S. Vertebrate and invertebrate carotenoid-binding proteins. Arch. Biochem. Biophys. 2007;458:121–127. doi: 10.1016/j.abb.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nolan J.M., Beatty S., Meagher K.A., Howard A.N., Kelly D., Thurnham D.I. Verification of Meso-Zeaxanthin in Fish. J. Food Process Technol. 2014;5:335. doi: 10.4172/2157-7110.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beaty S., Boulton M., Henson D., Koh H.H., Murray I.J. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999;83:867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akuffo K.O., Beatty S., Stack J., Dennison J., O’Regan S., Meagher K.A., Peto T., Nolan J. Central Retinal Enrichment Supplementation Trials (CREST): Design and methodology of the CREST randomized controlled trials. Ophthalmic. Epidemiol. 2014;21:111–123. doi: 10.3109/09286586.2014.888085. [DOI] [PMC free article] [PubMed] [Google Scholar]