Abstract
Purpose
To compare the effects of resistance training versus passive physical therapy on bone survival in the metastatic bone during radiation therapy (RT) as combined treatment in patients with spinal bone metastases. Secondly, to evaluate overall survival and progression-free-survival (PFS) as well as to quantify prognostic factors of bone survival after combined treatment.
Methods
In this randomized trial 60 patients were allocated from September 2011 until March 2013 into one of the two groups: resistance training (group A) or passive physical therapy (group B) with thirty patients in each group during RT. We estimated patient survival using Kaplan-Meier survival method. The Wald-test was used to evaluate the prognostic importance of pathological fracture, primary site, Karnofsky performance status, localization of metastases, number of metastases, and cerebral metastases.
Results
Median follow-up was 10 months (range 2–35). Bone survival showed no significant difference between groups (p = .303). Additionally no difference between groups could be detected in overall survival (p = .688) and PFS (p = .295). Local bone progression was detected in 16.7 % in group B, no irradiated bone in group A showed a local progression over the course (p = 0.019). In univariate analysis breast cancer, prostate cancer, and the presence of cerebral metastases had a significant impact on bone survival in group B, while no impact could be demonstrated in group A.
Conclusions
In this group of patients with spinal bone metastases we were able to show that guided resistance training of the paravertebral muscles had no essential impact on survival concomitant to RT. Importantly, no local bone progression in group A was detected, nevertheless no prognostic factor for combined treatment could be evaluated.
Trial registration
Clinical trial identifier NCT 01409720. Registered 8 February 2011.
Keywords: Bone metastases, Spine, Resistance training, Survival, Palliative radiotherapy
Background
Spinal bone metastases represent the most frequent site of skeletal metastasis [1], and two-thirds of all tumor patients are estimated to develop bone metastases in the course of their disease [2]. Bone metastases are a major clinical concern due to severe pain, pathological fractures, spinal cord compression and hypercalcaemia with a significant decrease of the quality of life (QoL) [3, 4]. Radiotherapy (RT) is the most common treatment option of bone metastases in advanced tumor disease [4–6], and is effective in reducing symptoms, increases subjective well-being, and has minimal side effects [7]. The classification into stable and unstable bone metastases and pathological fractures are of great clinical relevance regarding mobility and QoL in patients’ palliative care. Most patients with spinal metastases have a limited life expectancy [8]. The early initiation of therapy, which can generally be viewed as being given as palliative therapy, brings about a significant improvement of the QoL and appears to prolong the survival time [9]. The median overall survival varies from 7 to 32 months, depending on significant predictors e.g., Karnofsky performance score (KPS), primary tumor, and the absence of visceral metastases [8, 10–12]. Previously we showed that within our study guided resistance training of the paravertebral muscles could safety be practiced in palliative patients with stable bone metastases of the vertebral column; leading to an improved pain score and mobility as well as reduced fatigue and thereby an enhanced QoL [13, 14]. Secondary, we were able to show that resistance training concomitant to RT can improve pain relief, and improve bone density in the metastasis as a local response over a 6-months period [15, 16]. However, in our recent work, we analyzed the endpoints feasibility, QoL, local response, and pain of resistance training in patients with spinal bone metastases under RT until 6 months. The aim of this analysis was to compare the bone survival of patients with spinal bone metastases under resistance training versus passive physical therapy concomitant to RT. Secondary endpoints were overall survival, progression free survival, and to quantify prognostic factors to bone survival after combined treatment.
Methods
This is a randomized, controlled, two-armed intervention trial. A block randomization approach with block size 6 was used to ensure that the two groups were balanced. Inclusion criteria were an age of 18 to 80 years, KPS [17] ≥ 70, written consent to participate, and already initiated bisphosphonate therapy. The patients were subjected to a staging of their vertebral column within the context of the computed tomography (CT) designed to plan the radiation schedule prior to enrolment into the trial. In this examination metastases were classified as “stable” or “unstable”. This was diagnosed independently by a specialist for radiology as well as by a specialist for orthopedic surgery. The specifications for an unstable vertebral body were tumor occupancy more than 60 % of the vertebral body, and pedicle destruction [18]. Only a metastasis classified by both specialists as “stable” was suggested eligible for inclusion. After the baseline measurements, the patients with stable bone metastases were assigned to the respective treatment groups on a 1:1 basis according to the randomization list. Group A (intervention group, resistance training) and in group B (control group, passive physical therapy) each consisted of 30 patients. The primary endpoint was to compare bone survival between the two groups. Secondary endpoints were to quantify overall survival, progression free survival (PFS), and prognostic factors for bone survival. Local bone progression was defined as progressive treated bone metastasis, while systemic progressive bone was defined as additional bone metastases to the treated site. Progressive disease was defined as local progression and/or systemic progressive bone and/or death. PFS was the time between first diagnosis or existence of bone metastases (time equalized to the start of RT) until progressive disease or death. The progression of bone disease was estimated by CT scans 3, 6, 12 and 24 months after RT. The progressive treated bone metastases were classified by MDA criteria [19]. Bone survival was the time from first diagnosis or existence of bone metastases (time equalized to the start of RT) until death, and overall survival was the time from first diagnosis of primary site until death. Bone metastases distant from the irradiated site were not included. Patient-specific data was documented. The study was approved by the Heidelberg Ethics Committee (S-316/2011).
Radiotherapy
Radiotherapy was performed in the Department of Radiation Oncology at the University Hospital Heidelberg. After virtual simulation was performed for treatment planning, radiotherapy was carried out over a dorsal photon field of 6MV energy range. Primary target volume (PTV) covered the specific vertebral body affected as well as the ones immediately above and below. In group A twenty-four patients (80 %) were treated with 10 × 3 Gy, three patients (10 %) with 14 × 2.5 Gy, and three patients (10 %) with 20 × 2 Gy. In group B the dose fractions for twenty-eight patients (93.3 %) were 10 × 3 Gy, for one patient (3.3 %) 14 × 2.5 Gy, and for one patient (3.3 %) 20 × 2 Gy. The median individual dose in all patients was 3 Gy (range 2–3 Gy), the median total dose 30 Gy (range 20–35 Gy). The individual and total doses were decided separately for each individual patient, depending on histology, patient’s general state of health, current staging and the corresponding prognosis.
Exercise interventions
The interventions commenced on the same day as radiotherapy and were performed on each day of RT treatment (Monday through Friday) over a 2-week period, independent of the number of fractions. During the 2-week RT period, the patients in the resistance training group (group A) performed the exercises under the guidance of a trained physiotherapist. The patients were then instructed to perform all trainings at home three times per week until the endpoint assessment after 6 months. Self-reported training adherence was registered in a training diary. The resistance training lasted approx. 30 min, the passive physical therapy (group B) approx. 15 min. Since the site of the bone metastases differed from patient to patient, three different exercises were enacted to ensure an even resistance training of the muscles along the entire vertebral column. A detailed description of the exercise interventions was published earlier [16, 20].
Statistical approach
On account of the explorative character of this study it was not possible to estimate the total number of cases; with a scheduled number of 30 patients per group, it will, however, be possible to detect a standardized mean-value effect of 0.74 with a power of 80 % and an α significance level of 5 %. We calculated descriptive p-values of the corresponding statistical tests comparing the treatment groups. Wilcoxon u test was used for difference between groups. We estimated patient survival using Kaplan-Meier survival method. Patients were censored on the basis of whether they were alive. The Wald-test was used to evaluate the prognostic importance of pathological fracture (yes/no), primary site (non-small cell lung cancer (NSCLC), breast cancer, prostate cancer, other), KPS (70/>70), localization of metastases (thoracic/lumbar), number of metastases (1/>1), and cerebral metastases. The results were reported as survival times, p-values, hazard ratios including 95 % confidence intervals (CI). For all analysis, a p-value of 0.05 or less was considered significant. All statistical analyses were done using SAS software Version 9.3 (SAS Institute, Cary, NC, USA).
Results
From September 2011 through March 2013, consecutively 80 patients with a histologically confirmed cancer of any primary and spinal bone metastases of the thoracic or lumbar segments were considered in the Department of Radiation Oncology at the University Hospital Heidelberg. Fifteen patients were excluded due to unstable metastases, and five patients declined to participate in the study. Sixty patients fulfilled the inclusion criteria and were enrolled into the trial. Groups were balanced at baseline, and except for visceral metastases there were no group differences (Table 1). The median follow-up was 10 months (range 2–35) for both groups. All surviving patients completed all surveys. Mortality did not differ between groups.
Table 1.
Patient characteristics at baseline
| Intervention group A (n = 30) | Control group B (n = 30) | P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| Mean (SD) | 61.3 +/- 10.1 | 64.1 +/- 10.9 | 0.304 | ||
| Gender | |||||
| Male | 14 | 46.7 | 19 | 63.3 | 0.195 |
| Female | 16 | 53.3 | 11 | 36.7 | |
| Karnofsky-index (median, range) | 80 (70–100) | 80 (70–100) | 0.114 | ||
| Primary site | |||||
| Lung cancer | 12 | 40.0 | 8 | 26.6 | 0.320 |
| Breast cancer | 5 | 16.7 | 6 | 20.0 | 0.542 |
| Prostate cancer | 5 | 16.7 | 9 | 30.0 | 0.156 |
| Melanoma | 1 | 3.3 | 1 | 3.3 | 1.000 |
| Renal cancer | 1 | 3.3 | 2 | 6.7 | 0.875 |
| Other | 6 | 20.0 | 4 | 13.4 | 0.325 |
| Localization metastases | 0.717 | ||||
| Thoracic | 17 | 56.7 | 14 | 46.7 | |
| Lumbar | 9 | 30.0 | 13 | 43.3 | |
| Thoracic and lumbar | 2 | 6.7 | 2 | 6.7 | |
| Sacrum | 2 | 6.7 | 1 | 3.3 | |
| Number metastases | 0.257 | ||||
| Mean (range) | 1.4 (2–4) | 1.7 (1–5) | |||
| Solitary | 22 | 73.3 | 18 | 60.0 | |
| Multiple | 8 | 26.7 | 12 | 40.0 | |
| Type of metastases | 0.781 | ||||
| osteoblast | 9 | 30.0 | 10 | 33.3 | 0.956 |
| osteolytic | 21 | 70.0 | 20 | 66.7 | 0.935 |
| Distant metastases at baseline | |||||
| Visceral | 12 | 40.0 | 5 | 16.7 | 0.045 |
| brain | 3 | 10.0 | 4 | 13.4 | 0.688 |
| lung | 7 | 23.3 | 4 | 13.4 | 0.320 |
| tissue | 8 | 26.7 | 6 | 20.0 | 0.542 |
| Pathological fractures | 6 | 20.0 | 9 | 30.0 | 0.371 |
| Hormonotherapy | 10 | 33.3 | 16 | 53.3 | 0.118 |
| Immunotherapy | 7 | 23.3 | 5 | 16.7 | 0.519 |
| Chemotherapy | 25 | 83.3 | 20 | 66.7 | 0.136 |
| Neurological deficit | 0 | 0.0 | 2 | 6.7 | 0.150 |
| Orthopedic corset at baseline | 7 | 23.3 | 5 | 16.7 | 0.519 |
| Radiotherapy dose completed (Gy) | |||||
| single dose (median, range) | 3 (2–4) | 3 (2–4) | 1.000 | ||
| cumulative dose (median, range) | 30 (30–40) | 30 (30–40) | 1.000 | ||
Bone survival showed no significant difference between groups (p = 0.303); bone survival after 12 and 24 months was 58 and 42 % in group A, and 51 and 30 % in group B (Fig. 1). Overall survival after 12 and 24 months was 80 and 63 % in group A, and 70 and 57 % in group B respectively (p=0.688; Fig. 2). The PFS did not differ between groups; mean PFS was 24.3 months in group A and 20.5 months in group B (p = 0.295; Fig. 3).
Fig. 1.
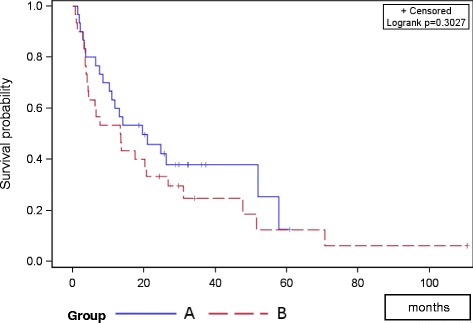
Progression free survival of both groups
Fig. 2.
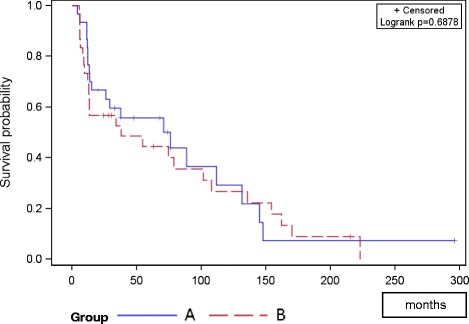
Overall survival of both groups
Fig. 3.
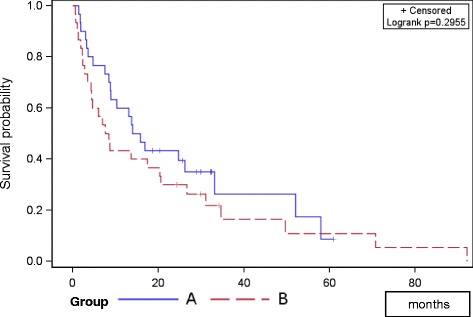
Bone survival of both groups
Local progression was detected in 16.7 % in group B, no irradiated bone in group A showed a local progression over the course (p = 0.019). Progressive disease and systemic bone progression showed no difference between groups (p = 0.095 and p = 0.108; Table 2).
Table 2.
Tumor progression of both groups
| Group A | Group B | ||||
|---|---|---|---|---|---|
| n | % | n | % | P-value | |
| Progressive disease | 22 | 73.3 | 27 | 90.0 | 0.095 |
| Local bone progression | 0 | 0.0 | 5 | 16.7 | 0.019 |
| Systemic bone progression | 8 | 26.7 | 14 | 46.7 | 0.108 |
In univariate analysis breast cancer (HR 0.103, 95 % CI 0.024–0.442, p = 0.002), prostate cancer (HR 0.160, 95 % CI 0.050–0.511, p=0.002), and the presence of cerebral metastases (HR 3.211, 95 % CI 1.063–9.695, p = 0.038) showed a significant impact on bone survival to group B, while no impact in group A could be demonstrated (Table 3).
Table 3.
Univariate analysis for prognostic factors of bone survival
| Intervention group (n = 30) | Control group (n = 30) | |||||
|---|---|---|---|---|---|---|
| HR | CI 95 % | P-value | HR | CI 95 % | P-value | |
| Pathological fracture | 1.288 | 0.371–4.467 | 0.690 | 0.833 | 0.343–2.020 | 0.686 |
| KPS | 0.527 | 0.216–1.289 | 0.161 | 0.872 | 0.372–2.043 | 0.752 |
| Localization | 0.588 | 0.209–1.655 | 0.315 | 1.166 | 0.504–2.694 | 0.720 |
| Number of metastases | 0.602 | 0.200–1.812 | 0.366 | 1.052 | 0.469–2.360 | 0.902 |
| Breast cancer | 0.230 | 0.028–1.923 | 0.175 | 0.103 | 0.024–0.442 | 0.002 |
| NSCLC | 2.442 | 0.834–7.145 | 0.103 | 0.968 | 0.346–2.712 | 0.951 |
| Prostate cancer | 0.950 | 0.237–3.804 | 0.942 | 0.160 | 0.050–0.511 | 0.002 |
| Cerebral metastases | 1.529 | 0.409–5.716 | 0.528 | 3.211 | 1.063 − 9.695 | 0.038 |
Discussion
The first results of this novel trial showed that guided resistance training of the paravertebral muscles can safely be practiced in palliative patients with stable bone metastases of the vertebral column. Furthermore improved pain and local response, reduced fatigue and enhanced QoL could be detected within 6 months.
In our current analysis, bone survival, overall survival, and PFS showed no significant differences between groups in long-term follow-up. The effect of resistance training showed an improved local response in group A [16], and no local bone progression at the irradiated bone metastases could be detected, while in group B 16.7 % of patients expanded a progression in the vertebral body. Nevertheless these data had no impact on PFS. Overall survival and bone survival showed no differences as well. We interpreted this result as minor impact of resistance training. On the one hand, at baseline visceral metastases were significantly higher in group A, which represents a prognostic factor for survival in the literature, on the other hand a positive tendency for group A could be shown in overall survival and bone survival. Additional small sample size, and different primary tumor types played a major role on the assessment. The most prevalent tumors were those of breast and prostate [21]. These tumor entities had an improved bone survival in group B, but showed no impact in group A. This result explained itself on account of several additional distant metastases which were detected in group A collectively. In a study by van der Linden et al. involving 342 patients with spinal metastases, the most important prognostic factors were performance status, metastatic involvement of other organs and primary site [8]. In the study by Katagiri et al. [22], primary tumor, performance status, number of bone metastases, metastatic involvement of other organs and previous chemotherapy regimens constituted important prognostic factors among 350 patients with bone metastases. In a retrospective analysis of 356 patients, Rades et al. [23] identified that improved survival was significantly associated with female gender, an Eastern Cooperative Oncology Group performance score (ECOG-PS) of 1–2, pre-RT ambulatory status, the absence of other bone metastases, the absence of visceral metastases, an interval from cancer diagnosis to RT of >15 months and slower (>7 days) development of motor deficits. Our trial was not able to demonstrate a survival benefit of resistance training concomitant to RT, and identified only tumor type and cerebral metastases as prognostic factors in our control group. However, the knowledge of prognostic factors and of the prognosis following bone metastasis is of critical importance. A paper by Sugiura et al. [24] considering 118 patients with bone metastases showed a 1-year survival rate of 31.6 % and a 2-year survival rate of 11.3 % for lung cancer. Overall survival rates of patients with renal cell carcinoma were described with 74 % after one year [25]. Correspondingly, the survival rates especially differ in the literature depending on the primary tumor. Based on the different primary types in our trial, these data are not comparable.
Bone metastases are among the most serious problems seen in tumor patients and bone pain, pathological fractures and neurological deficits can be life-threatening events [26]. Palliative RT constitutes one of the most important therapeutic options in these situations. In our recent work, we were able to demonstrate a benefit in QoL, pain response, local response, and reduced fatigue for patients after combined treatment with resistance training concomitant to RT. However, our results showed no differences in survival. In our opinion, the combined treatment with resistance training concomitant to RT is a very effective novel treatment. Future trial designs should stratify to primary tumor and visceral metastases. Further limitations of the study were the relatively small sample size, the variety of primary tumors and patient conditions, and the exclusion of patients presenting with cervical spine metastases. Among the strengths of our novel and original study were the randomized design and long-term follow-up among palliative patients with spinal bone metastases.
Conclusion
In this group of patients with spinal bone metastases we were able to show that guided resistance training of the paravertebral muscles had no essential impact on bone survival, overall survival, and progression free survival concomitant to RT. Importantly, the absence of local bone progression in group A could be detected, nevertheless no prognostic factor for combined treatment was evaluated.
Acknowledgements
This work was supported by a Heidelberg University young investigator grant to H. Rief. We thank our German Bone Cancer Research Group Members for their great effort. The authors thank all of the study participants for their participation.
Funding
Not applicable.
Availability of data and materials
The data used in this analysis is from publications available in the public domain.
Authors’contributions
HR and JD developed and planned this trial. TB was responsible for statistical considerations/basis of the analysis. TW estimated the stability of bone metastases. HR, IS, TBo, and RF performed the examinations and RT supervisions. HR made the data collection and performed the resistance training. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Heidelberg Ethics Committee approved this study on 1st August 2011 (S-316/2011).
Contributor Information
Harald Rief, Phone: 49-6221-56-8202, Email: harald.rief@med.uni-heidelberg.de.
Thomas Bruckner, Email: bruckner@imbi.uni-heidelberg.de.
Ingmar Schlampp, Email: ingmar.schlampp@med.uni-heidelberg.de.
Tilman Bostel, Email: tilmann.bostel@med.uni-heidelberg.de.
Thomas Welzel, Email: thomas.welzel@med.uni-heidelberg.de.
Jürgen Debus, Email: juergen.debus@med.uni-heidelberg.de.
Robert Förster, Email: robert.foerster@med.uni-heidelberg.de.
References
- 1.Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 2.Shaw B, Mansfield FL, Borges L. One-stage posterolateral decompression and stabilization for primary and metastatic vertebral tumors in the thoracic and lumbar spine. J Neurosurg. 1989;70:405–410. doi: 10.3171/jns.1989.70.3.0405. [DOI] [PubMed] [Google Scholar]
- 3.Wyne CM, Hu SS, Lotz JC. Biomechanically derived guidline equations for burst fracture risk prediction in the metastatically involved spine. J Spin Disorders & Techniques. 2003;16(2):180–185. doi: 10.1097/00024720-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Janjan N, Lutz ST, Bedwinek JM, et al. Therapeutic guidelines for the treatment of bone metastasis: a report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med. 2009;12:417–426. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 5.Mitera G, Probyn L, Ford M, Donovan A, Rubenstein J, Finkelstein J, Christakis M, Zhang L, Campos S, Culleton S, Nguyen J, Sahgal A, Barnes E, Tsao M, Danjoux C, Holden L, Yee A, Khan L, Chow E. Correlation of computed tomography imaging features with pain response in patients with spine metastases after radiation therapy. Int J Radiation Oncol Biol Phys. 2011;81(3):827–830. doi: 10.1016/j.ijrobp.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Wu JS, Monk G, Clark T, Robinson J, Eigl BJ, Hagen N. Palliative radiotherapy improves pain and reduces functional interference in patients with painful bone metastases: a quality assurance study. Clin Oncol. 2006;18:539–544. doi: 10.1016/j.clon.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.van Oorschot B, Schuler M, Simon A, Schleicher U, Geinitz H. Patterns of care and course of symptoms in palliative radiotherapy: a multicenter pilot study analysis. Strahlenther Onkol. 2011;187(8):461–466. doi: 10.1007/s00066-011-2231-9. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103(2):320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 9.Klimo P, Jr, Kestle JR, Schmidt MH. Clinical trials and evidence-based medicine for metastatic spine disease. Neurosurg Clin N Am. 2004;15:549–564. doi: 10.1016/j.nec.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76(8):1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::AID-CNCR2820760824>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T, Siemionow KB, Mroz TE, Podichetty V, Lieberman IH. A prospective analysis of prognostic factors in patients with spinal metastases: use of the revised Tokuhashi score. Spine (Phila Pa 1976) 2011;36(11):910–917. doi: 10.1097/BRS.0b013e3181e56ec1. [DOI] [PubMed] [Google Scholar]
- 12.Foerster R, Bruckner T, Bostel T, Schlampp I, Debus J, Rief H. Prognostic factors for survival of women with unstable spinal bone metastases from breast cancer. Radiat Oncol. 2015;10:144. doi: 10.1186/s13014-015-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rief H, Omlor G, Akbar M, Welzel T, Bruckner T, Rieken S, Haefner MF, Schlampp I, Gioules A, Habermehl D, von Nettelbladt F, Debus J. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy - first results of a randomized pilot trial. BMC Cancer. 2014;14:67. doi: 10.1186/1471-2407-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rief H, Akbar M, Keller M, Omlor G, Welzel T, Bruckner T, Rieken S, Häfner MF, Schlampp I, Gioules A, Debus J. Quality of life and fatigue of patients with spinal bone metastases under combined treatment with resistance training and radiation therapy- a randomized pilot trial. Radiat Oncol. 2014;9(1):151. doi: 10.1186/1748-717X-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rief H, Welzel T, Omlor G, Akbar M, Bruckner T, Rieken S, Haefner MF, Schlampp I, Gioules A, Debus J. Pain response of resistance training of the paravertebral musculature under radiotherapy in patients with spinal bone metastases-a randomized trial. BMC Cancer. 2014;14:485. doi: 10.1186/1471-2407-14-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rief H, Petersen LC, Omlor G, Akbar M, Bruckner T, Rieken S, Haefner MF, Schlampp I, Förster R, Debus J. Welzel T; German Bone Research Group. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients - A randomized trial. Radiother Oncol. 2014;112(1):133–139. doi: 10.1016/j.radonc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Karnofsky DA, Burchenal JH. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, editors. Evaluation of Chemotherapeutic Agents. New York; 1949. p. 191–205.
- 18.Taneichi H, Kaneda K, Takeda N, Abumi K, Satoh S. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine. 1997;22:239–245. doi: 10.1097/00007632-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Hamaoka T, Costelloe CM, Madewell JE, Liu P, Berry DA, Islam R, Theriault RL, Hortobagyi GN, Ueno NT. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102(4):651–657. doi: 10.1038/sj.bjc.6605546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rief H, Jensen AD, Bruckner T, Herfarth K, Debus J. Isometric muscle training of the spine musculature in patients with spinal bony metastases under radiation therapy. BMC Cancer. 2011;11:482. doi: 10.1186/1471-2407-11-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bollen L, van der Linden YM, Pondaag W, Fiocco M, Pattynama BP, Marijnen CA, Nelissen RG, Peul WC, Dijkstra PD. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1,043 patients. Neuro Oncol. 2014;16(7):991–998. doi: 10.1093/neuonc/not318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katagiri H, Takahashi M, Wakai K, Sugiura H, Kataoka T, Nakanishi K. Prognostic factors and a scoring system for patients with skleletal metastasis. J Bone Joint Surg (Br) 2005;87:698–703. doi: 10.1302/0301-620X.87B5.15185. [DOI] [PubMed] [Google Scholar]
- 23.Rades D, Douglas S, Veninga T, Bajrovic A, Stalpers LJ, Hoskin PJ, Rudat V, Schild SE. Metastatic spinal cord compression in non-small cell lung cancer patients. Prognostic factors in a series of 356 patients. Strahlenther Onkol. 2012;188:472–476. doi: 10.1007/s00066-012-0086-3. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466(3):729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Huang X, Sun F, Ma R, Wang H, Shao K, Zhu Y, Zhou W, Xu Z, Shen Z. Can Urol Assoc J. Prognostic factors for overall survival with targeted therapy in Chinese patients with metastatic renal cell carcinoma. Can Urol Assoc J. 2014;8(11–12):E821–E827. doi: 10.5489/cuaj.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–232. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this analysis is from publications available in the public domain.


