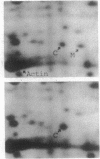
Abstract
The rate of spontaneous mutation resulting in electrophoretic variants per cell generation in a human lymphoblastoid cell line, on the basis of experiments described in this paper, is found to be 7.2 x 10(-8) per locus. A review of similar data on electrophoretic variants resulting from spontaneous mutation in the human germ line leads to an estimate of 3.3 x 10(-8) per locus per cell generation. It is argued that the similarity of these two estimates, despite an average cell generation time of 18.5 hr for the cultured somatic cells but about 26 days in the germ line, suggests that spontaneous mutation involving nucleotide substitutions is much more dependent on cell generation than on time. This finding permits the inference that environmental (exogenous) variables make a relatively small contribution to the rate of this type of human germinal spontaneous mutation. While in vitro somatic-cell mutation rates, such as derived in this study, provide a basis for modeling the contribution of nucleotide substitutions in multihit/clonal theories of carcinogenesis, it is also argued that the complex of events involved in carcinogenesis, including chromosomal rearrangements and mitotic recombination, could have very different individual probabilities. Estimates for the rates of these other types of mutation are needed to provide a better understanding of the manner in which multiple mutations accumulate in malignant cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awa A. A., Neel J. V. Cytogenetic "rogue" cells: what is their frequency, origin, and evolutionary significance? Proc Natl Acad Sci U S A. 1986 Feb;83(4):1021–1025. doi: 10.1073/pnas.83.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Preisinger A. C., Jessup J. M., Paraskeva C., Markowitz S., Willson J. K., Hamilton S., Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990 Dec 1;50(23):7717–7722. [PubMed] [Google Scholar]
- Bloom A. D., Neel J. V., Choi K. W., Iida S., Chagnon N. Chromosome aberrations among the Yanomamma Indians. Proc Natl Acad Sci U S A. 1970 Jul;66(3):920–927. doi: 10.1073/pnas.66.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R., Neel J. V. Description and validation of a method for simultaneous estimation of effective population size and mutation rate from human population data. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9407–9411. doi: 10.1073/pnas.86.23.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Boehnke M., Hanash S. M., Kuick R. D., Lamb B. J., Neel J. V., Niezgoda W., Pivirotto S., Sundling G. Estimation of mutation rates based on the analysis of polypeptide constituents of cultured human lymphoblastoid cells. Genetics. 1988 Jul;119(3):693–703. doi: 10.1093/genetics/119.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fox D. P., Robertson F. W., Brown T., Whitehead A. R., Douglas J. D. Chromosome aberrations in divers. Undersea Biomed Res. 1984 Jun;11(2):193–204. [PubMed] [Google Scholar]
- Hanash S. M., Boehnke M., Chu E. H., Neel J. V., Kuick R. D. Nonrandom distribution of structural mutants in ethylnitrosourea-treated cultured human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):165–169. doi: 10.1073/pnas.85.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanash S. M., Strahler J. R., Neel J. V., Hailat N., Melhem R., Keim D., Zhu X. X., Wagner D., Gage D. A., Watson J. T. Highly resolving two-dimensional gels for protein sequencing. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5709–5713. doi: 10.1073/pnas.88.13.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff V., Meadows A., Riccardi V. M., Strong L. C., Saunders G. F. Parental origin of de novo constitutional deletions of chromosomal band 11p13. Am J Hum Genet. 1990 Jul;47(1):155–160. [PMC free article] [PubMed] [Google Scholar]
- Jadayel D., Fain P., Upadhyaya M., Ponder M. A., Huson S. M., Carey J., Fryer A., Mathew C. G., Barker D. F., Ponder B. A. Paternal origin of new mutations in von Recklinghausen neurofibromatosis. Nature. 1990 Feb 8;343(6258):558–559. doi: 10.1038/343558a0. [DOI] [PubMed] [Google Scholar]
- Kuick R. D., Skolnick M. M., Hanash S. M., Neel J. V. A two-dimensional electrophoresis-related laboratory information processing system: spot matching. Electrophoresis. 1991 Oct;12(10):736–746. doi: 10.1002/elps.1150121007. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991 Jun 15;51(12):3075–3079. [PubMed] [Google Scholar]
- Ludwig M., Grimm T., Brackmann H. H., Olek K. Parental origin of factor IX gene mutations, and their distribution in the gene. Am J Hum Genet. 1992 Jan;50(1):164–173. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. Average locus differences in mutability related to protein "class": a hypothesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2062–2066. doi: 10.1073/pnas.87.6.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. Frequency of spontaneous and induced "point" mutations in higher eukaryotes. J Hered. 1983 Jan-Feb;74(1):2–15. doi: 10.1093/oxfordjournals.jhered.a109711. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Mohrenweiser H. W., Rothman E. D., Naidu J. M. A revised indirect estimate of mutation rates in Amerindians. Am J Hum Genet. 1986 May;38(5):649–666. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Goriki K., Fujita M., Takahashi N., Asakawa J., Hazama R. The rate with which spontaneous mutation alters the electrophoretic mobility of polypeptides. Proc Natl Acad Sci U S A. 1986 Jan;83(2):389–393. doi: 10.1073/pnas.83.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Thompson E. A. Founder effect and number of private polymorphisms observed in Amerindian tribes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1904–1908. doi: 10.1073/pnas.75.4.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Skolnick M. M., Neel J. V. An algorithm for comparing two-dimensional electrophoretic gels, with particular reference to the study of mutation. Adv Hum Genet. 1986;15:55–160. doi: 10.1007/978-1-4615-8356-1_2. [DOI] [PubMed] [Google Scholar]
- Stein W. D. Analysis of cancer incidence data on the basis of multistage and clonal growth models. Adv Cancer Res. 1991;56:161–213. doi: 10.1016/s0065-230x(08)60481-9. [DOI] [PubMed] [Google Scholar]
- Stewart F. M., Gordon D. M., Levin B. R. Fluctuation analysis: the probability distribution of the number of mutants under different conditions. Genetics. 1990 Jan;124(1):175–185. doi: 10.1093/genetics/124.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B. S. The origin of point mutations in human tumor cells. Cancer Res. 1992 Jan 15;52(2):249–253. [PubMed] [Google Scholar]
- Tawn E. J., Cartmel C. L., Pyta E. M. Cells with multiple chromosome aberrations in control individuals. Mutat Res. 1985 Dec;144(4):247–250. doi: 10.1016/0165-7992(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet. 1975;5:223–318. doi: 10.1007/978-1-4615-9068-2_4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wanner L. A., Neel J. V., Meisler M. H. Separation of allelic variants by two-dimensional electrophoresis. Am J Hum Genet. 1982 Mar;34(2):209–215. [PMC free article] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]