Abstract
Checkpoint inhibitors such as nivolumab represent a novel class of agents that are being increasingly used in the treatment of various cancers. Their toxicities represent unique challenges to the oncologists prescribing them, patients' primary care physicians and other specialists who may encounter these patients during consultations. It is important for physicians to remain vigilant and include autoimmune toxicities in the list of potential differential diagnoses in patients receiving novel cancer therapeutics who present with unusual toxicities. We report the unusual case of a 68-year-old woman with advanced lung cancer on the novel chemotherapeutic Nivolumab whom we suspect developed autoimmune myocarditis with significant cardiac conduction disease as an unintended, and as of yet unrecognised, side effect from this medication.
Background
Immunotherapy has rapidly become an important part of cancer treatment over the past several decades. Initial success has been primarily in the form of passive immunotherapy. However, with the US Food and Drug Administration (FDA) approval of the drug ipilimumab in 2011 for the treatment of patients with stage IV melanoma, T cell-directed immunotherapy with the goal of stimulating the immune system has assumed an increasing role.
Tumours cells are adept at evading the immune system through various mechanisms. One of these mechanisms involves downregulating T cell activation through the activation of immune checkpoint pathways and inhibitory T cell receptors. The first clinically validated checkpoint pathway was cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which is the target of and inhibited by ipilimumab.1 2 Programmed death-1 (PD-1) is another checkpoint receptor on activated T cells that mediates immune suppression when it encounters the PD-1 ligands, PD-L1 and PD-L.3 Blockade of this interaction via anti-PD-1 antibodies such as pembrolizumab and nivolumab has been shown to produce significant antitumour activity in multiple malignancies.4–10
Amplification of the immune system through immunotherapy has been associated with a unique panel of side effects known as immune-related adverse events (irAEs). Classic examples of these events include rash, pneumonitis, colitis, hepatitis and various endocrinopathies.11–14 We report a suspected case of autoimmune myocarditis with concomitant de novo conduction disease after treatment with the PD-1 inhibitor nivolumab.
Case presentation
This patient was a 68-year-old woman with refractory stage IV lung adenocarcinoma, deep venous thrombosis with pulmonary embolus on fondaparinux, a history of Wolff-Parkinson-White syndrome treated successfully with catheter ablation during her teenage and no other prior cardiac history who presented with altered mental status, nausea and vomiting. She was first diagnosed with metastatic lung cancer 4 years prior when she developed respiratory distress from a pulmonary embolus and required prolonged mechanical ventilation. She subsequently developed tracheal stenosis, and during the workup of her tracheal stenosis she was noted to have a 1.6×1.5 cm lung mass in the left lower lobe with evidence of extension into the fourth thoracic vertebrae. This was biopsied and confirmed to be metastatic lung adenocarcinoma with exon 21 epidermal growth factor receptor mutation positivity. She underwent surgical debulking followed by chemotherapy with carboplatin and pemetrexed with a modest tumour response.
About 1 year later, she had evidence of progression of her disease with new thoracic spinal metastases that were treated with radiation therapy. A trial of erlotinib was initiated. She developed severe gastrointestinal toxicity from erlotinib and had to stop therapy due to the side effects. She then had a similar reaction to afatinib. She received carboplatin and pemetrexed again with poor tumour response, and then erlotinib was tried once more but she developed unexplained thrombocytopenia. She continued to have progression of her lung cancer with additional metastases noted throughout her thoracic spine on repeated imaging. She wished for continued treatment and was deemed eligible for therapy with nivolumab given her progressive disease and failure of other agents. She received two doses of this medication 2 weeks apart. Almost 1 week after her second dose of nivolumab, she presented to our hospital experiencing altered mental status, nausea and vomiting.
Ten days prior to this admission, the results of a comprehensive metabolic panel were normal. Her previous surface ECG was about 1 year prior to admission and had no evidence of pre-excitation or intrinsic conduction disease. She had a left heart catheterisation performed 1 year prior for chest pain which showed non-obstructive disease. During her current admission, the patient was noted to have a markedly abnormal ECG with a right bundle branch block and a left posterior fascicular block. She had a heart rate of 150 bpm and was in moderate respiratory distress on initial examination. Other notable laboratory studies on admission were a modest elevation of the patient's liver enzymes with aspartate transaminase (AST) level of 240 U/L, alanine transaminase (ALT) level of 208 U/L and alkaline phosphatase (ALP) level of 181 U/L. She had significantly abnormal cardiac enzymes with serum troponin of 3.08 ng/mL, elevated creatinine kinase level of 1832 and isoenzyme CK-MB of 112.4 and CK index of 6.1 U/L. She also demonstrated pleural effusions on chest radiography. The patient was not found to be a candidate for immediate percutaneous coronary intervention as her altered mental status on admission in the setting of her widely metastatic disease gave concern for possible intracranial disease and placed her at an unacceptably high risk of intracerebral haemorrhage from anticoagulation.
The patient then began experiencing several electrical abnormalities. She had multiple ectopic beats that raised concern for ventricular tachycardia. She had a noted change in her ECG axis from a normal baseline to a wide rightward axis. She also experienced a gradual conversion from initial rightward vector to a greatly deviated rightward axis. Her ectopic beats became more frequent as well and did eventually become sustained ventricular tachycardia. Owing to this development, an urgent bedside transthoracic echocardiogram was performed which revealed no focal wall motion abnormalities and an ejection fraction of 65%. The patient's ECG series are shown in figures 1–3.
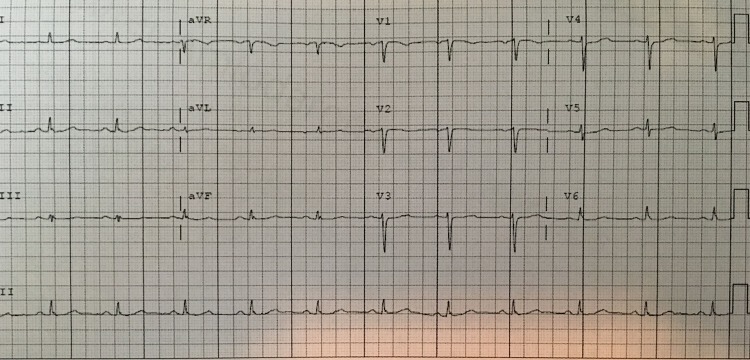
Figure 1.
Normal ECG 1 year prior. The patient's ECG 1 year prior to presentation.
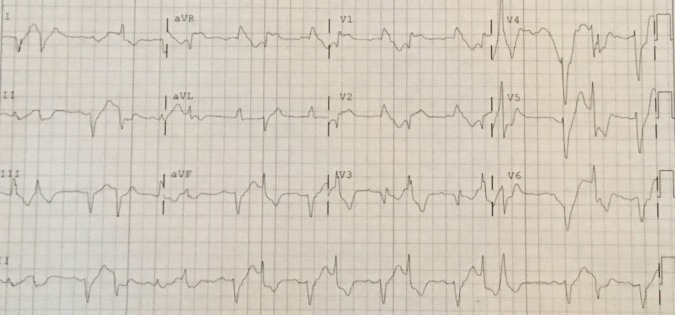
Figure 2.
ECG on presentation. The evolution of the patient's ECG shortly after presentation. Notable abnormalities include the presence of three different QRS complexes with a fourth complex (the 6th complex in the tracing) showing possible fusion. The patient also has a right bundle branch block with a wide right axis deviation.
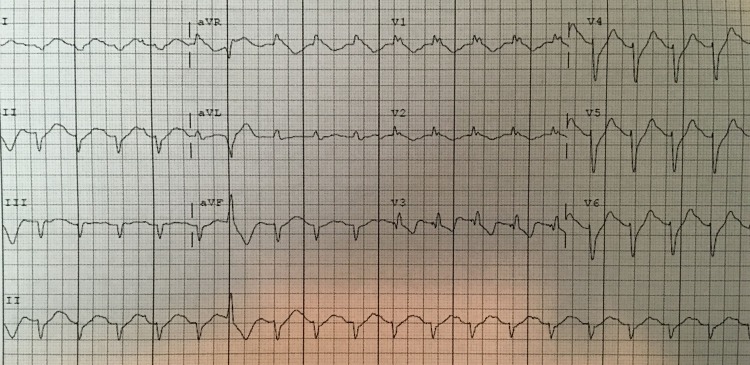
Figure 3.
ECG after amiodarone. The patient's ECG after amiodarone. Ectopy has lessened but the patient's right bundle branch block and right axis deviation persists.
Given the presence of abnormal cardiac markers and ventricular tachycardia in the absence of echocardiogram abnormalities and a recent cardiac catheterisation showing non-obstructive disease, as well as the temporal association to nivolumab administration, coronary ischaemia was not found to be the cause of her clinical status. Furthermore, given the known association of irAEs with nivolumab, and evidence of a current irAE with acute transaminitis and suspected pneumonitis, autoimmune myocarditis secondary to nivolumab was suspected.
Investigations
Basic science studies have demonstrated the propensity for both CTLA-4-deficient mice and PD-1-deficient mice to develop myocarditis. The mechanism of this is currently not understood. It has been suggested that PD-1 limits T-cell responses in the heart, and inhibition of this predisposes mice to fatal myocarditis.15–17 Additionally, animal models have shown that PD-1-deficient mice can develop autoantibodies against troponin I or other unidentified proteins.
Myocarditis is a clinically challenging condition to diagnose.18–20 This is in part due to the wide spectrum of presenting symptoms of the disease, ranging from non-specific and relatively benign symptoms, such as fatigue and malaise, to sudden cardiac death. Owing to this, myocarditis is a condition that is often diagnosed retrospectively. The currently accepted gold standard for diagnosis requires endomyocardial biopsy and evaluation with the Dallas criterion, which confirms the diagnosis with histopathology, immunochemical staining and PCR. However, endomyocardial biopsy is an invasive test that is difficult to perform in an unstable patient. Owing to this, alternate diagnostic criteria have been sought. Cardiac MRI is an alternative given its non-invasive nature. Abdel-Aty et al reported a set of criterion for diagnosing myocarditis with cardiac MRI based on three criteria: an increase in T1 and T2 signal activity consistent with oedema, an increase in myocardial contrast enhancement relative to skeletal muscle contrast enhancement consistent with hyperaemia and the presence of late gadolinium enhancement consistent with scar. When two of the above criteria were met the diagnosis of myocarditis was made with a specificity of 76% and a sensitivity of 96%.21
Differential diagnosis
In our patient's case, the aetiology of her marked electrical abnormalities was unable to be confirmed, but was presumed secondary to her myocarditis. Left heart catheterisation could not be performed due to her clinical status. However, on cardiac catheterisation 1 year prior, she had only minor coronary disease with no major obstructive lesions noted. Furthermore, due to the dual blood supply of the posterior fascicle, a simultaneous occlusion of the right-sided and left-sided circulations would have occurred causing this degree of compromise. She was unable to undergo endomyocardial biopsy because she was too ill, and she died before cardiac MRI could be performed. Additionally, autopsy was unable to be performed. Our diagnosis of nivolumab-induced myocarditis was supported clinically because of the strong temporal association with nivolumab exposure and the development of her symptoms. In addition, the cardiac biomarkers, electrical abnormalities, transaminitis and pneumonitis all improved with treatment with high steroids dose.
Treatment
The patient received amiodarone infusion and a taper of glucocorticoids starting with 80 mg of intravenous methylprednisolone after initially being given 250 mg in the emergency department. The values of troponin, CK-MB, AST and ALT trended downwards over the next several hours. The burden of ventricular tachycardia and ectopic ventricular beats lessened but did not fully resolve.
Outcome and follow-up
She had an urgent MRI of her brain that was performed to evaluate the changes in her mental status, which revealed the presence of a new intracranial metastatic disease. Subsequently, after discussion with the patient's family, the goals of treatment shifted to palliation and she was placed under hospice care. About 48 hours after initial presentation, the patient became bradycardic and hypotensive and she expired. An autopsy was offered to the family but was declined.
Discussion
We have reported a case of suspected autoimmune myocarditis and new conduction disease with nivolumab. Although this diagnosis could not be proven, we believe nivolumab to be the culprit for the patient's condition and ultimate death as evidenced by her presentation with concurrent transaminitis and pneumonitis, two commonly encountered irAEs with PD-1 inhibitors, and reports of cardiac toxicities with other novel immunotherapeutics. A literature review revealed four cases of myocarditis—one with ipilimumab, one with anti-PD-L1 antibody BMS-936559, one with pembrolizumab and one with combination ipilimumab and pembrolizumab—and one case of left ventricular dysfunction with ipilimumab.1 9 22–24 To the best of our knowledge, this is the first reported case of conduction abnormalities and myocarditis associated with nivolumab, and the third reported case of significant cardiac toxicity with the novel PD-1 inhibitors.25
Learning points.
Nivolumab is effective across multiple malignancies and is increasingly being used.
Like the other PD-1 inhibitors, nivolumab has unique autoimmune side effects.
We believe our patient experienced autoimmune myocarditis and we have presented a theoretical model for how this could occur.
Footnotes
Twitter: Follow Merry Markham at @DrMarkham
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 3.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015;37:764–82. 10.1016/j.clinthera.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page DB, Postow MA, Callahan MK et al. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185–202. 10.1146/annurev-med-092012-112807 [DOI] [PubMed] [Google Scholar]
- 5.Ansell SM, Lesokhin AM, Borrello I et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–19. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Ribas A, Wolchok JD et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 8.Hamid O, Robert C, Daud A et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Liberal J, Kordbacheh T, Larkin J. Safety of pembrolizumab for the treatment of melanoma. Expert Opin Drug Saf 2015;14:957–64. 10.1517/14740338.2015.1021774 [DOI] [PubMed] [Google Scholar]
- 12.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560–75. 10.3978/j.issn.2218-6751.2015.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbee MS, Ogunniyi A, Horvat TZ et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother 2015;49:907–37. 10.1177/1060028015586218 [DOI] [PubMed] [Google Scholar]
- 14.Kong YC, Flynn JC. Opportunistic autoimmune disorders potentiated by immune-checkpoint inhibitors anti-CTLA-4 and anti-PD-1. Front Immunol 2014;5:206 10.3389/fimmu.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Okazaki IM, Yoshida T et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol 2010;22: 443–52. 10.1093/intimm/dxq026 [DOI] [PubMed] [Google Scholar]
- 16.Tarrio ML, Grabie N, Bu DX et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 2012;188:4876–84. 10.4049/jimmunol.1200389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tivol EA, Borriello F, Schweitzer AN et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541–7. 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- 18.Okazaki T, Tanaka Y, Nishio R et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477–83. 10.1038/nm955 [DOI] [PubMed] [Google Scholar]
- 19.Angelini A, Calzolari V, Calabrese F et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart 2000;84:245–50. 10.1136/heart.84.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caforio AL, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–48. doi:48a-48d [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Aty H, Boyé P, Zagrosek A et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 2005;45:1815–22. 10.1016/j.jacc.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 22.Läubli H, Balmelli C, Bossard M et al. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 2015;3:11 10.1186/s40425-015-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patnaik A, Socinski MA, Gubens MA et al. Phase 1 study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second line therapy for advanced non-small cell lung cancer (NSCLC): Keynote-021 cohort D [abstract]. J Clin Oncol 2015;33:8011. [Google Scholar]
- 24.Roth ME, Muluneh B, Jensen BC et al. Left ventricular dysfunction after treatment with ipilimumab for metastatic melanoma. Am J Ther 2016. 10.1097/MJT.0000000000000430 [DOI] [PubMed] [Google Scholar]
- 25.Nishimura H, Okazaki T, Tanaka Y et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001;291:319–22. 10.1126/science.291.5502.319 [DOI] [PubMed] [Google Scholar]