Abstract
Insulin antibodies sometimes cause glucose instability. A 52-year-old male patient was admitted to our department for the treatment of diabetes mellitus. From October 2003, he received insulin treatment for autoimmune pancreatitis and diabetes mellitus, but his hemoglobin A1c (HbA1c) levels gradually reached 8.0% (64 mmol/mol IFCC). In January 2010, insulin glargine and insulin aspart were introduced. In April 2010, the insulin antibody titre rose to >13.6 U/mL. In July 2010, treatment was changed to insulin glargine, metformin and miglitol. In November 2011, a further change to insulin glargine, metformin and sitagliptin was made. The insulin antibody titres gradually decreased, but HbA1c levels remained high. In November 2014, liraglutide and insulin glargine were introduced and the HbA1c levels decreased dramatically to ∼7.5% (58 mmol/mol IFCC) despite increasing insulin antibody titres (from 32.6 to >50.0 U/mL). Liraglutide successfully improved glycaemic instability due to insulin antibodies without modulating plasma insulin levels.
Background
Glycaemic instability is an independent predictor of cardiovascular mortality in patients with type 2 diabetes mellitus.1 Insulin antibodies sometimes cause glucose instability such as nocturnal hypoglycaemia, postprandial hyperglycaemia and/or insulin allergy.2 3 Insulin antibodies directly bind to insulin and lower plasma ‘free’ insulin levels, which are unbound to insulin antibodies.2 3 Exogenous insulin administration is not always sufficient to lower plasma glucose levels in patients with diabetes having insulin antibodies.2 3 Several therapies have been tried, such as changing to insulin analogues, administering steroids or performing haemodialysis. However, these therapies do not always improve glycaemic control.2
Recently, antidiabetes drugs that independently potentiate insulin secretory capacity have been developed, including DPP-4 inhibitors, metformin and GLP-1 receptor agonists.4–7 These drugs suppress hepatic glucose production by the stimulation of intestinal GLP-1 signalling (gut–brain–liver axis) and the suppression of glucagon secretion. In this case report for improving glycaemic control in a person having diabetes with insulin antibodies, we tried three different therapies: (1) insulin analogues (insulin glargine and insulin aspart), (2) DPP-4 inhibitor (sitagliptin) and long-acting insulin (insulin glargine) and (3) GLP-1 analogue (liraglutide) and long-acting insulin (insulin glargine). Liraglutide might be a new approach to treating glycaemic instability owing to insulin antibodies independent of modulating insulin secretion.
Case presentation
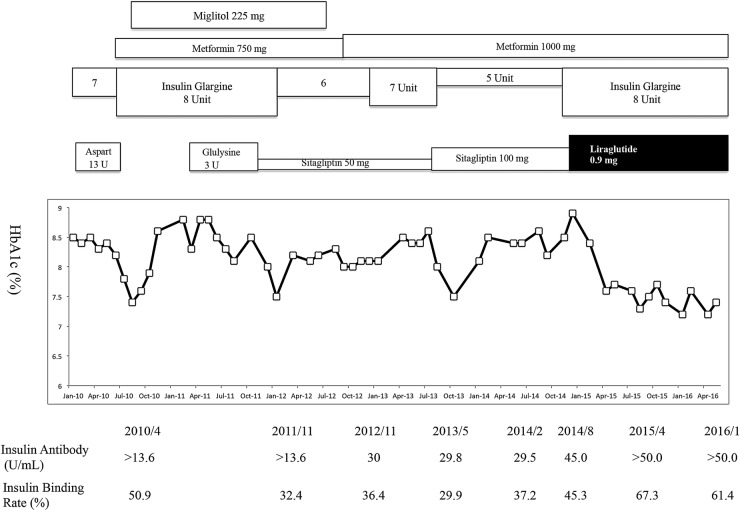
A 52-year-old male patient was admitted to our department to improve glycaemic control. In October 2003, he felt abdominal skin itching and had jaundice. He was admitted to the department of gastroenterology at our institution. An abdominal CT scan was performed and a pancreatic head mass (45×25 mm) was detected. Endoscopic ultrasound-guided fine-needle aspiration was performed for the diagnosis of autoimmune pancreatitis. In January 2004, administration of 30 mg prednisolone was started and gradually tapered off. At the same time, plasma glucose and hemoglobin A1c (HbA1c) level reached as high as 240 mg/dL and 10.3% (89.1 mmol/mol IFCC), respectively. The patient was then diagnosed with diabetes mellitus according to the Japan Diabetes Society criteria.8 Intensive insulin therapy with human insulin (morning 4 units, noon 6 units, evening 6 units) and Neutral Protamine Hagedorn (NPH) insulin (bedtime 6 units) was administrated and HbA1c levels were maintained to about 7.5% (58.5 mmol/mol IFCC). In July 2004, the pancreatic head mass was undetectable in a CT scan and steroid therapy for autoimmune pancreatitis was completely terminated. In 2005, plasma glucose levels gradually increased and HbA1c reached 8.0% (64 mmol/mol IFCC) (figure 1). In January 2006, he was hospitalised in our department to control plasma glycaemic levels. Insulin aspart (10 units) and premixed insulin aspart 30 (30% free and 70% protamine-bound biphasic aspart 30, biphasic insulin aspart (BIAsp) 30) (8 units) were administered (figure 1). However, the HbA1c levels were mostly higher than 7.5% (58 mmol/mol IFCC) and hypoglycaemia was often observed. In January 2010, BIAsp 30 was discontinued and he was prescribed insulin glargine (7 units) and insulin aspart (13 units) (figure 1). In April 2010, analysis of insulin antibody (insulin binding rate, %) was >13.6 µU/mL (51%). Free and total plasma insulin concentrations were 2.87 and 81.9 µU/mL, respectively (figure 1). Scatchard analysis showed that insulin antibodies were characterised by low affinity (K1: 4.55×10−2 (1/10−8 M)) and high binding capacity (R1: 3.45 (10−8 M)). The titre of the anti-insulin IgE reached 0.88 (<0.34 UA/mL). Fortunately, no symptoms of insulin allergy were observed. Therefore, we considered that glycaemic instability was due to insulin antibodies. In July 2010, he was again hospitalised for 2 weeks to improve glycaemic control. Insulin aspart was replaced with oral administration of metformin (750 mg/day) and miglitol (225 mg) was added to insulin glargine (8 units). After discharge, his HbA1c had transiently improved, but the HbA1c levels remained as high as 8% (63.9 mmol/mol IFCC). As the total dose of insulin was decreased from 20 to 8 units, the titres (% insulin binding) of insulin antibodies moderately decreased (>13.6 U/mL (53.7%), >13.6 U/mL (41.3%), to >13.6 U/mL (32.4%) in July 2010, July 2011 and October 2011, respectively; figure 1).
Figure 1.
Clinical treatment course of the patient.
In November 2011, he was yet again hospitalised for 2 weeks to improve glycaemic control. Sitagliptin (50 mg and later 100 mg) added to insulin glargine (6–8 units) and metformin 750 mg/day were administered. However, the insulin antibody titres (% insulin binding) were almost unchanged (30 U/mL (36.4%), 29.8 U/mL (29.9%), 29.5 U/mL (37.2%) and 45 U/mL (45.3%) in November 2012, May 2013, February 2014 and August 2014, respectively). After discharge, his HbA1c had transiently improved, but subsequently, the HbA1c levels remained unaffected (7.5–8.6% (58–70 mmol/mol IFCC); figure 1).
The patient had no family history of diabetes mellitus and diet or drug allergy except for allergy from insulin injections. He is an office worker and except for hospitalisation, his lifestyle remained unchanged, normally eating breakfast at 07:00, lunch at 12:00 and dinner at 22:00–23:00.
Outcome and follow-up
In November 2014, sitagliptin was switched to liraglutide. Combination therapy using insulin glargine (8 units), metformin (1000 mg), liraglutide (0.9 mg) and miglitol (50 mg) successfully improved his plasma HbA1c levels (from 8.5% (69 mmol/mol IFCC) to 7.2% (55 mmol/mol IFCC)) and hypoglycaemic episodes were not seen. In contrast, despite improvement in plasma HbA1c levels, insulin antibody titres increased remarkably following liraglutide treatment (>50 U/mL (67.3%) and >50 U/mL (61.3%) in April 2015 and January 2016, respectively; figure 1). In January 2016, his 2 hour postprandial plasma glucose and C peptide levels (196 mg/dL and 1.72 ng/mL, respectively) were similar to those in October 2013 (164 mg/dL and 0.91 ng/mL, respectively). Scatchard analysis also showed that insulin antibodies were characterised by low affinity (K1: 5.29×10−2 (1/10−8 M)) and high binding capacity (R1: 2.6 (10−8 M)), consistent with the previous patterns (K1: 4.55×10−2 (1/10−8 M) R1: 3.45 (10−8 M)) in April 2010. No symptoms of insulin allergy were observed.
Discussion
Insulin antibodies sometimes cause glucose instability in people with diabetes receiving insulin therapy. Treatment for glycaemic instability due to insulin antibodies may involve (1) switching to another insulin analogue, (2) switching from insulin to non-insulin therapy if endogenous β-cell function is preserved and (3) steroid administration.2
With the first treatment method, switching to another insulin analogue might be beneficial for reducing the insulin antibody titre, and consequently improving glycaemic control.9 However, in our case, a switch from BIAsp 30 to another insulin analogue (insulin glargine) did not improve glycaemic control, although the titres of insulin antibody decreased slightly. This suggests that after the insulin antibodies occurred, the switching from BIAsp 30 to insulin glargine might not be enough to improve glycaemic control.
With regard to the second treatment method, switching from insulin to non-insulin might be a better therapy if endogenous β-cell function is preserved. Previously, we reported a case where insulin was eliminated and replaced with oral therapy resulting in improved glycaemic control.3 However, in this present case, the insulin secretory capacity was low and we could not switch from insulin injection to non-insulin antidiabetes therapy.
Finally, it has been reported that prednisone at doses of 60–80 mg/day can result in lower insulin requirements in up to 75% of patients, which often occurs within a few days, even before a decline in antibody levels.2 Owing to this, we did not select steroid administration and considered that (1) the use of steroids might cause glycaemic control to deteriorate and (2) glucocorticoid therapy should be administered in a hospital, as previously recommended, because in some cases the response can be dramatic and severe hypoglycaemia can ensue.2 The patient did not want to be hospitalised due to his busy lifestyle.
Since titres of insulin antibodies are correlated with the dose of insulin injections, we considered that improvement of glycaemic control would have the effect of reducing insulin antibodies. Therefore, we considered the patient's earlier treatments as a hindrance to improving glycaemic control. Thus, therapies for glycaemic instability due to insulin antibodies may not always be effective.
Liraglutide is reported to have beneficial effects on glycaemic instability in insulin antibody-positive people with diabetes10 by improving glycaemic control through the potentiation of glucose-stimulated insulin secretion and suppression of glucagon secretion, through gastric emptying and through appetite.11 Moreover, other studies have reported that intestinal local GLP-1 signalling suppresses hepatic glucose output by stimulating vagal nerves (gut–brain–liver axis).4–7 The data from these reports suggest that liraglutide lowers plasma glucose levels independently of plasma insulin and glucagon levels. In our case, liraglutide successfully improved glycaemic control despite the postprandial plasma c peptide immunoreactivity (CPR) levels being very low (1.0–1.5 nmol/mL) at 10:00. Moreover, free insulin levels were much lower than total plasma insulin concentrations. For glucagon, plasma levels were not measured because of the difficulty in analysing ‘real glucagon’ concentrations.4–7 Considering the present and past studies that show liraglutide could suppress plasma glucagon levels, the improvement of glycaemic control in our case might be due to the insulin-independent suppression of hepatic glucose output. Sitagliptin also has a glucose lowering effect via the gut–brain–liver axis. However, sitagliptin was less effective than liraglutide because liraglutide is more potent in lowering glucose concentration.12 Therefore, liraglutide might be more useful for improving glycaemic instability in patients with diabetes with insulin antibodies.
We previously reported that the use of protamine-containing insulin (insulin NPH, premixed insulin and premixed insulin analogues) are risk factors for the occurrence of insulin antibodies in patients receiving insulin therapy.13 In this case, insulin NPH and premixed insulin aspart were used for at least 7 years. This suggests that the history of taking protamine-containing insulin might affect the occurrence of insulin antibodies. However, at present, we can use a coformulated insulin analogue that contains no protamine. Using coformulated insulin instead of premixed insulin might decrease the incidence of insulin antibody-positive diabetes mellitus. In addition, however, we should remember that inadequate use of insulin therapy could cause hypoglycaemia, body weight gain, iatrogenic hyperinsulinaemia, increased risk of cardiovascular events and glucose instability owing to insulin antibodies.
In conclusion, this case reports that liraglutide successfully improved glycaemic control in a patient with diabetes with insulin antibodies. This suggests that antidiabetes drugs suppressing hepatic glucose output through the insulin-independent pathway (gut–brain–liver axis) might be useful for improving glycaemic control in insulin antibody-positive patients with diabetes mellitus.
Learning points.
Insulin antibodies, which are often implicated as a cause of glucose instability in type 2 diabetes mellitus, are not amenable to any effective therapy as of now.
Liraglutide may improve glucose instability in patients with insulin antibodies.
Considering that free insulin levels unbound to insulin antibodies were much lower than total insulin levels, liraglutide lowered plasma glucose levels independent of increasing insulin secretion.
Footnotes
Contributors: KI, TK and HN wrote the manuscript. KI and JT made manuscript revisions.
Competing interests: KI has received lecture fees from Sanofi, Novo Nordisk and Eli Lilly. JT has received research lecture fee funding from Sanofi, Novo Nordisk and Eli Lilly.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Muggeo M, Zoppini G, Bonora E et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care 2000;23:45–50. 10.2337/diacare.23.1.45 [DOI] [PubMed] [Google Scholar]
- 2.Fineberg SE, Kawabata TT, Finco-Kent D et al. Immunological responses to exogenous insulin. Endocr Rev 2007;28:625–52. 10.1210/er.2007-0002 [DOI] [PubMed] [Google Scholar]
- 3.Iizuka K, Tomita R, Horikawa Y et al. A case of glycemic instability and insulin allergy due to anti-insulin antibodies in a patient with type 2 diabetes. Diabetol Int 2012;3:233–8. 10.1007/s13340-012-0077-8 [DOI] [Google Scholar]
- 4.Omar B, Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014;63:2196–202. 10.2337/db14-0052 [DOI] [PubMed] [Google Scholar]
- 5.Song R. Mechanism of metformin: a tale of two sites. Diabetes Care 2016;39:187–9. 10.2337/dci15-0013 [DOI] [PubMed] [Google Scholar]
- 6.Duca FA, Côté CD, Rasmussen BA et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 2015;21:506–11. 10.1038/nm.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jun LS, Millican RL, Hawkins ED et al. Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes 2015;64:819–27. 10.2337/db14-1052 [DOI] [PubMed] [Google Scholar]
- 8.Seino Y, Nanjo K, Tajima N et al. , The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010;1:212–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanai H, Adachi H, Hamasaki H. Diabetic ketosis caused by the insulin analog aspart-induced anti-insulin antibody: successful treatment with the newest insulin analog glulisine. Diabetes Care 2011;34:e108 10.2337/dc11-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawanami D, Ito T, Watanabe Y et al. Successful control of a case of severe insulin allergy with liraglutide. J Diabetes Investig 2013;4:94–6. 10.1111/j.2040-1124.2012.00239.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–42. 10.1038/nrendo.2012.140 [DOI] [PubMed] [Google Scholar]
- 12.Pratley RE, Nauck MA, Barnett AH et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–56. 10.1016/S0140-6736(10)60307-8 [DOI] [PubMed] [Google Scholar]
- 13.Nishimura H, Iizuka K, Takeda J. Protamine-containing insulin but not analog insulin and duration of insulin use are risk factors for the production of insulin autoantibodies in insulin-treated patients with diabetes mellitus. Endocr J 2014;61:635–40. 10.1507/endocrj.EJ13-0544 [DOI] [PubMed] [Google Scholar]