Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and coronary artery disease (CAD) is the cardiac manifestation of metabolic syndrome. NAFLD is strongly linked to CAD and hepatic steatosis is an independent risk factor for CAD and cardiac mortality. The pathogenic mechanism underlying this association remains poorly understood. In this study, we explored expression of circulating microRNAs (miRNAs) in patients with NAFLD and associated CAD.
Results
When compared to patients with NAFLD without CAD, patients with NAFLD and CAD had lower circulating levels of miR-132 (0.24±0.16 vs 0.30±0.11, p=0.03), while the circulating levels of miR-143 were higher (0.96±0.90 vs 0.64±0.77, p=0.02). The levels in circulation demonstrated trends opposite to previously observed intracellular levels in patients with CAD. In obese patients with NAFLD, lower circulating levels of miR-145 (1.42±1.00 vs 2.41±1.80), miR-211 (41.26±20.40 vs 57.56±25.45), miR-146a (2.13±1.40 vs 2.90±1.36) and miR-30c (6.92±4.99 vs 11.0±6.92) were detected when compared to lean patients with NAFLD. For miR-161 (0.59±1.19 vs 0.15±0.14) and miR-241 (0.28±0.29 vs 0.16±0.13), higher circulatory levels were detected in the obese patients with NAFLD. These observations suggest altered circulating levels of miRNAs that may serve to balance intracellular levels of miRNA in target tissues. Additional studies examining paired samples of target and producing tissues as well as respective plasma samples will help delineate the regulatory circuits governing the secretion and the uptake of miRNA in multitissue diseases.
Keywords: LIVER, CARDIOVASCULAR DISEASE, GENE REGULATION
Summary box.
What is already known about this subject?
-
▸
Non-alcoholic fatty liver disease (NAFLD) is strongly linked to coronary artery disease (CAD) and hepatic steatosis is an independent risk factor for CAD and cardiac mortality.
-
▸
The pathogenic mechanism underlying association between CAD and NAFLD remains poorly understood.
-
▸
Circulating, cell-free microRNAs (miRNAs) are attractive due to high stability in plasma, association with disease states and ease of sensitive measurement.
-
▸
Little is known about the origin of circulating miRNAs in either healthy or sick people or what factors influence levels of circulating miRNA biomarkers.
What are the new findings?
-
▸
This study examines the circulating levels of miRNA as a possible pathogenic route that explains the underlying association of NAFLD and CAD.
-
▸
The study shows an opposite trend in levels of circulating miRNA than previously reported intracellular levels in the tissues affected by pathogenic conditions.
-
▸
These observations suggest altered circulating levels of miRNAs may serve to balance intracellular levels of miRNA in target tissues.
-
▸
The findings suggest circulating miRNA as a link between multi-tissue crosstalk.
How might it impact on clinical practice in the foreseeable future?
-
▸
Altered miRNA secretion or uptake in pathogenic conditions may determine the adaptive response of the body to pathophysiological process.
-
▸
miRNA maybe indicators of multi-organ crosstalk.
-
▸
Targeted search for biomarkers in complex diseases like NAFLD need to consider influence of multiple peripheral tissues such as liver, vasculature and adipose participate in secretion driven regulatory milieu.
Background
MicroRNAs (miRNAs) are a large class of evolutionarily conserved non-coding RNAs. In mammals, miRNAs function as critical post-transcriptional regulators of a number of biological processes such as cell growth, tissue differentiation, cell proliferation, embryonic development and apoptosis.1 2 About 1–4% of the protein-encoding loci in the human genome also encodes one or more miRNAs. A single miRNA can regulate as many as 200 cognate mRNAs. Altered miRNA expression can thus influence development and progression of a variety of pathophysiological processes.2 3
Recently, circulating miRNAs have received increased attention as potential diagnostic and prognostic biomarkers that could be accessed in minimally invasive way.4 5 Several studies have examined their levels in a number of chronic diseases, including chronic liver disease.6–9 Serum miR-21, miR-122 and miR-223, for instance, have been shown to be elevated in patients with hepatitis B virus-related hepatocellular carcinoma and/or chronic type B hepatitis. These miRNAs have also been proposed as diagnostic biomarkers for liver injury and potential target for treatment.10
Unexpected stability of miRNAs in circulation hints at the presence of a signalling pathway in which miRNAs are selectively secreted by one cell and taken up by a distant, target cell, possibly to regulate gene expression. This intriguing idea of circulating miRNAs regulating distant cell-to-cell communication has been intensively investigated. Studies have also discovered an abundance of stable and functional miRNAs in other biological fluids including urine, tears, saliva and cerebrospinal fluid (CSF).11 12 However, the origin and the function of these circulating extracellular miRNAs remain poorly understood. Studies exploring the selective secretion and uptake of miRNA from tissues are also lacking.
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome.13 Pathologically, NAFLD represents a spectrum ranging from steatosis (fatty liver) to steatohepatitis, fibrosis and cirrhosis.14 The progression of NAFLD to cirrhosis and its complications is dependent on its histological subtypes and a number of other known and unknown factors.15 In a similar fashion, coronary artery disease (CAD) is the cardiac manifestation of metabolic syndrome.16 NAFLD is strongly linked to CAD and hepatic steatosis is an independent risk factor for CAD17–19 and cardiac mortality.20 The pathogenic mechanism underlying this association remains poorly understood. It is possible that miRNAs can provide some information about this elusive link.21–23 Therefore, in this study, our aim was to quantify the circulating levels of 42 preselected miRNAs in patients with NAFLD and associated CAD. The miRNA examined in this study have been shown to be dysregulated in metabolic syndrome in previous studies.
Methods
Sample collection
This study has been approved by Internal Review Board of Inova Fairfax Hospital. After informed consent, blood samples were collected from patients undergoing elective coronary angiography. In each sample, plasma was separated and processed by standard procedure, then immediately flash frozen in liquid nitrogen and added to the repository of specimens stored at −80°C. The samples were deidentified in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Clinical data were available for all the samples and included presurgery fasting measurements of the glucose, serum aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) and lipid panel. None of the included participants reported to have consumed alcohol in excess (>10 g/day in women and >20 g/day in men) in the past 5 years.
Diagnosis of NAFLD
For each patient, a hepatic ultrasound was performed just prior to cardiac angiography for the presence of NAFLD. The ultrasound was read by a single radiologist for the presence and severity of hepatic steatosis. Other causes of chronic liver disease (CLD) were excluded by history or blood testing. Diagnosis and severity of stenosis/CAD was determined by a cardiologist based on the number of diseased proximal arterial segments.24
RNA extraction and cDNA synthesis
Total RNA was extracted from plasma using miRNeasy kit (Qiagen) according to manufacturer's protocol, then converted to cDNA using Universal cDNA Synthesis kit (Exiqon, Denmark). For each reaction, 4 µl of total RNA isolated from 250 µl of plasma was used. Tissue and pathway-specific miRNAs (42 miRs) dysregulated in metabolic syndrome were shortlisted from literature; custom plates were designed using LNA-based primers (Exiqon).
Quantitative PCR
Quantitative PCR was performed using SYBR Green master mix (Exiqon). A total of 10 μL of the reaction mix (1:1 ratio of 50× diluted cDNA and 2× SYBR Green PCR Master Mix) was added to each well according to manufacturer's instructions (Exiqon, Denmark). Amplification was performed on the CFX96 thermocycler (Bio-Rad) using cycling parameters recommended by Exiqon (95°C for 10 min followed by 45 cycles of 95°C for 10 s and 60°C 60 s at a ramp rate of 1.6°C/s). A melting curve analysis (60–99°C) was performed after the thermal profile to ensure specificity in the amplification.
To determine the technical variation between the plates, the interplate calibrator (IPC) (UniSp3) analysis was performed. IPC (UniSp3) levels were highly similar among the samples with threshold cycle (Ct) values of 21.01±4.2. RNA-spike-in control (UniSp6) was used as the cDNA synthesis control. UniSp6 levels were highly similar among the samples with Ct values of 22.19±6.74. After global normalisation, group-wise comparisons of overall miRNA patterns were carried out. Ct values >35 were considered to be below the detection level of the assay.
Data analysis
All analyses were performed using SAS V.9.3 (SAS Institute, Cary, North Carolina, USA). Demographic and clinical variables of study patients were assessed using mean (±SDs) for continuous variables and frequencies (percentages) for categorical variables. Simple comparisons of continuous variables by NAFLD status were performed with non-parametric Wilcoxon tests and categorical variables with χ2 tests. The correlation between continuous variables was assessed using Pearson's correlation coefficient. Significance level was defined as two-sided p<0.05.
Results
miRNA concentrations were measured in the plasma samples collected from 44 patients with NAFLD and with or without CAD. Clinical and demographic characteristics of these patients are summarised in table 1.
Table 1.
Demographic and clinical variables in the cohort examined for expression of circulating miRNAs
| Demographic and clinical variables | Mean (SD) (N=44) or N (%) |
|---|---|
| Age (years) | 62.50±9.60 |
| BMI | 29.92±4.76 |
| ALT (U/L) | 25.50±13.75 |
| AST (U/L) | 21.65±8.31 |
| GGT | 22.96±17.39 |
| WCC (103/μL) | 9.26±13.79 |
| Platelet (103/μL) | 215.91±48.51 |
| Glucose (mg/dL) | 137.12±92.47 |
| Triglycerides (mg/dL) | 113.40±53.60 |
| Total cholesterol (mg/dL) | 150.73±31.88 |
| LDL (mg/dL) | 80.90±28.21 |
| HDL (mg/dL) | 43.40±14.72 |
| Gender—male | 39 (88.6%) |
| Race—white | 40 (90.9%) |
| CAD with NAFLD | 32 (72.7%) |
Values are presented as mean and SD or percentages, where appropriate.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; miRNA, microRNA; NAFLD, non-alcoholic fatty liver disease; WCC, white cell count.
Our results show that two miRNA species, miR-16 (reported to be enriched in red blood cells) and miR-223 (reported to be enriched in myeloid blood cells), were the most abundant in plasma. Interestingly, circulating levels of miR-122, a liver-specific miRNA, was also detected in patients with NAFLD (6.33±5.98).
miR-143 and miR-132 as markers of CAD
Comparison of patients with NAFLD and angiography-confirmed CAD (N=32) to those with NAFLD and without CAD (N=12) showed significant difference in circulating levels of miR-143 and miR-132. miR-132 had a lower expression in patients with NAFLD and CAD when compared to those without CAD (0.24±0.16 vs 0.30±0.11, p=0.03) and miR-143 had a higher expression in patients with NAFLD and CAD when compared to those without CAD (0.96±0.90 vs 0.64±0.77, p=0.02). Confounders such as body mass index (BMI, kg/m2) and age (years) were not statistically different in these patients (p>0.05; table 2).
Table 2.
Comparison of clinical variables and miRs in patients with NAFLD with and without cardiovascular disease
| NAFLD with CAD (N=32) | NAFLD without CAD (N=12) | p Value | |
|---|---|---|---|
| Age (years) | 64.22±8.23 | 57.92±11.74 | 0.14 |
| BMI (kg/m2) | 29.92±4.73 | 29.93±5.06 | 0.95 |
| WCC (103/μL) | 9.89±16.16 | 7.58±1.86 | 0.55 |
| Platelet (103/μL) | 207.31±42.93 | 238.83±56.73 | 0.07 |
| Glucose (mg/dL) | 133.27±95.05 | 146.75±88.95 | 0.25 |
| ALT (U/L) | 27.37±15.27 | 20.83±7.55 | 0.15 |
| AST (U/L) | 22.39±8.33 | 19.75±8.32 | 0.23 |
| Triglycerides (mg/dL) | 107.39±46.43 | 128.92±68.74 | 0.39 |
| LDL (mg/dL) | 80.31±29.63 | 82.45±25.34 | 0.70 |
| Total cholesterol (mg/dL) | 151.50±33.55 | 148.64±28.18 | 0.77 |
| HDL (mg/dL) | 44.52±15.10 | 40.50±13.87 | 0.45 |
| Gender (male) | 27 (84.4%) | 12 (100.0%) | 0.1458 |
| miR-132 | 0.24±0.16 | 0.30±0.11 | 0.039 |
| miR-143 | 0.96±0.90 | 0.64±0.77 | 0.028 |
p<0.05 is considered significant. Values are presented as mean and SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; miR, microRNA; NAFLD, non-alcoholic fatty liver disease; WCC, white cell count.
miRNA expression in plasma from patients with varying degree of obesity
To determine whether BMI independently influences the levels of circulating miRNAs, patients were grouped according to their BMI. Among the cohort, 20 patients were obese with BMI >30, and 24 patients had normal weight (lean) with BMI <26.46. The percentages of the lean and the obese patients with angiography-confirmed diagnosis of CAD and ultrasound-determined NAFLD were similar (p>0.05) (table 3). Among the metabolic features, triglyceride levels (mg/dL) (134.26±62.94 vs 96.88±38.74, p=0.035) and fasting glucose levels (mg/dL) (176.65±120.32 vs 101.18±27.47, p=0.027) were significantly higher in obese patients, while ALT and AST levels were similar (table 3).
Table 3.
Comparison of clinical variables and miRs in patients with BMI >30 and in patients with BMI <30
| Obese (N=20) | Lean (N=24) | p Value | |
|---|---|---|---|
| Age (years) | 61.05 (8.82) | 63.71 (10.23 | 0.3047 |
| BMI (kg/m2) | 34.08±3.48 | 26.46±2.22 | <0.0001 |
| WCC (103/μL) | 12.17±20.27 | 6.83±1.68 | 0.0527 |
| Platelet (103/μL) | 221.75±45.73 | 211.04±51.16 | 0.4477 |
| Glucose (mg/dL) | 176.65±120.32 | 101.18±27.47 | 0.0270 |
| ALT (U/L) | 24.10±10.07 | 26.77±16.55 | 0.9800 |
| AST (U/L) | 20.30±7.64 | 22.83±8.86 | 0.2522 |
| Triglycerides (mg/dL) | 134.26±62.94 | 96.88±38.74 | 0.0352 |
| LDL (mg/dL) | 74.50±27.61 | 86.14±28.23 | 0.1612 |
| Total cholesterol (mg/dL) | 149.53±36.89 | 151.77±27.69 | 0.5594 |
| HDL (mg/dL) | 41.95±17.04 | 44.54±12.85 | 0.2929 |
| Gender (male) | 18±90.0% | 21±87.5% | 0.7947 |
| CAD/NAFLD status: N (%) | |||
| CAD and NAFLD | 14 (70.0%) | 18 (75.0%) | 0.7108 |
| Only NAFLD | 6 (30.0%) | 6 (25.0%) | 0.7108 |
| miR-145 | 1.42±1.00 | 2.41±1.80 | 0.03 |
| miR-161 | 0.59±1.19 | 0.15±0.14 | 0.03 |
| miR-211 | 41.26±20.40 | 57.56±25.45 | 0.03 |
| miR-241 | 0.28±0.29 | 0.16±0.13 | 0.03 |
| miR-146a | 2.13±1.40 | 2.90±1.36 | 0.04 |
| miR-30c | 6.92±4.99 | 11.00±6.92 | 0.02 |
p<0.05 is considered significant. Values are presented as mean and SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; miR, microRNA; NAFLD, non-alcoholic fatty liver disease; WCC, white cell count.
In obese patients with NAFLD, lower circulating levels of miR-145 (1.42±1.00 vs 2.41±1.80), miR-211 (41.26±20.40 vs 57.56±25.45), miR-146a (2.13±1.40 vs 2.90±1.36) and miR-30c (6.92±4.99 vs 11.0±6.92) were detected when compared to lean patients with NAFLD. For miR-161 (0.59±1.19 vs 0.15±0.14) and miR-241 (0.28±0.29 vs 0.16±0.13), higher circulatory levels were detected in the obese patients (table 3).
Correlation analysis of miRNAs with the clinical data
The levels of miR-99a and miR-122 positively correlated with ALT and AST levels (see online supplementary table 1). Plasma concentrations of miR-211 correlated with AST levels only, while levels of miR-16, miR-211a, miR-29c, miR-320a and miR-133a negatively correlated with serum triglyceride levels. For several miRs, circulating levels were negatively correlated with total cholesterol levels (miR-7, miR-93, miR-98, miR-161, miR-191, miR-195, miR-203, miR-425, miR-422, miR-200a and miR-519d), while for other five miRs, namely miR-23b, miR-199a-5p, miR-199a-3p, miR-107 and miR-103, circulating levels were positively correlated with the same parameter. Some of these miRs, namely miR-23b, miR-199a-5p, miR-199a-3p and miR-103, were also positively correlated with serum low-density lipoprotein (LDL) levels. Among the miRs negatively correlated with total cholesterol, only miR-7, miR-93, miR-161, miR-191, miR-195 and miR-203 levels also negatively correlated with LDL levels. High-density lipoprotein (HDL) levels positively correlated with plasma concentration of miR-320b, miR-7 and miR-17 (see online supplementary table 1).
To verify whether the circulating miRNA originates in the blood cells, the correlations of miRNAs with blood cell counts25 26 were assessed. Notably, miR-17 was the only miRNA negatively correlated with platelet counts, while white cell counts negatively correlated with the levels of several miRs (miR-30c, miR-27b, miR-146a, miR-224, miR-223, miR-221, miR-211, miR-145, miR-24 and miR-21).
Discussion
CAD and the NAFLD are closely associated with metabolic syndrome.27 Studies exploring biological pathways that explain the association between the two common chronic diseases are scarce (reviewed in refs. 28–30). The system-wide changes in the levels of regulatory miRNAs may be the key to understanding metabolic dysfunction associated with NAFLD and CAD.
Interestingly, miRNAs secreted or released in biological fluids remain stable, making them suitable non-invasive biomarkers, applicable to early detection of the diseases, monitoring of disease progression and even response to treatment. Moreover, circulatory miRNAs remain functional. They could be carried across a variety of distant sites, where they have shown to play a role in maintaining homeostasis in the body by regulating the secretion of inflammatory cytokines,31 32 by directly modulating HDL/LDL ratio in plasma and by other relevant mechanisms.33 Therefore, the levels of plasma miRs may reflect the systemic response to pathological processes. In this study, we assessed the potential association of serum miRNA levels with CAD in patients with NAFLD.
When compared to NAFLD without CAD, patients with NAFLD and CAD had lower circulatory levels of miR-132, while the circulatory levels of miR-143 were higher. Interestingly, hsa-miR-132 has often been referred to as ‘neurimmiR’ given its involvement in neuronal functions.34 35 It is reported to be downregulated in lipopolysaccharide (LPS)-stimulated primary human macrophages and in splenocytes of LPS-treated mice, where it normally suppresses peripheral inflammation through regulation of acetylcholinesterase expression.36 Anti-inflammatory functions of this miR are also executed through the targeting of STAT4,37 HB-EGF,38 p30039 and SirT1.40 Interestingly, for both of these miRs, the levels in circulation demonstrated trends opposite to that previously observed in the tissues affected by pathogenic conditions (see below).
In cardiac tissue, intracellular expression of miR-132 is elevated under conditions of pathological hypertrophy.33 Cardiomyocyte-specific overexpression of the miR-132 leads to heart failure and death in mice. miR-132 functions by suppressing FoxO3 expression, an antihypertrophic and proautophagic protein, and increasing the expression of Atrogin-1 (Fbxo32), an E3-ubiquitin ligase that initiates the degradation of calcineurin.33 In our study, plasma levels of miR-132 were decreased in patients with CAD, contrary to reportedly higher levels of intracellular miR-132 observed in inflammatory and hypertrophic conditions. Similar trends were observed for miR-143 involved in the regulation of cell proliferation, angiogenesis, apoptosis and inflammation in adipose and vascular tissues. For this miR, higher levels were detected in patients with NAFLD with CAD when compared to those without CAD. In the heart, intracellular miR-143 regulates contractility of vascular smooth muscle cells (VSMCs)41 42 by modulating cytoskeletal dynamics and enabling responsiveness of VSMCs to injury.43 Studies in mice have shown that miR-143 expression decreases in experimental models of vascular stress, in patients with aortic aneurysm42 and in injured carotid arteries.44
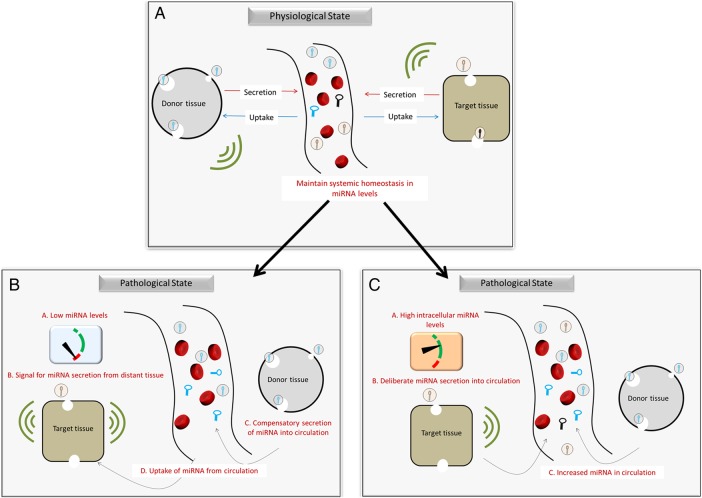
The observations from our study may be explained by the hypothesis that circulating levels of miRNAs serve to balance intracellular levels of miRNA in target tissues (figure 1A), as suggested by Hunter et al.45 The authors suggested that circulating miRNA may play a role in interorgan and intercellular communications. It is plausible that pathophysiological processes in the target tissues induce compensatory secretion of miRNA from distant tissues and/or cells, in order to maintain homeostasis. For instance, insufficient endogenous miRNA production in cardiac tissue to regulate vascular stress may result in a compensatory increase in the production of same miRNA in other distant tissues and its subsequent release into circulation (figure 1B). On the other hand, affected target tissues may deliberately secrete miRNA into interstitial fluid to balance excess of intracellular miRNA (figure 1C).46
Figure 1.
Compensatory mechanism of miRNA level regulation between tissues. (A) In physiological state, circulating miRNA plays a role in interorgan communications and help maintain balance in intracellular levels of miRNA. (B) Insufficient endogenous miRNA production in target tissue may result in a compensatory increase in production of same miRNA in other distant tissues and its subsequent release into circulation. (C) Increased intracellular levels in target cells may result in deliberate secretion of the miRNA by the cells into interstitial fluid. This would restore normal intracellular miRNA levels, while increasing the circulating levels. miRNA, microRNA.
The signalling pathways that ensure the secretion of certain miRNAs and their transfer to target tissues are as yet unknown. However, studies have shown that secretion and uptake of circulating miRNAs are non-random, ATP-dependent processes.46–50 Hence, these regulatory pathways may be modulated by the underlying metabolic states of cells.51 In the pathogenic conditions of NAFLD and CAD, multiple peripheral tissues such as liver, vasculature and adipose participate in secretion-driven regulatory milieu. Additional studies examining paired samples of target and producing tissues as well as respective plasma samples should be performed to delineate the regulatory circuits governing the secretion and the uptake of miRNA.
In short, our study indicates that circulating miRNAs may serve as an important regulatory circuit allowing the communication between the tissues and an adaptive response of the body to internally developed pathophysiological process.
Conclusion
In summary, our data on the levels of circulating miRNA demonstrated trends opposite to previously reported intracellular miRNA levels. Circulating levels of miRNA may be indicative of an adaptive response that balances intracellular miRNA levels in target tissues. Further studies exploring the source tissue of these miRNA will help elucidate interorgan communications in chronic diseases.
Footnotes
Contributors: RM conceived the study, participated in its design, carried out the experiments, coordinated all efforts and drafted the manuscript. MO performed statistical analysis. ZaY carried out sample collection, processing and storage. HA carried out the ultrasounds. BR determined diagnosis and severity of cardiac stenosis. ZoY participated in the design of the study and finalised the manuscript. All authors read and approved the final manuscript.
Competing interests: None declared.
Ethics approval: Inova IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33. doi:10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang TC, Mendell JT. microRNAs in vertebrate physiology and human disease. Annu Rev Genomics Hum Genet 2007;8:215–39. doi:10.1146/annurev.genom.8.080706.092351 [DOI] [PubMed] [Google Scholar]
- 3.Felekkis K, Touvana E, Stefanou Ch, et al. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010;14:236–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997–1006. doi:10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513–8. doi:10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumura T, Sugimachi K, Iinuma H, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer 2015;113:275–81. doi:10.1038/bjc.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Li J, Huang L et al. Serum microRNA profiles serve as novel biomarkers for the diagnosis of Alzheimer's disease. Dis Markers 2015;2015:e625659 doi:10.1155/2015/625659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers 2013;34:163–9. doi:10.1155/2013/259454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescador N, Pérez-Barba M, Ibarra JM, et al. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS One 2013;8:e77251 doi:10.1371/journal.pone.0077251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Wu C, Che X, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 2011;50:136–42. doi:10.1002/mc.20712 [DOI] [PubMed] [Google Scholar]
- 11.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733–41. doi:10.1373/clinchem.2010.147405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 2012;37:460–5. doi:10.1016/j.tibs.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Younossi ZM, Reyes MJ, Mishra A, et al. Systematic review with meta-analysis: non-alcoholic steatohepatitis—a case for personalised treatment based on pathogenic targets. Aliment Pharmacol Ther 2014;39:3–14. doi:10.1111/apt.12543 [DOI] [PubMed] [Google Scholar]
- 14.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010;103:71–83. doi:10.1093/qjmed/hcp158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224–9, 1229.e1–2 doi:10.1016/j.cgh.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Guize L, Pannier B, Thomas F, et al. Recent advances in metabolic syndrome and cardiovascular disease. Arch Cardiovasc Dis 2008;101:577–83. doi:10.1016/j.acvd.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 17.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007;13:1579–84. doi:10.3748/wjg.v13.i10.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gastaldelli A, Kozakova M, Højlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009;49:1537–44. doi:10.1002/hep.22845 [DOI] [PubMed] [Google Scholar]
- 19.Wong VW-S, Wong GL-H, Yip GW-K, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 2011;60:1721–7. doi:10.1136/gut.2011.242016 [DOI] [PubMed] [Google Scholar]
- 20.Agaç MT, Korkmaz L, Cavusoglu G, et al. Association between nonalcoholic fatty liver disease and coronary artery disease complexity in patients with acute coronary syndrome: a pilot study. Angiology 2013;64:604–8. doi:10.1177/0003319713479155 [DOI] [PubMed] [Google Scholar]
- 21.Estep M, Armistead D, Hossain N, et al. Differential expression of miRNAs in the visceral adipose tissue of patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2010;32:487–97. doi:10.1111/j.1365-2036.2010.04366.x [DOI] [PubMed] [Google Scholar]
- 22.Vacca M, Di Eusanio M, Cariello M, et al. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc Res 2016;109:228–39. doi:10.1093/cvr/cvv266 [DOI] [PubMed] [Google Scholar]
- 23.McClelland AD, Kantharidis P. MicroRNA in the development of diabetic complications. Clin Sci (Lond) 2014;126:95–110. doi:10.1042/CS20130079 [DOI] [PubMed] [Google Scholar]
- 24.Ringqvist I, Fisher LD, Mock M, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS). J Clin Invest 1983;71:1854–66. doi:10.1172/JCI110941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duttagupta R, Jiang R, Gollub J, et al. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS One 2011;6:e20769 doi:10.1371/journal.pone.0020769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard CC, Kroh E, Wood B, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 2012;5:492–7. doi:10.1158/1940-6207.CAPR-11-0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–23. doi:10.1053/jhep.2003.50161 [DOI] [PubMed] [Google Scholar]
- 28.Tarantino G, Caputi A. JNKs, insulin resistance and inflammation: a possible link between NAFLD and coronary artery disease. World J Gastroenterol 2011;17:3785–94. doi:10.3748/wjg.v17.i33.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nseir W, Shalata A, Marmor A, et al. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig Dis Sci 2011;56:3439–49. doi:10.1007/s10620-011-1767-y [DOI] [PubMed] [Google Scholar]
- 30.Lonardo A, Sookoian S, Pirola CJ, et al. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016;65:1136–50. doi:10.1016/j.metabol.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 31.Chen T, Li Z, Tu J, et al. MicroRNA-29a regulates pro-inflammatory cytokine secretion and scavenger receptor expression by targeting LPL in oxLDL-stimulated dendritic cells. FEBS Lett 2011;585:657–63. doi:10.1016/j.febslet.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 32.Miyata R, Kakuki T, Nomura K, et al. Poly(I:C) induced microRNA-146a regulates epithelial barrier and secretion of proinflammatory cytokines in human nasal epithelial cells. Eur J Pharmacol 2015;761:375–82. doi:10.1016/j.ejphar.2015.04.031 [DOI] [PubMed] [Google Scholar]
- 33.Neppl RL, Wang DZ. The myriad essential roles of microRNAs in cardiovascular homeostasis and disease. Genes Dis 2014;1:18–39. doi:10.1016/j.gendis.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanet A, Tacheny A, Arnould T, et al. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 2012;40:4742–53. doi:10.1093/nar/gks151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol 2013;10:542–52. doi:10.1038/nrgastro.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaked I, Meerson A, Wolf Y, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009;31:965–73. doi:10.1016/j.immuni.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Lei Y, Zhang H, et al. MicroRNA regulation of STAT4 protein expression: rapid and sensitive modulation of IL-12 signaling in human natural killer cells. Blood 2011;118:6793–802. doi:10.1182/blood-2011-05-356162 [DOI] [PubMed] [Google Scholar]
- 38.Molnár V, Érsek B, Wiener Z, et al. MicroRNA-132 targets HB-EGF upon IgE-mediated activation in murine and human mast cells. Cell Mol Life Sci 2012;69:793–808. doi:10.1007/s00018-011-0786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez-Saavedra M, Antoun G, Yanagiya A, et al. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum Mol Genet 2011;20:731–51. doi:10.1093/hmg/ddq519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strum JC, Johnson JH, Ward J, et al. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol 2009;23:1876–84. doi:10.1210/me.2009-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, et al. miR-143 and miR-145 molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet 2011;4:197–205. doi:10.1161/CIRCGENETICS.110.958702 [DOI] [PubMed] [Google Scholar]
- 42.Elia L, Quintavalle M, Zhang J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 2009;16:1590–8. doi:10.1038/cdd.2009.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 2009;23:2166–78. doi:10.1101/gad.1842409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res 2007;100:1579–88. doi:10.1161/CIRCRESAHA.106.141986 [DOI] [PubMed] [Google Scholar]
- 45.Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008;3:e3694 doi:10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Zhang S, Weber J, et al. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 2010;38:7248–59. doi:10.1093/nar/gkq601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohshima K, Inoue K, Fujiwara A, et al. Let-7 MicroRNA Family Is Selectively Secreted into the Extracellular Environment via Exosomes in a Metastatic Gastric Cancer Cell Line. PLoS ONE 2010;5:e13247 doi:10.1371/journal.pone.0013247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pigati L, Yaddanapudi SCS, Iyengar R, et al. Selective release of microrna species from normal and malignant mammary epithelial cells. PLoS One 2010;5:e13515 doi:10.1371/journal.pone.0013515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010;39:133–44. doi:10.1016/j.molcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 50.Iguchi H, Kosaka N, Ochiya T. Secretory microRNAs as a versatile communication tool. Commun Integr Biol 2010;3:478–81. doi:10.4161/cib.3.5.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diehl P, Fricke A, Sander L, et al. Microparticles: major transport vehicles for distinct miRNAs in circulation. Cardiovasc Res 2012;93:633–44. http://dx.doi.org/10.1093/cvr/cvs007 [DOI] [PMC free article] [PubMed] [Google Scholar]