Abstract
Some dog breeds, including the German shepherd dog (GSD), are predisposed to immune-related disorders. The authors prospectively described development of serum and faecal IgA and serum IgE in GSD from puppies until adulthood and the relationship between mothers and their offspring. Further, the authors tested whether dogs with lower serum IgA also have low faecal IgA and/or serum IgE. To reveal whether any of the parameters could be proven to influence the immune response, the authors also measured serum IgG against canine distemper virus (CDV). To test their hypothesis, the authors used linear mixed models to investigate the relationship of serum IgA, serum IgE and faecal IgA levels in litters and their mothers. Fifteen GSD bitches beginning at 42 days of pregnancy and subsequently all of their offspring (n=83 puppies), reared under well-controlled conditions, were included. All dogs came from the kennel of the Swedish Armed Forces.
Serum IgE, serum IgA and faecal IgA levels were lower in seven-week-old puppies than at one year of age. There was no relationship in Ig concentrations between bitches and their puppies at seven weeks of age. Dogs with higher faecal IgA had higher IgG titres against CDV, indicating a favourable systemic immune status.
Keywords: Dogs, Immunology, Immune mediated diseases
Introduction
Some dog breeds, including the German shepherd dog (GSD), are predisposed to immune-related disorders (Whitbread and others, 1984, Wisselink and others, 1985, Day and others, 1986, Batt and others, 1991, Griot-Wenk and others, 1999, Nodtvedt and others, 2006). In a retrospective study based on insurance data, the authors described the disease pattern in GSD in Sweden and found that this breed was over-represented for a number of immune-related disorders (Vilson and others, 2013). To reveal whether there are differences in immunological parameters in GSD from what is reported in other breeds, the authors performed an extensive screening for Igs in GSD reared under well-controlled conditions.
The concentrations of serum Igs develop during the first year of life and do not reach adult levels until 12 months of age. Schreiber and others (1992) measured serum IgG, IgM and IgA concentrations in 10-month-old beagles, and these concentrations were less than those in older dogs. There are no breed-specific reference ranges for Ig concentration for dogs younger than one year old (Day and others, 2007).
It has previously been documented that serum IgA deficiency is more common and that faecal IgA (Batt and others, 1991, Willard and others, 1994) levels commonly are lower in GSD than in other breeds (Batt and others, 1991, German and others, 2000, Littler and others, 2006). Furthermore, low serum IgA levels have been associated with atopy in GSD (Tengvall and others, 2013).
Faecal IgA stimulate mucosal secretions more than other Ig isotypes in dogs (Heddle and others, 1975). In a study by German and others (1998), no correlation was shown between serum IgA and secretory IgA in tears and saliva, but the correlation between IgA in serum and faeces has not yet been described in GSD or any other breeds.
To establish well-defined data for comparison in further studies on the effect of genetic variations as well as interventions in the environment, the authors have followed changes in serum and faecal IgA and serum IgE from birth to young adult age and evaluated the relationship between dams and their offspring reared under well-controlled conditions. Further, the authors tested whether dogs with lower serum IgA also have low faecal IgA and/or serum IgE. To reveal whether any of the parameters could be proven to influence the immune response, the authors also measured serum IgG against canine distemper virus (CDV).
Materials and methods
Animals
Fifteen pregnant German shepherd bitches from the kennel of the Swedish Armed Forces were recruited at 42 days of pregnancy. All offspring alive at seven weeks of age were included (n=83 puppies). The bitches lived with families and arrived at the kennel at least five days before the start of the study. Upon arrival, they were gradually introduced to the dieti subsequently fed to them as well as to the puppies throughout the entire study. Twelve sires were used for the 15 litters. Eleven of the bitches were imported, mainly from other European countries (one from the USA, the rest from Europe), while the rest were born at the kennel. Three of the sires were imported, five were from other Swedish kennels and the rest were born at the kennel. All bitches and their litters were housed and treated with same routines at the kennel. When the puppies were eight weeks old, they were moved from the kennel to live with families throughout Sweden where living areas were registered as countryside (an area localised between cities) (n=27), small city (<200,000 citizens) (n=38) or big city (>200,000 citizens) (n=18).
The mean age of the bitches was 3.6 years at whelping and they gave birth to on average 6.3 pups/litter. The average weight at birth was 516 g. Ninety-four puppies were born alive, and 18 were stillborn. Another 11 of the puppies died before seven weeks of age (Table 1). Sex distribution of the remaining 83 living puppies was 39 females and 44 males. Mothers and their litters were separated from other dogs at the kennel and they were not exposed to other food. All dogs followed the same vaccination programme. The bitches were routinely vaccinated every third year, with the last vaccination within three years. Puppies were vaccinated with the live vaccine Nobivac DHHPi vet. at 7 weeks of age (after sample collection), at 12 weeks and at 12–13 months of age (after sample collection). The study was approved by the Local Animal Ethical Committee in Uppsala, Sweden (C355/9).
TABLE 1:
Number of puppies alive at birth and seven weeks of age, number of stillborn puppies and average birth weight in each of the 15 study litters
| Litter numbers: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of alive at birth | 5 | 7 | 9 | 4 | 5 | 7 | 3 | 9 | 8 | 3 | 6 | 7 | 4 | 9 | 8 | 94 |
| Number of stillborn | 3 | 0 | 0 | 0 | 1 | 2 | 5 | 0 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 18 |
| Number of alive at 7 weeks | 4 | 7 | 9 | 4 | 4 | 7 | 3 | 9 | 8 | 3 | 5 | 6 | 3 | 5 | 6 | 83 |
| Average birth weight (g) | 390 | 517 | 533 | 625 | 505 | 551 | 613 | 518 | 500 | 420 | 564 | 547 | 487 | 472 | 470 | 516 |
Sample collection
Faeces were collected from mothers at day 42 of pregnancy, at 12–24 hours after completed whelping, at four weeks post partum and at seven weeks post partum. Blood samples were collected from mothers at seven weeks post partum. Blood and faeces were collected from puppies at 7 weeks of age, at 12–13 months of age (median 12 months, 3 weeks) and at 15–18 months (median 16 months, 3 weeks) of age. Parameters measured at the different time points are presented in Table 2.
TABLE 2:
Parameters measured at different time points
| Pregnancy day 42 | Partum | 4 weeks post partum | 7 weeks post partum | 12–13 months | 15–18 months | |
|---|---|---|---|---|---|---|
| Mothers | Faecal IgA | Faecal IgA | Faecal IgA | Faecal IgA Serum IgA Serum IgE |
||
| Puppies | Faecal IgA Serum IgA Serum IgE IgG (CDV) |
Faecal IgA Serum IgA Serum IgE IgG (CDV) |
Faecal IgA Serum IgA Serum IgE IgG (CDV) |
CDV, canine distemper virus
Measurement of Igs
Antibodies in faecal contents
Faecal IgA was extracted from fresh (mothers) or frozen (puppies) faeces. The frozen faecal samples were frozen within two hours of collection and extracted in association with analysis, while the fresh faecal samples were extracted within 45 minutes and then frozen. All faecal and serum samples were frozen at −80°C within 48 hours. Between collection and freezing at −80°C, the samples were stored in dry ice or in −25°C freezer.
Using 1.5 ml of the extraction buffer (50 mM-EDTA and 100 µg/l soybean trypsin inhibitor in PBS/1 per cent bovine serum albumin from Sigma), 0.5 g of faeces were vortexed. Phenylmethanesulphonyl fluoride (25 µl, 350 mg/l from Sigma) was added to each tube, and the samples were centrifuged for 10 minutes. The supernatants were collected and frozen at −80°C until assayed for IgA by ELISA (Bethyl Laboratories) within 45 months as follows: a 96-well plate was coated overnight at 4°C with a 1:100 dilution of goat anti-canine IgA Affinity purified in 50 µl of borate buffer (6.2 g H3BO3/l, 9.54 g Na2B4O7 10 H2O/l and 4.4 g NaCl/l, pH 7) and then washed with PBS-Tween-20. Free binding sites were blocked with 100 µl of PBS containing 5 per cent fetal calf serum and 0.1 per cent Tween-20 (ELISA buffer) for one hour at 37°C. Duplicate faecal extracts were incubated with ELISA buffer (final volume 50 µl) for two hours at 37°C and then washed with PBS-Tween-20. The plate was incubated with a 1:10,000 dilution of polyclonal goat anti-canine IgA conjugated with horseradish peroxidase in ELISA buffer (final volume 50 µl) for one hour at 37°C, washed with PBS-Tween-20 and developed with 50 µl of the 3,3′,5,5′ -tetramethylbenzidine (TMB) peroxidase substrate system according to the manufacturer’s instructions. The reaction was stopped with 50 µl of 1M-phosphoric acid. Colour development was read at 450 nm, and results expressed as µg/ml using a canine IgA standard. The concentration of faecal IgA was adjusted against total protein (TP) content in faeces and expressed as µg IgA/µg TP.
Antibodies in serum
Blood samples were collected from the cephalic vein. Before centrifugation, samples were left to clot for at least 30 minutes in room temperature. After centrifugation (10 minutes, 7200 rpm), serum were collected and frozen. All samples were frozen at –80°C within 48 hours. Between collection and freezing at –80°C, the samples were stored in dry ice or in –25°C freezer.
Serum were assayed for IgA total IgE (Bethyl Laboratories) and IgG against CDV (VMRD) within 45 months by ELISA (Bethyl Laboratories) as follows: a 96-well plate was coated overnight at 4°C with a 1:100 dilution of goat anti-canine IgA, IgE (Bethyl Laboratories) or CDV (VMRD) Affinity purified in 50 μl of borate buffer (6.2 g H3BO3/l, 9.54 g Na2B4O7 10 H2O/l and 4.4 g NaCl/l, pH 7) and then washed with PBS-Tween-20. Free binding sites were blocked with 100 μl of PBS containing 5 per cent fetal calf serum and 0.1 per cent Tween-20 (ELISA buffer) for one hour at 37°C. Duplicate serum samples were incubated with ELISA buffer (final volume 50 µl) for two hours at 37°C and then washed with PBS-Tween-20. The plate was incubated with a 1:10,000 dilution of polyclonal goat anti-canine IgA conjugated with horseradish peroxidase in ELISA buffer (final volume 50 µl) for one hour at 37°C, washed with PBS-Tween-20 and developed with 50 µl of the TMB peroxidase substrate system according to the manufacturer’s instructions. The reaction was stopped with 50 μl of 1M-phosphoric acid. Colour development was read at 450 nm, and results expressed as µg/ml using a canine standard. The vaccine response was calculated as the difference in concentration of IgG against CDV between 7 weeks and 13 months. All results are expressed in this paper as g/l.
Statistics
R (R Core Team, 2012) and lme4 (Bates and others, 2012) were used to perform linear mixed effect analyses of (1) the relationships between Ig concentrations in mother and offspring, (2) the relationships between the different Igs, (3) the change of Igs over time, (4) the impact of sex on Igs and (5) the impact of litter size on Igs. It was necessary to analyse these relationships in linear mixed effects analyses because the data were non-independent (i.e. the puppies were nested in litters) and linear mixed effects models allowed the authors to control for this non-independence. For all the linear mixed models, litter membership was entered as a random effect where the intercept was allowed to vary between litters. The results were presented as the estimated population mean differences based on the model (β). P values were obtained by Wald Z-tests.
To examine the relationship between Ig concentrations in mother and offspring, bitch Ig value was entered as a fixed effect and the litter membership intercept was entered as a random effect.
To examine the relationship between the different Igs, puppy Ig was entered as a fixed effect and litter membership was entered as a random effect.
Intraclass coefficients (ICCs) were used to quantify the degree to which individuals with a fixed degree of relatedness resemble each other in terms of a quantitative trait (Ig concentrations). The closer to 1, the stronger is the litter effect.
For all analyses, data with a Z-score less than –3 or greater than 3 were regarded as an outlier and were not included in the analyses. Level of significance was set to P=0.05.
Results
In total, 74 out of 83 dogs completed the study. Nine excluded dogs were euthanased due to unrelated medical reasons (discospondylitis (n=1), intestinal volvulus (n=2), elbow dysplasia (n=2), hip dysplasia (n=1), hit by car (n=2) and pulmonary abscess (n=1)). Seven out of the nine euthanased puppies died before 13 months of age. Four serum IgA values at 12–13 months had Z-scores higher than 3 and were thereby regarded as outliers, although one of these dogs had also a high outlier value at 15–18 months. Six faecal IgA values and two serum IgE, all at 15–18 months, were regarded as high outliers (Z-score >3) and thereby excluded from the analysis. One dog had high values (Z-score >3) of serum IgG against CDV both at 12–13 months and 15–18 months, and these values were excluded. In the bitches, one serum IgA value had Z-score <3 and one serum IgE had Z-score >3 and were excluded.
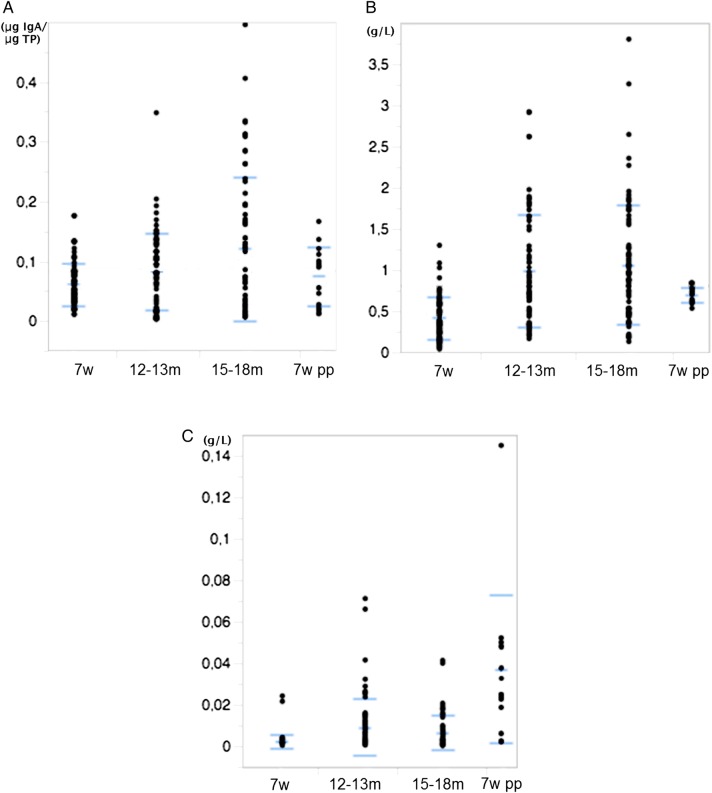
Faecal IgA increased from 7 weeks to 18 months (β=−0.052, P<0.001). Serum IgA (β=−0.576, P<0.001) and serum IgE increased (β=5.404, P<0.001) from 7 weeks to 13 months and was then stabilised between 13 and 18 months (Fig 1). At 12–13 months, faecal IgA levels ranged between 0.002 and 0.347 µg IgA/µg TP to be compared with the maternal levels (0.011–0.165 µg IgA/µg TP, 7 weeds post partum). Serum IgA levels at 12–13 months ranged between 0.165 and 2.921 to be compared with the maternal levels between 0.534 and 0.845 g/l. At 12–13 months, serum IgE ranged from 0.0005 to 0.071 g/l to be compared with the maternal levels (0.002–0.145 g/l).
FIG 1:
Concentrations of (A) faecal IgA (µg IgA/µg TP), (B) serum IgA (g/l) and (c) serum IgE (g/l) in puppies at 7 weeks, 12–13 months and 15–18 months of age and their mothers at 7 weeks pp. Mean and sds are indicated in blue. pp, postpartum; TP, total protein
There was a positive relationship between faecal IgA and vaccine response at 7 weeks (β=0.056, P=0.042), 12–13 months (β=0.032, P=0.008) and 15–18 months (β=0.022, P=0.007) while serum IgA was positively related to vaccine response only at 15–18 months (β=0.003, P=0.0384). Serum IgE and IgA was positively related to each other at 15–18 months (β=0.017, P=0.021). Faecal IgA and serum IgA was not related to each other.
There was no relationship between the mother and her puppies at seven weeks regarding serum IgE, serum IgA or faecal IgA. The correlation within litters (ICC) for faecal IgA and serum IgG against CDV decreased with age, while serum IgA increased by age. In serum IgE, the ICC was close to 0 at all time points (Table 3).
TABLE 3:
Intraclass coefficients, how strongly the puppies within a litter resemble each other
| 7 weeks | 12–13 months | 15–18 months | Mean | |
|---|---|---|---|---|
| Faecal IgA | 0.62 | 0.12 | −0.04 | 0.24 |
| Serum IgA | 0.25 | 0.48 | 0.45 | 0.43 |
| Serum IgE | −0.01 | 0 | 0.1 | 0.11 |
| IgG against CDV | 0.61 | 0.27 | 0.20 | 0.41 |
The closer to 1, the stronger is the litter effect
CDV, canine distemper virus
Male puppies had higher mean faecal IgA than females at seven weeks of age (β=0.014, P=0.006). No other sex differences were detected in any of the Igs (data not shown). Litter size was negatively related to faecal IgA at 12–13 months (β=−0.010, P=0.007). Serum IgA was positively related to birth weight at 12–13 months of age (β=0.003, P=0.005) but not at 7 weeks or 15–18 months. No other Igs were related to birth weight. Delivery mode (caesarean section or not), birth month and living area did not affect the parameters (data not shown).
Discussion
In this extensive study of long-term changes of different Igs in a well-defined population of growing GSDs, the authors identified a relationship between faecal IgA and serum IgG against CDV that might have clinical implications. The authors also found that the levels of serum IgE, serum IgA and faecal IgA are significantly lower in puppies and that levels are stabilised after one year of age. This study may supply data for comparison in further studies on the genetic and environmental interventions on the canine immune system.
The finding of lower levels of serum IgE, serum IgA and faecal IgA in puppies that stabilises after one year of age is in accordance with previous studies (Glickman and others, 1988, Schreiber and others, 1992, Racine and others, 1999, Zaine and others, 2011, Olsson and others, 2014). The variation increased by age, which could be a result of increased exposure to varied environmental factors. The authors could not detect any sex differences in any of the serum Igs. This was neither detected by Olsson and others (2014) for serum IgA levels in adult dogs (n=1247) in a large population of different dog breeds, including GSDs (n=319). This is also in accordance with Griot-Wenk and others (1999), who did not find any sex differences in serum IgA in a study of 233 dogs of different breeds. However, in a study on 147 healthy colonised beagles, Racine and others (1999) found that females had higher levels of total serum IgE compared with males.
Litter size or birth weight did not affect the levels of the Igs measured in this study at seven weeks of age. At 12–13 months of age, there was a negative relationship between faecal IgA and litter size, indicating that dogs from large litters have lower faecal IgA concentrations. There was a positive relationship between serum IgA and birth weight at 12–13 months. However, the relevance of these findings is equivocal since this relation was absent at 7 weeks as well as 15–18 months.
The authors measured CDV vaccine response as a marker for the systemic immune status. Vaccine responses demonstrate clinically relevant alternations in an immune response to a challenge under well-controlled conditions and therefore are often used as a surrogate for responses to an infectious challenge. Faecal IgA was positively related to vaccine response at all ages, which means that dogs with high faecal IgA have a better vaccine response also indicating a favourable systemic immune status. Satyaraj and others (2013) showed that colostrum supplementation increased faecal IgA as well as vaccine response, but it is unknown whether the increased vaccine response resulted from the increase in faecal IgA.
Griot-Wenk and others (1999) showed a negative correlation between serum IgE and serum IgA, and the authors suggested that increased IgE levels might represent an important immune response to reduced IgA levels. The authors could only find a relationship between these Igs at 15–18 months (positive) and not in the other age groups, which is in accordance with a study by Hill and others (1995), where they did not find any correlations between serum IgA, IgE and IgG in a population of adult dogs.
Dogs have an endotheliochorial placenta, which constitutes a relatively impermeable barrier between the maternal and fetal circulation. Puppies are therefore born hypo-gammaglobulinemic and they receive assistance from the mother by antibodies transferred through colostrum. The variation in the efficiency colostral Ig uptake may be related to the size and vigour of the newborn puppy and the maternal abilities of the bitch, as well as the concentration of specific antibodies in the colostrum of the individual bitch. How the concentration of maternal Igs in serum and faeces affects the Ig levels in their offspring is not well studied in dogs. The authors could not find any relationship in Ig concentrations between bitches and their puppies when the puppies were seven weeks old. The puppies, however, strongly resembled each other in faecal IgA, indicating that even if there is no relationship between mother and puppies, there is a strong relation between litter mates. This could be a result of an oral transfer through ingestion of each other’s faeces within the litters. The ICC for IgG against CDV was high in the seven-week-old puppies, but decreased when the dogs were older. Since the puppies were unvaccinated at sample collection at seven weeks of age, IgG are supposed to have maternal origin and the transfer through colostrum should be equal within the litter. However, when the dogs are older, there might be environmental factors that affect the results and the increased variation might influence the ICC.
Delivery mode did not affect the levels of Igs; however, only one litter with two puppies were born trough caesarean section and this small number makes it difficult to draw any conclusions from these results.
It has been indicated that IgA deficiency is more common in GSD than in other breeds (Whitbread and others, 1984, Batt and others, 1991, Olsson and others, 2014). In this study, the authors did not reveal any significant differences between GSD and other breeds regarding serum IgA that would explain a predisposition for immune-related disorders in GSD. Only one dog had a serum IgA at a level below 0.07; the cut-off value for IgA deficiency in man (Olsson and others, 2014). Differences in serum IgA levels between studies could be due to differences between subpopulations of GSDs related to their function as indicated by Tengvall and others (2013). The authors neither noted any clinical signs of faecal IgA deficiency in this study population. The authors could not prove a relationship between serum IgA and faecal IgA in the dogs studied.
This study was strengthened by being based on a large population that was born and raised at the same kennel with standardised daily routines, diet, deworming and vaccination schedules. Additionally, all samples were collected by the same person and analysed at the same laboratory, minimising human-induced variation.
From eight weeks of age, puppies lived with families, which resulted in an increase in the variation arising from environmental factors. These results thereby would be more applicable to pet dogs living in the society compared with data from laboratory dogs and of great value when comparing Ig-level studies on immune stimulation in natural populations of growing dogs.
Further research based on these results could focus on how genetics and environmental factors might affect the immune response in growing dogs.
Conclusion
In conclusion, the levels of serum IgE, serum IgA and faecal IgA were lower in seven-week-old puppies than at one year of age and there was no relationship in Ig concentrations between bitches and their puppies when the puppies were seven weeks old.
The authors showed that dogs with high faecal IgA had a better vaccine response indicating a favourable systemic immune status.
The authors did not identify any significant differences in the Igs of GSD explaining their predisposition for a number of immune-related disorders.
Contributors: ÅV, HH-H, ÅH, AR, JS, BB participated in the design of the study. ÅV collected the samples. ES and RP performed the lab work and prepared the data. CR performed the statistical analysis. All authors participated in manuscript preparation. All authors also read and approved the final manuscript.
Funding: The present study was funded by Nestlé Purina PetCare and made possible by access to dogs born and raised at Kennel of the Swedish Armed Forces.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
Nestlé Purina Pro Plan Puppy Sensitive, Salmon and Rice (32 per cent protein, 20 per cent fat, 1.2 per cent omega 3).
References
- Bates D. M., Maechler M., Bolker B. (2012) lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0 [Google Scholar]
- Batt R. M., Barnes A., Rutgers H. C., Carter S. D. (1991) Relative IgA deficiency and small intestinal bacterial overgrowth in German shepherd dogs. Research in Veterinary Science 50, 106–111 doi:10.1016/0034-5288(91)90062-S [DOI] [PubMed] [Google Scholar]
- Day M. J. (2007) Immune system development in the dog and cat. Journal of Comparative Pathology 137(Suppl 1), S10–S15 doi:10.1016/j.jcpa.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Day M. J., Penhale W. J., Eger C. E., Shaw S. E., Kabay M. J., Robinson W. F., Huxtable C. R., Mills J. N., Wyburn R. S. (1986) Disseminated aspergillosis in dogs. Australian Veterinary Journal 63, 55–59 doi:10.1111/j.1751-0813.1986.tb02924.x [DOI] [PubMed] [Google Scholar]
- German A. J., Hall E. J., Day M. J. (1998) Measurement of IgG, IgM and IgA concentrations in canine serum, saliva, tears and bile. Veterinary Immunology and Immunopathology 64, 107–121 doi:10.1016/S0165-2427(98)00132-9 [DOI] [PubMed] [Google Scholar]
- German A. J., Hall E. J., Day M. J. (2000) Relative deficiency in IgA production by duodenal explants from German shepherd dogs with small intestinal disease. Veterinary Immunology and Immunopathology 76, 25–43 doi:10.1016/S0165-2427(00)00191-4 [DOI] [PubMed] [Google Scholar]
- Glickman L. T., Shofer F. S., Payton A. J., Laster L. L., Felsburg P. J. (1988) Survey of serum IgA, IgG, and IgM concentrations in a large beagle population in which IgA deficiency had been identified. American Journal of Veterinary Research 49, 1240–1245 [PubMed] [Google Scholar]
- Griot-Wenk M. E., Busato A., Welle M., Racine B. P., Weilenmann R., Tschudi P., Tipold S. (1999) Total serum IgE and IgA antibody levels in healthy dogs of different breeds and exposed to different environments. Research in Veterinary Science 67, 239–243 doi:10.1053/rvsc.1999.0314 [DOI] [PubMed] [Google Scholar]
- Heddle R. J., Rowley D. (1975) Dog immunoglobulins. I. Immunochemical characterization of dog serum, parotid saliva, colostrum, milk and small bowel fluid. Immunology 29, 185–195 [PMC free article] [PubMed] [Google Scholar]
- Hill P. B., Moriello K. A., Deboer D. J. (1995) Concentrations of total serum IgE, IgA, and IgG in atopic and parasitized dogs. Veterinary Immunology and Immunopathology 44, 105–113 doi:10.1016/0165-2427(94)05298-7 [DOI] [PubMed] [Google Scholar]
- Littler R. M., Batt R. M., Lloyd D. H. (2006) Total and relative deficiency of gut mucosal IgA in German shepherd dogs demonstrated by faecal analysis. Veterinary Record 158, 334–341 doi:10.1136/vr.158.10.334 [DOI] [PubMed] [Google Scholar]
- Nødtvedt A., Egenvall A., Bergvall K., Hedhammar Å. (2006) Incidence of and risk factors for atopic dermatitis in a Swedish population of insured dogs. Veterinary Record 159, 241–246 doi:10.1136/vr.159.8.241 [DOI] [PubMed] [Google Scholar]
- Olsson M., Frankowiack M., Tengvall K., Roosje P., Fall T., Ivansson E., Bergvall K., Hansson-Hamlin H., Sundberg K., Hedhammar Å., Lindblad-Toh K., Hammarström L. (2014) The dog as a genetic model for immunoglobulin A (IgA) deficiency: identification of several breeds with low serum IgA concentrations. Veterinary Immunology and Immunopathology 160, 255–259 doi:10.1016/j.vetimm.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Racine B. P., Marti E., Busato A., Weilenmann R., Lazary S., Griot-Wenk M. E. (1999) Influence of sex and age on total immunoglobulin E concentration in Beagles. American Journal of Veterinary Research 60, 93–97 [PubMed] [Google Scholar]
- Satyaraj E., Reynolds A., Pelker R., Labuda J., Zhang P., Sun P. (2013) Supplementation of diets with bovine colostrum influences immune function in dogs. The British Journal of Nutrition 110, 2216–2221 doi:10.1017/S000711451300175X [DOI] [PubMed] [Google Scholar]
- Schreiber M., Kantimm D., Kirchhoff D., Heimann G., Bhargava A. S. (1992) Concentrations in serum of IgG, IgM and IgA and their age-dependence in beagle dogs as determined by a newly developed enzyme-linked-immuno-sorbent-assay (ELISA). European Journal of Clinical Chemistry and Clinical Biochemistry 30, 775–778 [DOI] [PubMed] [Google Scholar]
- Tengvall K., Kerczak M., Bergvall K., Olsson M., Frankowiack M., Farias F. H., Pielberg G., Carlborg Ö., Leeb T., Andersson G., Hammarström L., Hedhammar Å., Lindblad-Toh K. (2013) Genome-wide analysis in German Shepherd dogs reveals association of a locus on CFA27 with atopic dermatitis. PLoS Genetics 9, e1003475 doi:10.1371/journal.pgen.1003475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilson Å., Bonnett B., Hansson-Hamlin H., Hedhammar Å. (2013) Disease patterns in 32,486 insured German shepherd dogs in Sweden: 1995–2006. Veterinary Record 173, 116 doi:10.1136/vr.101577 [DOI] [PubMed] [Google Scholar]
- Whitbread T. J., Batt R. M., Garthwaite G. (1984) Relative deficiency of serum IgA in the German shepherd dog: a breed abnormality. Research in Veterinary Science 37, 350–352 [PubMed] [Google Scholar]
- Willard M. D., Simpson R. B., Fossum T. W., Cohen N. D., Delles E. K., Kolp D. L., Carey D. P., Reinhart G. A. (1994) Characterization of naturally developing small intestinal bacterial overgrowth in 16 German shepherd dogs. Journal of the American Veterinary Medical Association 204, 1201–1206 [PubMed] [Google Scholar]
- Wisselink M. A., Willemse A., Koeman J. P. (1985) Deep pyoderma in the German shepherd dog. Journal of American Animal Hospital Assocciation 21, 773–776 [Google Scholar]
- Zaine L., Ferreira C., Gomes Mde O., Monti M., Tortola L., Vasconcellos R. S., Carciofi A. C. (2011) Fecal IgA concentration is influenced by age in dogs. The British Journal of Nutrition 106, 183–186 doi:10.1017/S0007114511000559 [DOI] [PubMed] [Google Scholar]