Abstract
Polyarteritis nodosa (PAN) is a systemic necrotising vasculitis that affects medium-sized and small-sized arteries. The spectrum of disease ranges from involving a single organ to polyvisceral failure. We report a case of a 75-year-old male presented with solely hypertension and mild renal function impairment as a consequence of diffuse renal necrotising vasculitis with occlusive but non-stenotic lesions associated with PAN. The overall prognosis of PAN has been improved in recent decades, primarily reflecting early diagnosis and more effective treatments. Therefore, early diagnosis is critical and it warrants full investigations even in those patients without obvious multiorgan manifestations. In those instances, with mild disease, steroid monotherapy has been shown to be effective with excellent response. Our patient responded well to steroid monotherapy and we were able to gauge his response by improvement in his blood pressure.
Background
Polyarteritis nodosa (PAN) is a systemic necrotising vasculitis that typically affects medium-sized muscular arteries, with occasional involvement of small muscular arteries.1 The affected organs could include the kidneys, gastrointestinal tract, heart, peripheral, central nervous system and skin.2 As there are no known markers, the accurate diagnosis of this disease remains challenging. Renal involvement generally appears with hypertension, haematuria or proteinuria,3 and would frequently result in variable degrees of renal failure, hypertension and perirenal haematomas due to rupture of renal artery aneurysms. In most cases, the incomplete luminal narrowing of the inflamed arteries leads to glomerular ischaemia but not inflammation or necrosis. Therefore, these patients usually have subnephrotic and often minimal proteinuria and modest haematuria, but red blood cell casts are usually absent.1 We report a case of a patient with isolated renal involvement of PAN with no constitutional symptoms or urinary manifestations, nor involvement of any other organ. The patient was treated with merely a corticosteroid-based regimen with improvement of his renal function and hypertension.
Case presentation
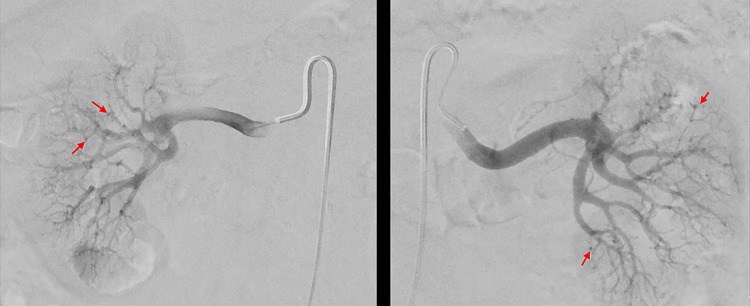
A 75-year-old man with hypertension and coronary artery disease was referred for further evaluation and management of uncontrolled hypertension as well as worsening renal function. Patient had a history of long-standing essential hypertension for 15 years that was previously controlled on long-acting metoprolol 50 mg/day. However, in November 2015, his hypertension worsened and he developed uncontrolled hypertension (HTN) with systolic blood pressures of 200 mm Hg with, at the time, normal renal function. His metoprolol dose was increased to 100 mg/day and he was also started on losartan 100 mg/day. A CT scan of abdomen and pelvis was obtained to evaluate adrenal adenoma and revealed partially obstructed left renal artery, for which no intervention was performed since his blood pressure was improved. The plasma aldosterone/renin ratio and potassium levels were 4.5 and 3.6 mmol/L, respectively, which were within the normal limits. In April 2015, the patient once again developed uncontrolled hypertension with systolic blood pressures in mid-200 mm Hg, his long-acting metoprolol dose was increased to 200 mg/day and his losartan continued at 100 mg/day. At this time, his creatinine level was noted to be elevated to 1.5 mg/dL from a baseline of <1.0 mg/dL. Further work-up of his uncontrolled hypertension demonstrated an elevated chromogranin of 156 ng/mL (reference range 1.9–15 ng/mL) raising concern for carcinoid syndrome. An OctreoScan performed in May 2015 and was negative for carcinoid syndrome. Further laboratory tests including parathyroid hormone (PTH), thyroid and liver function tests as well as 24-hour urine metanephrines were carried out to rule out other aetiologies of elevated chromogranin levels which were unremarkable. Then a renal angiogram was obtained in June 2015, which revealed patent renal arteries, marked irregularity of the interlobar branches of the renal arteries bilaterally with multiple areas of strictures. Numerous microaneurysms involving interlobar branches of renal arteries were found (figure 1), suspicious for polyarthritis nodosa. He was noted to have a normal erythrocyte sedimentation rate (ESR) of 6 mm/hour and mildly elevated C-reactive protein (CRP) of 4.68 mg/L. His hepatic function test, complement levels, other serological tests including antinuclear antibody (ANA) and ANCA levels were all normal. His blood urea nitrogen (BUN) and creatinine (Cr) levels were 50 and 1.93 mg/dL, respectively. The patient was started on prednisone 0.5 mg/kg/day as prescribed by his primary care physician and was referred to our clinic for further management. On presentation to our clinic, he denied any systemic symptoms. His Cr was 1.5 mg/dL, and his urinalysis was negative for haematuria and proteinuria. As the patient denied any systemic symptoms, and had angiogram findings suspicious for PAN, his prednisone dose was increased to 1 mg/kg/day, which was continued for 6 weeks.
Figure 1.
Angiogram of both kidneys in our case of PAN involving the kidneys, arterial wall irregularity along with distal microaneurysm formation in vessels with abrupt termination can be seen. PAN, polyarteritis nodosa.
Outcome and follow-up
With increased dose of steroids, patient's blood pressures improved to 140 mm Hg systolic. Subsequently, metoprolol was discontinued and losartan dose was decreased to 50 mg daily. Patient also had improvement of his renal function with subsequent Cr levels decreasing to 1.51 mg/dL from 1.76 to 1.9 mg/dL. As he had improvement in his hypertension and renal function, he did not require any other immunosuppressant medications. His prednisone dose was decreased to 0.5 mg/kg/day with the plan to taper off in 4 months. His blood pressures became well controlled with systolic blood pressures in the range of 110–120 mm Hg on no antihypertensive medications. Over the course of follow-up, he did not have significant weight change with his weight being 87.9 kg on the first visit and 87.7 kg on the last visit.
Discussion
PAN is a vasculitis of small-sized and medium-sized arteries with an estimated prevalence of 2–33 cases per million people.4 5 PAN is categorised as primary (idiopathic) or secondary (as a complication of a viral infection such as hepatitis B virus). It typically presents with weight loss, livedo reticularis, myalgia, muscle weakness, hypertension or renal dysfunction.6 Unfortunately, to date, no definitive diagnostic marker has been developed, which makes the diagnosis challenging. Diagnosis of PAN is based on the recognition of a vasculitic syndrome with supportive evidence deriving from radiological or pathological studies or both.1
The demonstration of medium-sized vessel microaneurysms is the hallmark of PAN. PAN is diagnosed when characteristic angiographic changes are detected, even in the absence of histological confirmation.7–9 Biopsy of an organ with clinical or laboratory evidence of dysfunction is ideal for verifying the diagnosis of vasculitis. However, angiography is similarly valuable or even preferable to tissue sampling for diagnosis. Travers et al10 reviewed 17 cases of PAN and reported that hepatic, renal and mesenteric arteriography yielded comparable diagnostic information and assisted in the diagnosis of 13 of the 17 patients.
The differential diagnosis of PAN is broad and includes systemic rheumatic diseases, such as systemic lupus erythematosus (SLE), atherosclerotic disease, infections and malignancies.11 12 While it is beyond the scope of this case report to provide a comprehensive list of all possible alternative diagnoses, we need to mention that our patient did not meet the criteria for other diagnoses such as SLE, an infectious or malignant process.
The prognosis of PAN varies based on the degree of major organ system involvement. Prior studies have established the five-factor score (FFS), in order to predict PAN-associated mortality. These criteria initially included proteinuria >1 g/day, renal insufficiency defined as serum creatinine >1.58 mg/dL, gastrointestinal tract surgery, cardiomyopathy and central nervous system involvement;13 however, these criteria were revised in 2009 and include age >65 years, cardiac symptoms, gastrointestinal involvement and renal insufficiency.14 Our patient had an FFS of 1 based on elevated creatinine.
Hypertension is a common finding in patients with PAN and renal involvement, which is postulated to be secondary to activation of the renin–angiotensin system due to renal ischaemia.15
We believe that our patient's worsening renal function and uncontrolled hypertension resulted from diffuse renal necrotising vasculitis with occlusive but non-stenotic lesions of small-sized arteries. On initiation of prednisone, due to the lack of microalbuminuria, we had planned to follow a reduction in his blood pressure or reduction of his creatinine to gauge a response to prednisone. Since his blood pressure and creatinine improved, as we tapered the steroids, we were able to follow his blood pressure and creatinine to evaluate whether the pace of the taper is too rapid.
Glucocorticoids and cyclophosphamide represent the cornerstone of PAN therapy. The distribution of the involved organs and disease progression are the two principal determinants for treating patients with PAN. Current therapeutic approaches consider treating mild forms of primary PAN with corticosteroids alone. Prednisone or prednisolone is typically used at doses of 1 mg/kg/day with subsequent tapering when remission is achieved.16 17 In the presence of persistent critical organ involvement, cyclophosphamide is administered in addition to corticosteroids.16 At present, cyclophosphamide is recommended to induce remission and a safer immunosuppressive agent, such as azathioprine or methotrexate, is advised to maintain remission.18 Given that our patient only had mild worsening renal function with no further organ involvement, it was decided to treat the patient with steroid monotherapy. Our case report shows successful treatment of isolated renal involvement of PAN with steroid monotherapy.
Patient's perspective.
It started for me when I was miserable, very fatigued and jittery. My family physician was alarmed by my blood pressure (BP) which was 200+ over 100+. I had been taking 50 mg of Toporol-XL for years and maintaining a normal BP range. He immediately doubled the Toporol and added Losartan and counselled me to carefully monitor my BP. He began the search to find out the reason for the rapid rise in BP. I had numerous blood tests and a body scan over the next several months.
In late April, my BP again skyrocketed to 200+ over 100+ again. The BP medications were doubled (now quadruple the amount in December). This was now getting serious! Some of the blood test numbers were going crazy and were indicative of a possible carcinoid tumour. The plan was to confirm or rule out the carcinoid tumour, and move on to the kidneys. A nuclear scan proved negative for the carcinoid and a renal angiogram was ordered. The renal angiogram was concerning for a ‘rosary bead’ pattern in the mid-sized arteries of the kidneys, which was reported as ‘highly suspicious of PAN’.
I was started on a treatment of 40 mg of prednisone per day around 1 July 2015 and referred me to Nephrology clinic at University of Florida. After an extensive consultation/examination by nephrologist and rheumatologist at University of Florida, the prednisone was increased to 80 mg per day.
By the first week of August, my BP readings improved and one of my BP medications was discontinued. As the BP was still quite low, my family physician began to methodically reduce the BP medications. Later in August, the prednisone was tapered. By September, I was completely off all BP medications and tapering on prednisone. BP numbers were excellent.
As of this writing (11 April 2016), I am off of all prednisone, off of all prescription drugs and have superior BP readings. I have been playing golf again and getting involved in more outdoor activities. Side effects of the prednisone are lessening, but still lingering. I have been told that my kidney function has improved significantly, but I must continue to monitor my BP carefully.
As I look back on this experience which began about 16 months ago, I thank God for a wonderful team of doctors and the loving care of my wife of 47 years. I have read enough to understand that PAN is very rare and very deadly if not detected early and treated properly. Most people with PAN never know that they have the disease and die of a stroke or heart attack. I am most fortunate and blessed!
Never once did I fear for my life or become discouraged. That is not to say that I have not been troubled and inconvenienced by the side effects of the prednisone. They are very real and debilitating, but for the most part, temporary. Actually, the shakes and confusion presented me and my family with many opportunities for a good laugh!
Learning points.
Polyarteritis nodosa (PAN) is a systemic necrotising vasculitis predominantly targeting medium-sized or small arteries. Renal failure and uncontrolled hypertension could represent renal involvement in PAN and warrants further investigation including consideration of an angiography.
The spectrum of disease ranges from involving a single organ to polyvisceral failure. Except for some forms of gastrointestinal or aortic single organ involvement that can be severe or even fatal, the prognosis of single organ vasculitis is usually very good.
Diagnosis requires the integration of clinical, angiographic and biopsy findings. PAN is diagnosed when characteristic angiographic changes are detected, even in the absence of histological confirmation. Typical arteriographic lesions are arterial saccular or fusiform microaneurysms.
Monotherapy with glucocorticoids has been shown to be effective in those patients with mild disease and often leads to an excellent response. Generalised PAN should be treated with a combination of glucocorticoids and cyclophosphamide.
Footnotes
Contributors: Case was seen by NP under supervision of MS and ES. NP drafted the manuscript. MS and ES contributed to revision of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Balow JE. Renal vasculitis. Kidney Int 1985;27:954–64. 10.1038/ki.1985.104 [DOI] [PubMed] [Google Scholar]
- 2.Hiraike Y, Kodaira M, Sano M et al. Polyarteritis nodosa diagnosed by surgically resected jejunal necrosis following acute abdomen. World J Gastroenterol 2013;19:2830–4. 10.3748/wjg.v19.i18.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagnoux C, Seror R, Henegar C et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum 2010;62:616–26. 10.1002/art.27240 [DOI] [PubMed] [Google Scholar]
- 4.Mahr A, Guillevin L, Poissonnet M et al. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 2004;51:92–9. 10.1002/art.20077 [DOI] [PubMed] [Google Scholar]
- 5.Haugeberg G, Bie R, Bendvold A et al. Primary vasculitis in a Norwegian community hospital: a retrospective study. Clin Rheumatol 1998;17:364–8. 10.1007/BF01450893 [DOI] [PubMed] [Google Scholar]
- 6.Lightfoot RW, Michel BA, Bloch DA et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 1990;33:1088–93. 10.1002/art.1780330805 [DOI] [PubMed] [Google Scholar]
- 7.Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg-Strauss syndrome. Lupus 1998;7:238–58. 10.1191/096120398678920055 [DOI] [PubMed] [Google Scholar]
- 8.Greco A, De Virgilio A, Rizzo MI et al. Microscopic polyangiitis: advances in diagnostic and therapeutic approaches. Autoimmun Rev 2015;14:837–44. 10.1016/j.autrev.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 9.Watts R, Lane S, Hanslik T et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. 10.1136/ard.2006.054593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travers RL, Allison DJ, Brettle RP et al. Polyarteritis nodosa: a clinical and angiographic analysis of 17 cases. Semin Arthritis Rheum 1979;8:184–99. 10.1016/S0049-0172(79)80007-4 [DOI] [PubMed] [Google Scholar]
- 11.Bateman H, Rehman A, Valeriano-Marcet J. Vasculitis-like syndromes. Curr Rheumatol Rep 2009;11:422–9. 10.1007/s11926-009-0062-9 [DOI] [PubMed] [Google Scholar]
- 12.Molloy ES, Langford CA. Vasculitis mimics. Curr Opin Rheumatol 2008;20:29–34. 10.1097/BOR.0b013e3282f1dcf2 [DOI] [PubMed] [Google Scholar]
- 13.Guillevin L, Lhote F, Gayraud M et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore) 1996;75:17–28. 10.1097/00005792-199601000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Guillevin L, Pagnoux C, Seror R et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011;90:19–27. 10.1097/MD.0b013e318205a4c6 [DOI] [PubMed] [Google Scholar]
- 15.White RH, Schambelan M. Hypertension, hyperreninemia, and secondary hyperaldosteronism in systemic necrotizing vasculitis. Ann Intern Med 1980;92(Pt 1):199–201. 10.7326/0003-4819-92-2-199 [DOI] [PubMed] [Google Scholar]
- 16.Guillevin L, Cohen P, Mahr A et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty-five patients. Arthritis Rheum 2003;49:93–100. 10.1002/art.10922 [DOI] [PubMed] [Google Scholar]
- 17.Ribi C, Cohen P, Pagnoux C et al. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: a prospective randomized study of one hundred twenty-four patients. Arthritis Rheum 2010;62:1186–97. 10.1002/art.27340 [DOI] [PubMed] [Google Scholar]
- 18.Guillevin L, Lhote F, Amouroux J et al. Antineutrophil cytoplasmic antibodies, abnormal angiograms and pathological findings in polyarteritis nodosa and Churg-Strauss syndrome: indications for the classification of vasculitides of the polyarteritis Nodosa Group. Br J Rheumatol 1996;35:958–64. 10.1093/rheumatology/35.10.958 [DOI] [PubMed] [Google Scholar]