Abstract
Granulomatosis with polyangiitis is an uncommon condition characterised by vasculitis and associated granuloma formation with a highly specific autoantibody, namely proteinase 3-anti-neutrophil cytoplasmic antibody (ANCA). The respiratory tract and kidneys are the organ systems most often involved. Symptoms can be non-specific, and isolated hearing loss can predate other symptoms by months, leading to lengthy delays in diagnosis and treatment. Left untreated, hearing loss can be irreversible, and therefore early diagnosis is crucial. We present a case study of severe hearing impairment in an attempt to raise awareness of ear involvement as an early feature of this unusual condition.
Background
Granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, is a systemic inflammatory condition characterised by vasculitis and necrotising granuloma that most commonly affects the upper respiratory tract and lower respiratory tract and the kidneys. It is a member of the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV), along with microscopic polyangiitis and Churg-Strauss syndrome.1 Typical features of the disease include fatigue, weight loss, recurrent sinusitis and haemoptysis. In addition, ear involvement is a common, but non-specific finding that occurs along a spectrum of severity, from mild inflammation to profound and sometimes to irreversible hearing loss. We present a case of severe hearing impairment in the setting of GPA, in an attempt to raise awareness of ear involvement as an early feature of this uncommon disorder.
Case presentation
A 38-year-old female became unwell while travelling abroad on a holiday, with pain in her right ear and associated sweats. On her return, she presented to an ear, nose and throat (ENT) specialist, and was given a diagnosis of ear infection that was treated with antibiotics. Two weeks later, she again felt unwell and developed right-sided facial pain. She was again treated with antibiotics, and X-rays revealed the presence of wisdom teeth. As her pain continued, extraction of two wisdom teeth was performed. She subsequently noted hearing loss in her right ear and sinus pain, and this led to a second ENT review where a diagnosis of otitis media was made. A grommet was inserted into her right ear, which led to temporary improvement in her hearing. Her facial pain was ongoing, however, despite treatment with gabapentin, ibuprofen and tramadol.
After 4 months from her initial symptoms, she became increasingly symptomatic with right-sided facial pain, sweats, loss of taste and smell, and bilateral hearing loss. She had also developed shortness of breath and haemoptysis. She was assessed by a second ENT specialist, who observed severe nasal crusting, bilateral tympanic membrane perforations and granulation of the right tympanic membrane. The nasal mucosa was cobbled and friable, and it bled easily. Biopsies of the nasal mucosa revealed focal ulceration with a diffuse, mixed inflammatory infiltrate within the submucosa, as well as active granuloma formation and vasculitis. The features were highly suspicious for GPA and so further workup, including vasculitis screen, chest X-ray and CT sinuses, were performed and a prompt review by the immunology service was set up.
Investigations
The patient's initial laboratory investigations revealed a normal full blood count and renal profile, a C reactive protein (CRP) of 176 mg/L (normal <5) and erythrocyte sedimentation rate (ESR) of 108 mm/hour (normal <10). Autoantibody tests revealed a positive cytoplasmic ANCA pattern (titre 1:160) and strongly positive antiproteinase 3 (PR3) antibodies (96 IU/mL). Urine analysis was positive for red cell casts, indicating likely glomerular involvement. A chest X-ray showed multiple cavitating lesions, and CT sinus demonstrated mucosal thickening and nasal septum deviation (figure 1). Audiometry testing revealed profound bilateral hearing loss, up to more than 100 dB at higher frequencies (figure 2). She was referred to the immunology department and started on oral cyclophosphamide 125 mg/day, together with prednisolone 60 mg daily. Additional therapy included a bisphosphonate for bone protection, a proton-pump inhibitor and cotrimoxazole for prophylaxis of Pneumocystis pneumonia.
Figure 1.
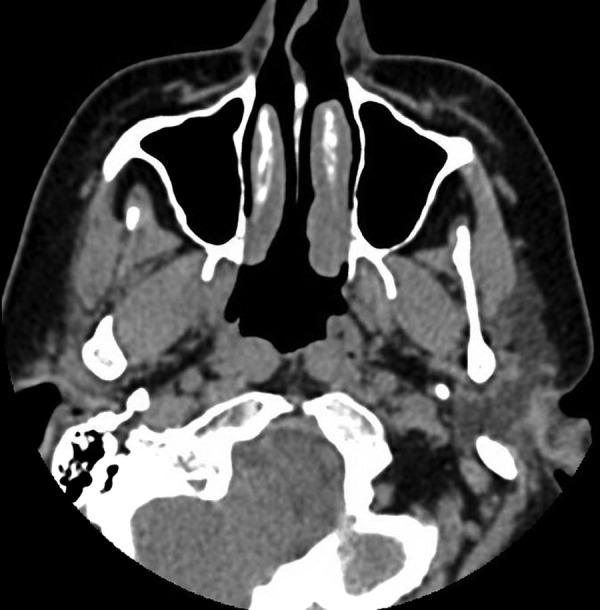
Axial CT sinus demonstrating mild mucosal thickening of the right maxillary sinus and septal deviation. Sinus disease is a non-specific finding that should alert clinicians to the possibility of a vasculitic disorder.
Figure 2.
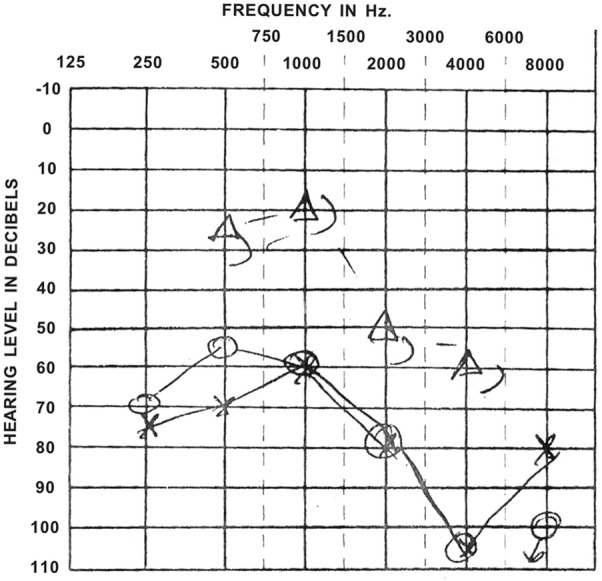
Audiometry recording, taken prior to diagnosis, showing significant bilateral hearing impairment, particularly at 4000 Hz.
Differential diagnosis
- Common causes of hearing loss in adults;
- Cerumen build-up;
- Otitis media;
- Tympanic membrane perforation;
- Cholesteatoma;
- Otosclerosis;
- Meniere’s disease;
- Acoustic neuroma;
- Autoimmune hearing loss (including GPA).
Outcome and follow-up
Two weeks following her initial treatment, she reported a marked improvement in her systemic symptoms, including reduced facial pain and nasal discharge, and a return of her sense of taste and smell. Although her hearing in one-to-one conversation was satisfactory, she still experienced difficulty in the context of any significant background noise. She continued to have close clinical follow-up and 4 weeks later, she reported an improvement in all of her symptoms except for her hearing loss, which had not changed. Repeat laboratory testing revealed a reduction in inflammatory markers and a fall in PR3-ANCA levels. She was continued on immunosuppressive therapy, and cyclophosphamide was replaced by azathioprine after 3 months of therapy.
As her hearing did not improve, she was equipped with bilateral hearing aids. She continues to experience episodes of moderate disease relapse, which are treated with short courses of prednisolone as well as with azathioprine dose escalation. At her last review in March 2016, she was clinically well on a maintenance dose of 100 mg of azathioprine daily. Her hearing has not recovered.
Discussion
GPA is a member of the ANCA-associated vasculitides, characteristically associated with cANCA positivity against PR3.1 Most commonly, patients experience constitutional symptoms such as fatigue, fever and night sweats, and respiratory symptoms such as epistaxis, sinusitis, cough and haemoptysis. Although not specific, hearing loss can be a presenting feature in an important minority of cases, and can often predate other more characteristic symptoms by several months.2 The broad spectrum of non-specific symptoms that occur in GPA can make diagnosis difficult, leading to delays in treatment. If a diagnosis of GPA is suspected, relevant investigations include a full blood count, renal profile, ESR, CRP and cANCA titre. A urine dipstick should be performed to assess for glomerulonephritis; relevant radiological tests include chest X-rays and CTs of the thorax and sinuses.
Ear involvement in GPA can vary considerably in its severity, with hearing loss occurring as a result of conductive and sensorineural mechanisms.2 In the retrospective analysis of the frequency of hearing loss in patients with GPA, it was found in 56% of the patients. In addition, outcomes were worse in cases of sensorineural hearing loss (where 3 of 17 cases improved) compared with conductive hearing loss (7 of 12 improved). As our case study has shown, hearing loss can be irreversible even when other symptoms have fully resolved.
Treatment of GPA generally consists of an induction phase and a maintenance phase.3 Induction is most commonly achieved with a combination of cyclophosphamide and prednisolone. In Fauci's original study, this regimen was successful in achieving complete remission in 93% of cases.4 As a result of the potential significant toxicity of cyclophosphamide and the greater cumulative drug exposure when taken orally, pulsed intravenous cyclophosphamide is preferred over the oral route. In the CYCLOPS (pulsed versus continuous oral CYClophosphamide as therapy for systemic vasculitis) study, pulsed intravenous cyclophosphamide was as effective as daily oral cyclophosphamide in inducing remission,5 although it was associated with a higher rate of relapse in a subsequent long-term follow-up study.6 More recent studies have suggested that the anti-B-cell antibody rituximab can be equally effective in inducing remission.7 8 Options for maintenance therapy include azathioprine, methotrexate and rituximab, with increasing use of the latter drug. In a prospective study comparing maintenance rituximab with azathioprine following induction with cyclophosphamide and glucocorticoids, patients who received maintenance rituximab at 6 monthly intervals were less likely to relapse compared with those on a maintenance daily dose of azathioprine.9
One problem that clinicians face is distinguishing disease flares from intercurrent illness. While CRP and ESR are useful for the monitoring of inflammation, these are non-specific and cannot reliably distinguish between active disease and intercurrent infection. Development of biomarkers that can predict relapse of GPA is a major goal of research; however, this has remained problematic. In an earlier study, we demonstrated that a significant rise in a combination of three blood parameters—neutrophil count, CRP and PR3-ANCA level can predict relapse in some 59% of patients.10 However, identifying a reliable biomarker for disease activity is still a goal for researchers.
This case highlights the often unusual and non-specific features that can occur in early GPA. Our patient reported earache initially, and then non-specific unilateral facial pain, which was attributed to the presence of wisdom teeth. It was only when her symptoms worsened that the diagnosis of GPA was considered. Unfortunately, this is a common pitfall for clinicians. Given the highly variable pattern of presenting symptoms in GPA and the fact that isolated symptoms, such as hearing loss, can predate other symptoms by many months, opportunities for early diagnosis may be missed, leading to potentially irreversible and life-altering consequences for patients. Therefore, we suggest that the combination of new onset ear or facial pain with unexplained hearing loss in an otherwise healthy adult should raise suspicion for a vasculitic disorder such as GPA.
Learning points.
Granulomatosis with polyangiitis (GPA) has a highly variable and non-specific pattern of presenting symptoms.
Isolated ear pain or hearing loss can predate other symptoms by months, resulting in opportunities for early diagnosis and treatment to be missed.
New onset, unexplained hearing loss in an otherwise healthy adult should raise suspicion for GPA.
Footnotes
Contributors: PB authored the first draft of the manuscript, and obtained patient consent for publication. CF coauthored and edited the manuscript for publication. NC has given significant input into the care of the patient and was instrumental in preparing the revised manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lally L, Spiera R. Current landscape of antineutrophil cytoplasmic antibody-associated vasculitis: classification, diagnosis, and treatment. Rheum Dis Clin North Am 2015;41:1–19. 10.1016/j.rdc.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Bakthavachalam S, Driver MS, Cox C et al. Hearing loss in Wegener's granulomatosis. Otol Neurotol 2004;25:833–7. 10.1097/00129492-200409000-00030 [DOI] [PubMed] [Google Scholar]
- 3.Smith RM. Update on the treatment of ANCA associated vasculitis. La Presse Médicale 2015;44:241–9. 10.1016/j.lpm.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Fauci AS, Haynes BF, Katz P et al. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983;98:76–85. 10.7326/0003-4819-98-1-76 [DOI] [PubMed] [Google Scholar]
- 5.de Groot K, Harper L, Jayne DR et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Int Med 2009;150:670–80. 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 6.Harper L, Morgan MD, Walsh M et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis 2012;71:955–60. 10.1136/annrheumdis-2011-200477 [DOI] [PubMed] [Google Scholar]
- 7.Jones RB, Tervaert JW, Hauser T et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363:211–20. 10.1056/NEJMoa0909169 [DOI] [PubMed] [Google Scholar]
- 8.Stone JH, Merkel PA, Spiera R et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillevin L, Pagnoux C, Karras A et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014;371:1771–80. 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 10.Hogan PC, O'Connell RM, Scollard S et al. Biomarkers predict relapse in granulomatosis with polyangiitis. J Biomark 2014;2014:596503 10.1155/2014/596503 [DOI] [PMC free article] [PubMed] [Google Scholar]


