Abstract
Objective
To examine whether older age was associated with lower health-related quality of life (HRQOL) among patients with systemic lupus erythematosus (SLE) and whether differential disease-related damage and activity explained these associations.
Methods
We used cross-sectional data on 684 patients with SLE aged ≥20 years from the Georgians Organized Against Lupus cohort to estimate the associations between age (categorised as 20–39, 40–59 and ≥60 years) and HRQOL (Short Form-12 norm-based domain and physical component summary (PCS) and mental component summary (MCS) scores), using multivariable linear regression. We then examined the effect of disease-related damage and activity on these associations.
Results
The mean age of the cohort was 48.2±13.1 years (range, 20–88 years), with 28.0%, 52.9% and 19.1% of participants being aged 20–39, 40–59 and ≥60 years, respectively; 79.0% were African-American and 93.7% were female. The mean PCS score was 39.3 (41.8, 38.7 and 37.4 among those aged 20–39, 40–59 and ≥60 years, respectively), while the mean MCS score was 44.3 (44.2, 43.8 and 46.1, respectively). In general, lower physical but not mental HRQOL scores were associated with older age. With adjustment, older ages (40–59 and ≥60, respectively, vs 20–39) remained associated (β (95% CI)) with lower PCS (−2.53 (−4.58 to −0.67) and −3.57 (−6.19 to −0.96)) but not MCS (0.47 (−1.46 to 2.41) and 1.20 (−1.52 to 3.92)) scores. Associations of age with HRQOL domain and summary scores were not substantially changed by further adjustment for disease-related damage and/or activity.
Conclusions
Nearly one in five participants in this large, predominantly African-American cohort of patients with SLE was at least 60 years old. The associations of older age with lower physical, but not mental, HRQOL were independent of accumulated SLE damage and current SLE activity. The results suggest that studies of important geriatric outcomes in the setting of SLE are needed to inform patient-centred clinical care of the ageing SLE population.
Keywords: Quality of LIfe, Epidemiology, Disease Activity
Introduction
Systemic lupus erythematosus (SLE) was historically considered a disease of young women.1 However, as a complex, chronic disease, SLE can also be thought of as analogous to ‘geriatric syndromes’,2 which are multifactorial health conditions that involve morbidity across multiple organ systems and confer susceptibility to poor outcomes, including low health-related quality of life (HRQOL).3 4 Furthermore, SLE also affects older individuals,5 and the increasing pool of older individuals in the US population6 who can develop late-onset SLE (up to 18% of patients with SLE7) and longer life expectancy for all patients with SLE7 8 will likely result in a substantial population of older patients with SLE over time. As patients with SLE age, they are likely to accumulate disease-related damage as well as experience changes in SLE activity. Lower HRQOL may be associated with both increased disease activity and greater accumulated damage,9–13 although this evidence is not consistent across time or all HRQOL domains.4 13 However, independent of SLE-specific disease activity and accumulated organ damage, patients with SLE may also experience changes in their HRQOL that are related to age itself.
Despite this, few studies have explicitly examined the independent association of age with HRQOL in SLE. Older age has been shown to be a potential linear predictor of lower HRQOL in patients with SLE,4 13 but previous studies of patients with SLE generally have not examined categorisations of age that might reveal important non-linear patterns in the association of HRQOL with age. Knowledge of how HRQOL differs across the lifespan (ie, young, middle-aged and older) in this population could support care by providing context for results from clinically recommended assessment of HRQOL.14 It is also important to understand to what extent clinical factors such as SLE-associated organ damage and SLE activity, which are also recommended for comprehensive SLE assessment,14 drive associations between age and HRQOL. As individuals age, their reported HRQOL may be less related to current health status,15 and disease severity may have differential effects with age. For example, in a population-based study, higher degree of renal insufficiency was shown to have disproportionately higher impact on physical aspects of HRQOL, but disproportionately lower impact on mental aspects of HRQOL, among older versus younger adults.16 In SLE, similar differential effects may exist, and disease factors are also likely to differ over the lifespan: while SLE-associated organ damage is likely to increase with age,17 SLE activity may be lower among the oldest individuals.18
Greater understanding of the associations of age with HRQOL—and the relative contributions of cumulative disease damage and disease activity to these associations—may inform this recommended care for patients with SLE of all ages. Furthermore, many previous studies of HRQOL have targeted predominantly white populations,19 20 despite African-Americans having greater susceptibility to SLE and poorer outcomes of SLE, including greater disease activity and damage.21–25 Thus, examining these questions in a cohort with better representation of African-Americans is needed to advance our understanding of the effects of age, disease activity and damage on HRQOL in SLE. Using cross-sectional data from the ongoing, predominantly African-American Georgians Organized Against Lupus (GOAL) cohort, which enrolled large numbers of adult patients with SLE of young, middle and older ages, we examined whether these age categories are independently associated with various domains of HRQOL among patients with SLE and whether these associations are independent of SLE-specific changes in accumulated disease damage and disease activity over time.
Methods
Study design, population and data sources
We used data from the ongoing GOAL cohort study of patients with SLE in metropolitan Atlanta, Georgia. GOAL recruitment and data collection details have been published previously.26 Briefly, participants of the GOAL study were recruited primarily from the existing Georgia Lupus Registry, a population-based registry funded by the Centers for Disease Control and Prevention, in order to estimate the incidence and prevalence of SLE in metropolitan Atlanta.27 Patients not included in the registry but who were receiving SLE treatment at Emory University, at Grady Memorial Hospital (safety net hospital in Atlanta) or from community rheumatologists in metropolitan Atlanta at the time of study recruitment were additionally recruited to further enrich this population-based cohort. All participants were recruited by mail, by telephone and in person, and assessments have been performed annually since Wave 1 (baseline; September 2011–September 2012). A total of 850 adult participants (aged ≥18 years) with a documented diagnosis of SLE (more than four revised American College of Rheumatology (ACR) criteria28 or three ACR criteria plus a diagnosis of SLE by the attending board-certified rheumatologist) were included in Wave 1.
We used a cross-sectional design to describe the association of age at survey with HRQOL reported during a single wave (Wave 3; September 2013–September 2014). A total of 714 adult patients with SLE with known age (aged ≥20 years at Wave 3) participated in Wave 3 of GOAL. For analyses, patients were excluded if they were missing HRQOL summary scores (n=11) or any other covariates (n=19), leaving 684 patients in the final models for summary scores. The Emory University Institutional Review Board, Grady Health System Research Oversight Committee and Georgia Department of Public Health Institutional Review Board approved the GOAL study protocol. All GOAL participants provided informed consent.
Study variables
Age at survey
Self-reported age at survey, which served as the exposure of interest, was grouped into categories of 20–39 (young), 40–59 (middle-aged) and ≥60 (oldest) years for analyses, to estimate associations of HRQOL with different phases of the adult lifespan.29 Age at survey was also examined as a continuous exposure in sensitivity analyses (see below).
HRQOL
HRQOL was obtained from the self-administered SF-12 questionnaire,30 which is a validated 12-item version of the SF-36 questionnaire31 32 recommended for use in SLE.14 Overall summary HRQOL scores (mental component summary (MCS) and physical component summary (PCS) scores; scale, 0–100, with higher scores representing better HRQOL, and 50 representing the average score for 45–54 year olds in the general US population33) were calculated based on the responses to the 12 items33 and served as the primary outcomes. Norm-based scoring of individual domain scores (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) was used, such that the scales and interpretation of domain scores were the same as those for MCS and PCS, and the domain scores can be meaningfully compared.34
SLE-related organ damage and activity
SLE-related organ damage was assessed via the Brief Index of Lupus Damage (BILD)35 (range, 0–30, with higher scores indicating greater levels of damage); the self-administered BILD was further validated in GOAL.36 Current SLE activity was assessed via the Systemic Lupus Activity Questionnaire (SLAQ)37 (range, 0–44, with higher scores indicating greater SLE-related disease activity.
Other variables
Age at SLE onset, sex, race, ethnicity, body mass index (BMI), education, employment status, income and marital status were self-reported. SLE duration was calculated as the number of years between age at SLE onset and age at survey. Because older adults with SLE could have developed SLE as a child, as a young adult or as an older adult, we also categorised age at onset as paediatric (<18 years), early adult (18–49 years) and late adult (≥50 years) onset, as in prior studies.38 Emotional support was determined by the survey item ‘How often do you get the emotional support you need?’ and categorised as always/usually versus sometimes/rarely/never. Renal involvement in SLE was determined by whether renal ACR criteria28 were met in the diagnosis of SLE. Impact of SLE was assessed via the Lupus Impact Tracker (LIT)39 instrument, which has scores scaled to 0–100, with higher scores indicating greater impact of SLE. The presence of depressive symptoms was assessed via the nine-item Patient Health Questionnaire (PHQ-9) depression severity screener.40 PHQ-9 scores (range 0–27, with higher scores indicating more severe depression) were categorised as 5 or more (mild to severe depressive symptoms) versus 0–4 (none or minimal depressive symptoms).
Statistical analysis
Participant characteristics, including sociodemographic and clinical factors, were summarised overall and by age group. HRQOL scores were also summarised overall and by age group. Slopes (βs) and 95% CIs for the associations between age group and HRQOL scores were estimated with multivariable linear regression models. Adjustment for a priori confounders sex and race (categorised as African-American vs not African-American) and for potential mediators between age and HRQOL (employment, marital status, emotional support and SLE duration) was performed; adjustment for SLE duration alone was also performed. Because the associations of SLE-related damage and SLE activity with age may differ in direction, the effects of further adjustment for damage (BILD score), activity (SLAQ score) and both were examined in this fully adjusted model, to explore the separate and combined influence of each on the association of age with HRQOL. In sensitivity analyses, we examined the effects of: (i) further adjustment for BMI, depressive symptoms and LIT score, which were excluded from main models because they are likely on the causal pathways between BILD, SLAQ and HRQOL; (ii) further adjustment for income, which were excluded from the main models due to missing data and likelihood of being on the pathway from employment and marital status to HRQOL; (iii) further adjustment for renal involvement in SLE, to account for the lack of renal components in the SLAQ score; (iv) using continuous versus categorical age, to address whether age cut-offs mask linear effects of age on HRQOL and (v) the addition of an age-squared term to the continuous age models (to assess whether the effects of age on HRQOL were potentially continuous but non-linear). Stata V.13 (StataCorp, College Station, Texas, USA) was used for all analyses, and the threshold for statistical significance was set at α=0.05.
Results
Characteristics of the SLE cohort
Table 1 shows that 29.0%, 52.9% and 18.1% of GOAL participants included in our study were aged 20–39, 40–59 and ≥60 years, respectively, with the mean age being 47.8 years (range 20–88 years). A total of 26 (3.8%) and 4 (0.6%) participants were ≥70 and ≥80 years, respectively. Overall, 5.6% of participants were male and 78.5% were African-American, and there were no differences in sex or race by age group (table 1). Participants who were older were more likely to be or to have been married, to report always or usually having emotional support and to have higher annual income, compared with younger patients (table 1). While the oldest patients were most likely to report being unemployed (primarily due to retirement), 35.9%, 44.5% and 21.8% of young, middle-aged and older participants, respectively, reported not working due to disability.
Table 1.
Characteristics of the Georgians Organized Against Lupus cohort of patients with SLE in September 2013–November 2014, overall and by categorised age at survey
| Age at survey, years |
||||||
|---|---|---|---|---|---|---|
| Characteristic | N | Overall | 20–39 N=198 (29.0%) |
40–59 N=362 (52.9%) |
≥60 N=124 (18.1%) |
p Value* |
| Sociodemographic | ||||||
| Mean (SD) age at survey | 684 | 47.8 (13.0) | 32.3 (5.1) | 49.8 (5.7) | 66.9 (5.7) | <0.001 |
| % Male | 684 | 5.6% | 5.6% | 5.8% | 4.8% | 0.92 |
| Race, % | 684 | 0.16 | ||||
| White | 19.6% | 19.2% | 18.0% | 25.0% | ||
| African-American | 78.5% | 77.3% | 81.0% | 74.2% | ||
| Other | 1.9% | 3.5% | 1.4% | 0.8% | ||
| % Hispanic/Latino | 680 | 5.0% | 7.1% | 4.7% | 2.4% | 0.16 |
| Marital status, % | 684 | <0.001 | ||||
| Never married | 32.5% | 58.1% | 26.26% | 9.7% | ||
| Married | 32.6% | 18.2% | 37.1% | 42.7% | ||
| Separated, divorced, widowed | 34.9% | 23.7% | 36.7% | 47.6% | ||
| Mean (SD) years of education | 673 | 14.6 (3.1) | 14.6 (2.9) | 14.6 (3.0) | 14.9 (3.5) | 0.64 |
| Employment, % | 684 | <0.001 | ||||
| Employed | 37.3% | 45.0% | 38.7% | 21.0% | ||
| Not employed† | 24.9% | 19.2% | 16.9% | 57.3% | ||
| Disabled | 37.9% | 35.9% | 44.5% | 21.8% | ||
| Income, % | 630 | <0.001 | ||||
| <US$20 000 | 41.1% | 47.6% | 42.9% | 24.6% | ||
| US$20 000–$49 999 | 27.0% | 30.2% | 22.7% | 34.6% | ||
| ≥US$50 000 | 31.9% | 22.2% | 34.4% | 40.9% | ||
| % Always/usually have emotional support | 684 | 56.7% | 57.6% | 53.3% | 65.3% | 0.06 |
| Clinical | ||||||
| Mean (SD) age at onset | 684 | 32.4 (12.2) | 21.8 (6.1) | 33.8 (9.5) | 45.3 (11.8) | <0.001 |
| Age at onset, % | 684 | <0.001 | ||||
| Paediatric (<18 years) | 10.5% | 26.3% | 4.7% | 2.4% | ||
| Early adult (18–49 years) | 81.0% | 73.7% | 91.7% | 61.3% | ||
| Late adult (≥50 years) | 8.5% | 0.0% | 3.6% | 36.3% | ||
| Mean (SD) duration of SLE, years | 684 | 15.4 (9.6) | 10.5 (5.9) | 15.9 (9.3) | 21.6 (11.3) | <0.001 |
| % SLE onset within past 10 years | 684 | 33.6% | 49.5% | 31.2% | 15.3% | <0.001 |
| Mean (SD) BMI | 644 | 29.3 (7.8) | 28.1 (7.7) | 30.0 (8.1) | 29.3 (6.8) | 0.03 |
| Renal involvement‡, % | 633 | 30.2% | 41.5% | 27.7% | 21.3% | <0.001 |
| Median (IQR) PHQ-9 score | 683 | 7 (3–12) | 7 (3–12) | 8 (4–13) | 6 (2–10) | 0.004 |
| Median (IQR) BILD score | 684 | 3 (1–5) | 2 (1–3) | 3 (1–5) | 4 (2–5) | <0.001 |
| Median (IQR) SLAQ score | 684 | 15 (9–23) | 15 (9–23) | 17 (10–24) | 15 (8–20) | 0.22 |
| Mean (SD) LIT score | 682 | 41.8 (24.1) | 40.9 (24.0) | 43.9 (24.4) | 37.1 (22.6) | 0.02 |
*By χ2, Fisher's exact, analysis of variance or non-parametric equality-of-medians test, as appropriate.
†Includes retired, student and homemaker population.
‡From American College of Rheumatology diagnostic criteria.
BILD, Brief Index of Lupus Damage; BMI, body mass index; LIT, Lupus Impact Tracker; PHQ-9, nine-item Patient Health Questionnaire; SLAQ, Systemic Lupus Activity Questionnaire; SLE, systemic lupus erythematosus.
Older age at SLE onset was seen among older participants, with mean ages of SLE onset of 21.8 versus 45.3 for the youngest versus oldest age groups (table 1). About one-third of patients aged ≥60 years had late-onset SLE (table 1). BMI and BILD scores were higher with older age, whereas PHQ-9 and LIT scores were lower with older age; SLAQ scores were highest in the middle-age, but scores were not statistically significantly different across age groups (table 1).
In a comparison of participants included in the analysis (n=684) and participants excluded from the analysis due to missing data on HRQOL scores or covariates (n=30; 4.2%), 40.0% of those excluded were ≥60 years old, versus 18.1% in the included cohort (p<0.001). Related, mean SLE duration was longer (19.4 vs 15.4 years; p=0.03) among excluded versus included participants. Other characteristics that differed by excluded versus included status were: male sex (23.3% vs 5.6%; p<0.001), BMI (26.2 vs 29.3 kg/m2; p=0.04) and education (13.2 vs 14.6 years; p=0.01). No other characteristics listed in table 1, including BILD and SLAQ scores, differed by exclusion status.
Association of HRQOL with age in a cohort of patients with SLE
The SF-12 norm-based scores were <50 for all HRQOL domains, overall and across age categories (table 2). Overall, scores reflecting physical aspects of HRQOL were lower in older age groups: PCS scores were 41.8, 38.7 and 37.4 among the young, middle-age and oldest age groups, respectively, and patterns were similar for the physical functioning, role physical and bodily pain scores (table 2). In contrast, general health, vitality, social functioning and role emotional scores were similar across age groups, and MCS and mental health scores were highest in the oldest group, although the differences were not statistically significant (table 2). A spider plot by age group (figure 1) demonstrates that the youngest age group had the highest mean scores related to physical aspects of HRQOL, but lower scores related to mental aspects of HRQOL, relative to the oldest patients. The oldest patients had the lowest and highest scores on physical and mental aspects of HRQOL, respectively; and the middle-aged patients had the lowest MCS and mental health scores (figure 1).
Table 2.
Health-related quality of life scores among the Georgians Organized Against Lupus cohort of patients with systemic lupus erythematosus in September 2013–November 2014, overall and by categorised age at survey
| SF-12 domain | N | Mean (SD) norm-based SF-12 scores*: |
p Value† | ρ value‡ | p Value‡ | |||
|---|---|---|---|---|---|---|---|---|
| Overall | Age at survey |
|||||||
| 20–39 years | 40–59 years | ≥60 years | ||||||
| Physical component summary | 703 | 39.3 (10.9) | 41.8 (11.7) | 38.7 (10.5) | 37.4 (10.0) | <0.001 | −0.18 | <0.001 |
| Physical functioning | 703 | 39.6 (11.3) | 41.9 (11.5) | 39.1 (11.2) | 37.6 (11.0) | <0.001 | −0.17 | <0.001 |
| Role physical | 702 | 39.7 (10.9) | 41.7 (10.9) | 39.0 (10.9) | 38.9 (10.5) | 0.01 | −0.10 | 0.01 |
| Bodily pain | 693 | 39.4 (11.4) | 40.7 (11.6) | 38.9 (11.5) | 38.6 (10.6) | 0.15 | −0.11 | 0.004 |
| General health | 698 | 41.8 (10.7) | 43.5 (11.3) | 40.9 (10.4) | 41.9 (10.2) | 0.02 | −0.08 | 0.03 |
| Mental component summary | 703 | 44.3 (11.4) | 44.2 (11.7) | 43.8 (11.4) | 46.1 (11.2) | 0.14 | 0.06 | 0.09 |
| Mental health | 703 | 45.1 (10.9) | 45.1 (11.3) | 44.5 (10.9) | 46.9 (10.1) | 0.08 | 0.06 | 0.10 |
| Role emotional | 700 | 40.2 (13.4) | 41.1 (13.6) | 39.4 (13.3) | 40.9 (13.3) | 0.30 | −0.01 | 0.85 |
| Social functioning | 698 | 41.4 (11.0) | 42.3 (10.6) | 40.7 (11.1) | 41.7 (11.4) | 0.29 | −0.03 | 0.36 |
| Vitality | 701 | 43.7 (10.2) | 44.3 (10.2) | 43.5 (10.2) | 43.4 (10.3) | 0.63 | −0.03 | 0.42 |
*Among patients with both mental component summary and physical component summary scores.
†By analysis of variance, across indicated categories of age.
‡Pearson's correlation coefficients (ρ) and p values for correlations between domain scores and continuous age.
Figure 1.
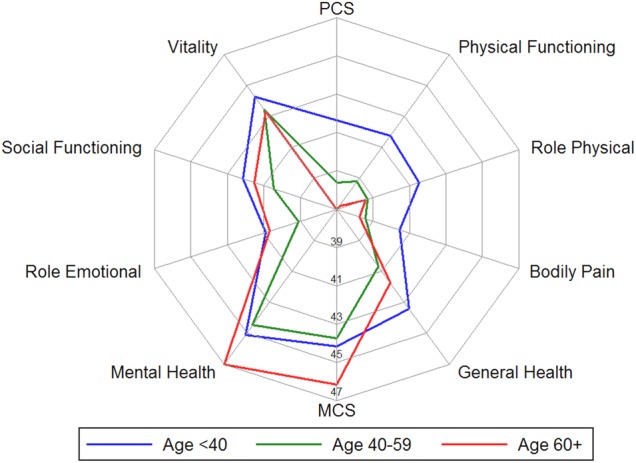
Overall summary and individual component health-related quality of life scores among patients with systemic lupus erythematosus by age group, in the Georgians Organized Against Lupus cohort, September 2013–November 2014. MCS, mental component summary; PCS, physical component summary.
In crude regression analyses of HRQOL scores by categorised age, PCS scores were lower among those aged 40–59 and ≥60 years (by 3.3 and 4.1 points, on average), compared with those aged 20–39 years (table 3). Adjustment for SLE duration alone generally resulted in very similar results (table 3). Adjustment for sex, race, employment, marital status, emotional support and SLE duration attenuated the associations, particularly for those aged 40–59 versus 20–39, but the associations remained negative and statistically significant. Further adjustment for BILD and SLAQ scores did not change the results substantially (table 3). A similar pattern was seen for the associations of physical functioning, role physical, bodily pain and general health subdomain scores with categorised age, but only the association of age with physical functioning remained statistically significant after adjustment (table 3).
Table 3.
Overall associations of categorised age at survey with summary health-related quality of life scores among patients with SLE in the Georgians Organized Against Lupus cohort, September 2013–November 2014
| Domain/model | Difference in score (95% CI), compared with age 20–39 years |
|
|---|---|---|
| Age 40–59 years | Age ≥60 years | |
| Physical component summary (N=684) | ||
| Crude | −3.26 (−5.12 to −1.39) | −4.13 (−6.55 to −1.71) |
| Adjusted for SLE duration only | −3.28 (−5.21 to −1.35) | −4.18 (−6.80 to −1.56) |
| Multivariable-adjusted* | −2.53 (−4.38 to −0.67) | −3.57 (−6.19 to −0.96) |
| Multivariable+BILD score | −2.10 (−3.93 to −0.28) | −3.43 (−5.99 to −0.87) |
| Multivariable+SLAQ score | −2.15 (−3.87 to −0.43) | −3.13 (−5.55 to −0.71) |
| Multivariable+BILD and SLAQ score | −1.85 (−3.55 to −0.15) | −3.05 (−5.43 to −0.66) |
| Physical functioning (N=684) | ||
| Crude | −3.04 (−4.99 to −1.09) | −3.97 (−6.49 to −1.44) |
| Adjusted for SLE duration only | −3.09 (−5.11 to −1.07) | −4.07 (−6.80 to −1.33) |
| Multivariable-adjusted* | −2.12 (−4.04 to −0.19) | −3.42 (−6.13 to −0.72) |
| Multivariable+BILD score | −1.76 (−3.67 to 0.14) | −3.30 (−5.97 to −0.63) |
| Multivariable+SLAQ score | −1.76 (−3.57 to 0.05) | −3.01 (−5.55 to −0.47) |
| Multivariable+BILD and SLAQ score | −1.52 (−3.32 to 0.27) | −2.94 (−5.46 to −0.42) |
| Role physical (N=683) | ||
| Crude | −2.83 (−4.71 to −0.95) | −2.57 (−5.00 to −0.13) |
| Adjusted for SLE duration only | −3.09 (−5.04 to −1.15) | −3.10 (−5.74 to −0.47) |
| Multivariable-adjusted* | −2.05 (−3.90 to −0.19) | −2.58 (−5.18 to 0.02) |
| Multivariable+BILD score | −1.70 (−3.53 to 0.14) | −2.46 (−5.03 to 0.11) |
| Multivariable+SLAQ score | −1.67 (−3.38 to 0.04) | −2.13 (−4.53 to 0.27) |
| Multivariable+BILD and SLAQ score | −1.45 (−3.15 to 0.25) | −2.07 (−4.45 to 0.31) |
| Bodily pain (N=674) | ||
| Crude | −1.87 (−3.85 to 0.11) | −1.75 (−4.33 to 0.82) |
| Adjusted for SLE duration only | −1.88 (−3.93 to 0.17) | −1.77 (−4.57 to 1.02) |
| Multivariable-adjusted* | −0.88 (−2.83 to 1.07) | −1.70 (−4.45 to 1.05) |
| Multivariable+BILD score | −0.52 (−2.46 to 1.41) | −1.61 (−4.32 to 1.10) |
| Multivariable+SLAQ score | −0.34 (−2.07 to 1.40) | −1.15 (−3.60 to 1.29) |
| Multivariable+BILD and SLAQ score | −0.15 (−1.88 to 1.58) | −1.12 (−3.55 to 1.31) |
| General health (N=679) | ||
| Crude | −2.65 (−4.50 to −0.80) | −1.56 (−3.97 to 0.85) |
| Adjusted for SLE duration only | −2.80 (−4.72 to −0.89) | −1.87 (−4.48 to 0.74) |
| Multivariable-adjusted* | −2.14 (−4.03 to −0.25) | −1.63 (−4.29 to 1.03) |
| Multivariable+BILD score | −1.82 (−3.69 to 0.06) | −1.53 (−4.16 to 1.10) |
| Multivariable+SLAQ score | −1.71 (−3.44 to 0.01) | −1.15 (−3.58 to 1.27) |
| Multivariable+BILD and SLAQ score | −1.52 (−3.24 to 0.20) | −1.11 (−3.52 to 1.31) |
| Mental component summary (N=684) | ||
| Crude | −0.33 (−2.31 to 1.65) | 2.10 (−0.46 to 4.67) |
| Adjusted for SLE duration only | −0.73 (−2.77 to 1.32) | 1.29 (−1.48 to 4.07) |
| Multivariable-adjusted* | 0.47 (−1.46 to 2.41) | 1.20 (−1.52 to 3.92) |
| Multivariable+BILD score | 0.49 (−1.45 to 2.43) | 1.20 (−1.51 to 3.92) |
| Multivariable+SLAQ score | 0.86 (−0.94 to 2.65) | 1.65 (−0.87 to 4.17) |
| Multivariable+BILD and SLAQ score | 0.76 (−1.04 to 2.56) | 1.62 (−0.90 to 4.15) |
| Mental health (N=684) | ||
| Crude | −0.41 (−2.29 to 1.47) | 2.04 (−0.39 to 4.48) |
| Adjusted for SLE duration only | −0.76 (−2.70 to 1.18) | 1.32 (−1.31 to 3.96) |
| Multivariable-adjusted* | 0.50 (−1.35 to 2.35) | 1.36 (−1.25 to 3.96) |
| Multivariable+BILD score | 0.52 (−1.34 to 2.38) | 1.36 (−1.24 to 3.97) |
| Multivariable+SLAQ score | 0.87 (−0.84 to 2.59) | 1.79 (−0.62 to 4.20) |
| Multivariable+BILD and SLAQ score | 0.79 (−0.93 to 2.51) | 1.77 (−0.64 to 4.18) |
| Role emotional (N=681) | ||
| Crude | −1.62 (−3.94 to 0.71) | 0.14 (−2.87 to 3.16) |
| Adjusted for SLE duration only | −1.94 (−4.35 to 0.46) | −0.52 (−3.79 to 2.74) |
| Multivariable-adjusted* | −0.86 (−3.11 to 1.40) | −0.85 (−4.01 to 2.32) |
| Multivariable+BILD score | −0.69 (−2.95 to 1.57) | −0.79 (−3.95 to 2.37) |
| Multivariable+SLAQ score | −0.42 (−2.53 to 1.69) | −0.34 (−3.30 to 2.63) |
| Multivariable+BILD and SLAQ score | −0.38 (−2.50 to 1.74) | −0.33 (−3.30 to 2.64) |
| Social functioning (N=680) | ||
| Crude | −1.72 (−3.64 to 0.21) | −0.42 (−2.91 to 2.06) |
| Adjusted for SLE duration only | −1.91 (−3.90 to 0.08) | −0.82 (−3.52 to 1.88) |
| Multivariable-adjusted* | −0.71 (−2.62 to 1.21) | −0.50 (−3.19 to 2.18) |
| Multivariable+BILD score | −0.47 (−2.38 to 1.44) | −0.41 (−3.08 to 2.26) |
| Multivariable+SLAQ score | −0.26 (−1.99 to 1.46) | 0.01 (−2.41 to 2.43) |
| Multivariable+BILD and SLAQ score | −0.16 (−1.89 to 1.57) | 0.04 (−2.37 to 2.46) |
| Vitality (N=682) | ||
| Crude | −0.94 (−2.72 to 0.83) | −0.86 (−3.16 to 1.43) |
| Adjusted for SLE duration only | −1.33 (−3.16 to 0.50) | −1.66 (−4.15 to 0.82) |
| Multivariable-adjusted* | −0.51 (−2.34 to 1.33) | −1.25 (−3.83 to 1.33) |
| Multivariable+BILD score | −0.41 (−2.25 to 1.43) | −1.22 (−3.79 to 1.36) |
| Multivariable+SLAQ score | −0.19 (−1.92 to 1.54) | −0.84 (−3.27 to 1.59) |
| Multivariable+BILD and SLAQ score | −0.18 (−1.92 to 1.55) | −0.84 (−3.27 to 1.59) |
Complete case analysis was used (only individuals with complete information on the outcome and all covariates included). All subdomain scores are norm-based SF-12 scores.
*Adjusted for sex, race (African-American vs not African-American), employment, marital status, social support and lupus duration.
BILD, Brief Index of Lupus Damage; SLAQ, Systemic Lupus Activity Questionnaire; SLE, systemic lupus erythematosus.
In contrast, MCS scores did not differ for participants who were 40–59 and 20–39 years of age; those aged >60 years had higher MCS scores (by 2.1 points, on average) than those aged 20–39 years, but the difference was not statistically significant (table 3). Adjustment for sex, race, employment, marital status, emotional support and SLE duration rendered the association between age ≥60 versus 20–39 with MCS non-statistically significant (table 3). Further adjustment for SLE damage (BILD score) and SLE activity (SLAQ score) did not change the results. A nearly identical pattern was seen for the mental health subdomain score (table 3). Middle-aged (but not older) participants appeared to have lower (0.9–1.6 points) scores than younger patients on vitality, social functioning and role emotional subdomain scores in crude analyses, but these associations were not statistically significant and were rendered null by full adjustment (table 3).
Sensitivity analyses
Further adjustment for BMI, depression and lupus impact score generally did not substantially change the patterns seen in the primary analyses, although there was a suggestion of slightly stronger associations after this adjustment among those aged ≥60 years (see online supplementary table S1). Adjustment for income showed similar results to those in the primary analyses (see online supplementary table S1). Adjustment for renal involvement in SLE resulted in slightly higher magnitude of negative associations of age with physical aspects of HRQOL (see online supplementary table S1). Similar to the results with categorised age, lower PCS and physical subdomain scores remained statistically significantly associated with older, continuous age after adjustment, whereas MCS and mental subdomain scores were generally not associated with continuous age (see online supplementary table S2). Age-squared (in addition to continuous age) was only statistically significantly (and positively) associated with MCS and mental health scores in these continuous models, but these associations were not robust to adjustment (data not shown).
lupus-2016-000161supp_tables.pdf (42KB, pdf)
Discussion
In this cross-sectional study of a cohort of patients with SLE of all ages, we examined the associations of age with HRQOL and the contributions of cumulative disease damage and disease activity to these associations. We found that, on average, patients with SLE in the middle-aged and older versus younger age groups had lower scores on physical aspects of HRQOL. However, older patients had similar or even slightly higher scores on mental aspects of HRQOL, relative to both young and middle-aged patients. These associations were generally robust to adjustment with potential confounders and independent of the effect of both cumulative SLE-related damage and current SLE activity.
Regardless of age or HRQOL domain, scores in this predominantly African-American cohort of patients with SLE were low, with all HRQOL norm-based scores being <50, indicating poorer health in SLE than in general population. This observation is consistent with prior studies, showing that HRQOL is worse among patients with SLE than similarly aged counterparts with other chronic conditions, including hypertension, diabetes and myocardial infarction, across domains of HRQOL.3 The patients with in our study actually had HRQOL scores similar to those of patients with end-stage renal disease treated with dialysis, for example, the PCS and MCS scores reported for US patients on dialysis are 33.1 and 46.6,41 respectively, compared with 39.3 and 44.3 in our cohort.
Despite these generally low scores, there was variation in reported HRQOL scores by age group. The directions of the crude associations of HRQOL domains with older age were similar to those reported in the general population, in whom consistently lower physical HRQOL scores are seen for older versus younger individuals, but higher mental HRQOL scores are seen among older (and younger) versus middle-aged individuals.29 42 With adjustment for confounders and mediators, only the negative association of older age with lower physical HRQOL remained statistically significant. To our knowledge, the only prior study to examine the association of categorised age with HRQOL showed that older (≥65) versus younger (<65) patients with rheumatic diseases including SLE had lower HRQOL across all domains.43 However, the study did not separate the middle-aged patients and did not examine the association specifically in patients with SLE, likely due to small numbers of patients with SLE at older ages. We were also somewhat limited in our ability to compare HRQOL in the oldest patients with our younger patients (given that <4% and <1% of the patients in our cohort were ≥70 and ≥80 years old). However, for SLE, being >60 years old may be reasonably considered ‘older’, given relatively recent increases in longevity, similar to the HIV population, where ≥50 years is considered older.44
We also found that greater cumulative SLE-related damage was associated with older age but that SLE activity was similar across age groups. In a 2-year prospective study, Mok et al13 showed that disease damage but not disease activity was associated with lower HRQOL; however, we found that disease damage and disease activity had similar individual effects on the associations of age with HRQOL, after adjustment for other important factors, such as race, sex, employment and lupus duration. Furthermore, these effects were modest, with neither disease damage nor disease activity (or both in combination) explaining the observed associations of age with HRQOL in middle-aged or older versus younger patients with SLE. Interestingly, we found that the modest effects of disease damage and activity may have differed by age, with effects of these factors being stronger in the middle-aged patients: for example, compared with the model adjusted for all other confounding and mediating factors, further adjustment for BILD and SLAQ scores gave effect estimates that were reduced by 27% versus 11% in the middle-aged versus older adults. Thus, the contributions of SLE-related damage and activity to HRQOL may be less important to consider in older patients, who may benefit from a more innovative and patient-centred approach to their health and well-being.
Collectively, we found that associations of age with HRQOL among patients with SLE were not necessarily straightforward and, further, were not entirely explained by disease burden and activity in patients with SLE. These findings suggest that the effect of older age on HRQOL is mediated by non-disease factors, consistent with prior studies in both healthy individuals15 and individuals with renal insufficiency.16 Thus, in patients with SLE, a multidisciplinary, patient-centred approach, which emphasises syndromes analogous to geriatric syndromes2 45 and includes assessment of physical, mental, emotional and social functioning across all life stages,46 may be preferable to the traditional disease-centred approach, which primarily emphasises the treatment of disease-related signs and symptoms. In the heterogeneous population of older patients with SLE, which includes survivors of early-onset SLE and patients with relatively recent late-onset SLE, who may have different care needs, this approach may be particularly important. In the clinical setting, more patient-centred, goal-oriented care could lead to better, more appropriate shared decision-making and a focus on the outcomes most important to individual patients.47 48 Furthermore, future health services research that includes comprehensive geriatric assessment across patients with SLE of all ages, including assessment of HRQOL and other important syndromes such as impaired physical and cognitive function and frailty, has the potential to inform both clinical care and successful ageing in setting of SLE.49
There are several limitations of this study that deserve mention. First, this is a cross-sectional study, which limits causal inference. Additionally, the lack of long-term follow-up data means that we do not know individual trajectories of HRQOL over time.22 50 Thus, we cannot rule out survivor effects with age. Exclusions due to missing data may have led to selection bias, given observed differences between excluded and included participants; however, missingness was minimal (<5%). As with all observational studies, it is possible that we have not accounted for unknown or unmeasured confounders or mediators. Misclassification is also possible; for example, disease activity may be underestimated with SLAQ, which does not include items related to renal disease. While we adjusted for renal involvement in SLE in sensitivity analyses, renal activity at the time of survey is unknown. Finally, generalisability of the results may be limited due to the single location and potential healthy cohort effects. However, the study also has several strengths, including relatively large sample sizes within older age groups; a population-based sample of patients with SLE and adequate representation of African-American patients, who are at disproportionate risk of SLE and poor outcomes in SLE, in each age group.
HRQOL is low in SLE, regardless of age. Further, older age is associated with lower physical, but not mental, HRQOL among patients with SLE. These associations are independent of accumulated SLE damage and current SLE activity, suggesting that studies of the associations between other important geriatric outcomes in addition to HRQOL in the setting of SLE are warranted. Furthermore, the results highlight the need to establish patient-centred clinical strategies to prepare for an ageing SLE population.
Acknowledgments
The GOAL study was supported by Human Genome Science and GlaxoSmithKline (GHO-11-3366). SSL and CD are supported by the NIH (R01AR065493) and the CDC (U01DP005119).
Footnotes
Contributors: All contributors meet the criteria for authorship and approved the submitted version of the manuscript.
Funding: GlaxoSmithKline (GHO-11-3366), Centers for Disease Control and Prevention (U01DP005119), National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR065493).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Emory University Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: GOAL is an ongoing cohort study, and data are not available.
References
- 1.Brunsting LA. Disseminated (systemic) lupus erythematosus. Proc Mayo Clinic 1952;27:410–12. [PubMed] [Google Scholar]
- 2.Inouye SK, Studenski S, Tinetti ME et al. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007;55:780–91. doi:10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. [PubMed] [Google Scholar]
- 4.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus 2006;15:633–43. doi:10.1177/0961203306071710 [DOI] [PubMed] [Google Scholar]
- 5.Lim SS, Drenkard C. Epidemiology of lupus: an update. Curr Opin Rheumatol 2015;27:427–32. doi:10.1097/BOR.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 6.Howden LM, Meyer JA.2011. Age and Sex Composition: 2010. 2010 Census Briefs: U.S. Department of Commerce. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- 7.Boddaert J, Huong DL, Amoura Z et al. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83:348–59. doi:10.1097/01.md.0000147737.57861.7c [DOI] [PubMed] [Google Scholar]
- 8.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85:147–56. doi:10.1097/01.md.0000224709.70133.f7 [DOI] [PubMed] [Google Scholar]
- 9.Khanna S, Pal H, Pandey RM et al. The relationship between disease activity and quality of life in systemic lupus erythematosus. Rheumatology (Oxford) 2004;43:1536–40. doi:10.1093/rheumatology/keh376 [DOI] [PubMed] [Google Scholar]
- 10.Stoll T, Gordon C, Seifert B et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol 1997;24:1608–14. [PubMed] [Google Scholar]
- 11.Hanly JG. Disease activity, cumulative damage and quality of life in systematic lupus erythematosus: results of a cross-sectional study. Lupus 1997;6:243–7. doi:10.1177/096120339700600305 [DOI] [PubMed] [Google Scholar]
- 12.Meacock R, Dale N, Harrison MJ. The humanistic and economic burden of systemic lupus erythematosus: a systematic review. Pharmacoeconomics 2013;31:49–61. doi:10.1007/s40273-012-0007-4 [DOI] [PubMed] [Google Scholar]
- 13.Mok CC, Ho LY, Cheung MY et al. Effect of disease activity and damage on quality of life in patients with systemic lupus erythematosus: a 2-year prospective study. Scand J Rheumatol 2009;38:121–7. doi:10.1080/03009740802415527 [DOI] [PubMed] [Google Scholar]
- 14.Gladman D, Urowitz M, Fortin P et al. Systemic Lupus International Collaborating Clinics conference on assessment of lupus flare and quality of life measures in SLE. Systemic Lupus International Collaborating Clinics Group. J Rheumatol 1996;23:1953–5. [PubMed] [Google Scholar]
- 15.Covinsky KE, Wu AW, Landefeld CS et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med 1999;106:435–40. doi:10.1016/S0002-9343(99)00052-2 [DOI] [PubMed] [Google Scholar]
- 16.Chow FYF, Briganti EM, Kerr PG et al. Health-related quality of life in Australian adults with renal insufficiency: a population-based study. Am J Kidney Dis 2003;41:596–604. doi:10.1053/ajkd.2003.50121 [DOI] [PubMed] [Google Scholar]
- 17.Rivest C, Lew RA, Welsing PM et al. Association between clinical factors, socioeconomic status, and organ damage in recent onset systemic lupus erythematosus. J Rheumatol 2000;27:680–4. [PubMed] [Google Scholar]
- 18.Yazdany J, Yelin EH, Panopalis P et al. Validation of the systemic lupus erythematosus activity questionnaire in a large observational cohort. Arthritis Rheum 2008;59:136–43. doi:10.1002/art.23238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alarcon GS, McGwin G Jr, Uribe A et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum 2004;51:465–74. [DOI] [PubMed] [Google Scholar]
- 20.Urowitz M, Gladman DD, Ibañez D et al. Changes in quality of life in the first 5 years of disease in a multicenter cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2014;66:1374–9. doi:10.1002/acr.22299 [DOI] [PubMed] [Google Scholar]
- 21.Urowitz MB, Gladman DD, Ibañez D et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2012;64:132–7. doi:10.1002/acr.20648 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, González LA, Roseman JM et al. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010;19:727–33. doi:10.1177/0961203309359289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon GS, Calvo-Alen J, McGwin G Jr et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006;65:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce IN, O'Keeffe AG, Farewell V et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. doi:10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petri M, Purvey S, Fang H et al. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum 2012;64:4021–8. doi:10.1002/art.34672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drenkard C, Rask KJ, Easley KA et al. Primary preventive services in patients with systemic lupus erythematosus: study from a population-based sample in Southeast U.S. Semin Arthritis Rheum 2013;43:209–16. doi:10.1016/j.semarthrit.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Lim SS, Bayakly AR, Helmick CG et al. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: the Georgia Lupus Registry. Arthr Rheumatol (Hoboken, NJ) 2014;66:357–68. doi:10.1002/art.38239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725 doi:10.1002/1529-0131(199709)40:9<1725::AID-ART29>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- 29.Mishra GD, Hockey R, Dobson AJ. A comparison of SF-36 summary measures of physical and mental health for women across the life course. Qual Life Res 2014;23:1515–21. doi:10.1007/s11136-013-0586-3 [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson C, Layte R, Jenkinson D et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997;19:179–86. doi:10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 31.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 32.Brazier JE, Harper R, Jones NM et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. doi:10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware JE Jr, Kosinski M, Keller SD. SF-36 physical & mental health summary scales: a user's manual. Boston: New England Medical Center, 1994. [Google Scholar]
- 34.Ware JE Jr, Kosinski M, Turner-Bowker DM et al. User's manual for the SF-12v2 health survey. Lincoln, RI: Quality Metric Incorporated, 2002. [Google Scholar]
- 35.Yazdany J, Trupin L, Gansky SA et al. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63:1170–7. doi:10.1002/acr.20503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drenkard C, Yazdany J, Trupin L et al. Validity of a self-administered version of the brief index of lupus damage in a predominantly African American systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2014;66:888–96. doi:10.1002/acr.22231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlson EW, Daltroy LH, Rivest C et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus 2003;12:280–6. doi:10.1191/0961203303lu332oa [DOI] [PubMed] [Google Scholar]
- 38.Pons-Estel GJ, Alarcón GS, Scofield L et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010;39:257–68. doi:10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jolly M, Garris CP, Mikolaitis RA et al. Development and validation of the Lupus Impact Tracker: a patient-completed tool for clinical practice to assess and monitor the impact of systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2014;66:1542–50. doi:10.1002/acr.22349 [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. doi:10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuhara S, Lopes AA, Bragg-Gresham JL et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2003;64:1903–10. doi:10.1046/j.1523-1755.2003.00289.x [DOI] [PubMed] [Google Scholar]
- 42.Ware JE., Jr SF-36 health survey: manual & interpretation guide. Boston, MA: The Health Institute, New England Medical Center, 1993. [Google Scholar]
- 43.Goulia P, Voulgari PV, Tsifetaki N et al. Comparison of health-related quality of life and associated psychological factors between younger and older patients with established rheumatic disorders. Aging Ment Health 2010;14:819–27. doi:10.1080/13607861003781809 [DOI] [PubMed] [Google Scholar]
- 44.Simone MJ, Appelbaum J. HIV in older adults. Geriatrics 2008;63:6–12. [PubMed] [Google Scholar]
- 45.Flacker JM. What is a geriatric syndrome anyway? J Am Geriatr Soc 2003;51:574–6. doi:10.1046/j.1532-5415.2003.51174.x [DOI] [PubMed] [Google Scholar]
- 46.Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington DC: U.S. Department of Health and Human Services, 2011. https://www.healthypeople.gov [Google Scholar]
- 47.McElhone K, Abbott J, Gray J et al. Patient perspective of systemic lupus erythematosus in relation to health-related quality of life concepts: a qualitative study. Lupus 2010;19:1640–7. doi:10.1177/0961203310378668 [DOI] [PubMed] [Google Scholar]
- 48.Gallop K, Nixon A, Swinburn P et al. Development of a conceptual model of health-related quality of life for systemic lupus erythematosus from the patient's perspective. Lupus 2012;21:934–43. doi:10.1177/0961203312441980 [DOI] [PubMed] [Google Scholar]
- 49.Kane RL. The contribution of geriatric health services research to successful aging. Ann Intern Med 2003;139(Pt 2):460–2. doi:10.7326/0003-4819-139-5_Part_2-200309021-00016 [DOI] [PubMed] [Google Scholar]
- 50.Kuriya B, Gladman DD, Ibañez D et al. Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum 2008;59:181–5. doi:10.1002/art.23339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2016-000161supp_tables.pdf (42KB, pdf)


