Abstract
Paradoxical adverse events (PAEs) have been reported during biological treatment for chronic immune-mediated diseases. PAEs are defined as the occurrence during biological agent therapy of a pathological condition that usually responds to this class of drug. A wide range of PAEs have been reported including dermatological, intestinal and ophthalmic conditions, mainly with antitumour necrosis factor α (TNF-α) agents. True PAEs include psoriasis, Crohn's disease and hidradenitis suppurativa. Other PAEs may be qualified as borderline and include uveitis, scleritis, sarcoidosis and other granulomatous diseases (granuloma annulare, interstitial granulomatous dermatitis), vasculitis, vitiligo and alopecia areata. Proposed hypotheses to explain these PAEs include an imbalance in cytokine production, the differential immunological properties between the monoclonal antibodies and TNF-α soluble receptor, an unopposed type I interferon production and a shift towards a Th1/Th2 profile. Data from registries suggest that the risk for paradoxical psoriasis is low and non-significant. We discuss management of these PAEs, which depends on the type and severity of the adverse events, pre-existing treated conditions and the possibility of alternative therapeutic options for the underlying disease. Paradoxical adverse events are not restricted to anti-TNF-α agents and close surveillance of new available biological drugs (anti-interleukin-17/23, anti-integrin) is warranted in order to detect the occurrence of new or as yet undescribed events.
Keywords: Anti-TNF, DMARDs (biologic), Sarcoidosis, Treatment
Key messages.
What is already known about this subject?
Different paradoxical adverse events have been described under biological agents, mainly tumour necrosis factor α inhibitors.
What does this study add?
A wide range of paradoxical adverse events have been reported including dermatological, intestinal and ophthalmic conditions, but their relationship with the biological agent exposition remains still debated.
How might this impact on clinical practice?
The clinician must know these paradoxical adverse events as well as the therapeutic strategy to have when such event occurs in a patient under a biological agent.
The introduction of biological agents on the market has dramatically changed the therapeutic approach to a variety of systemic immune-mediated diseases, such as chronic inflammatory rheumatic diseases (rheumatoid arthritis (RA) and spondyloarthritis (SpA)), plaque psoriasis and inflammatory bowel diseases (Crohn's disease (CD) and ulcerative colitis (UC)). Currently, five tumour necrosis factor α (TNF-α) blocking agents are available: three monoclonal antibodies (infliximab, adalimumab, golimumab), a p75 TNF-α soluble receptor (etanercept) and a Fab’ fragment associated with a pegol molecule (certolizumab). With the improved understanding of the pathophysiology of immune-mediated diseases, new relevant therapeutic targets have been identified, leading to the development of new biological drugs. In this setting, anti-CD20 (rituximab), anti-interleukin (IL)-1 (anakinra), anti-IL-6 (tocilizumab) and a fusion protein inhibiting the costimulatory pathway (abatacept) have been developed for the treatment of RA. It has also been shown that the Th17/ IL-23 pathway plays an important role in psoriasis and psoriatic arthritis (PsA), and thus ustekinumab, an anti-p40 IL-12/23 monoclonal antibody, has become available. Vedolizumab is a new biological agent directed against the α4β7 integrin that has been recently licensed in the treatment of CD.
Intriguingly, unexpected side effects have been reported with the use of biological agents in clinical practice. Indeed, dermatological, intestinal and ophthalmological paradoxical adverse events (PAEs) have been described, mainly with anti-TNF-α agents. In this review, we will focus on the different PAEs that have been described with anti-TNF-α and other biological agents. We will also attempt to analyse the potential mechanisms that may explain this immunological phenomenon, and finally we propose management strategies.
Definition and general considerations
PAEs may be defined as the occurrence during therapy with a biological agent, of a pathological condition that usually responds to this class of drug. In this regard, the incriminated biological agent must have previously proven its efficacy in the treatment of the induced condition. In this case, the PAE is qualified as ‘true’ (or “authentic”). This is well illustrated by the onset of (de novo) psoriasis during anti-TNF-α therapy.1 In parallel, the biological agent may worsen a pre-existing condition (for instance, psoriasis may worsen when an anti-TNF-α agent is started for psoriasis or PsA). In addition, some PAEs are in fact extra-articular manifestations of the disease (for instance, uveitis during anti-TNF-α therapy for SpA). On the other hand, ‘borderline’ PAEs can be defined as the development of certain immune-mediated conditions that are observed during a biological treatment that has not proven its efficacy in this specific condition, despite a rationale for its use. For instance, sarcoidosis may occur during anti-TNF-α therapy, but anti-TNF-α agents are not approved for the treatment of this granulomatous disease.2 On the contrary, some specific adverse events occurring with biological drugs (for instance, demyelinating diseases with etanercept, systemic lupus erythematosus or antiphospholipid syndrome with anti-TNF-α agents) are not considered as paradoxical.
PAEs are uncommon and were not observed during the development programme of the biological agents. They were subsequently reported as isolated cases or case series. PAEs were mainly described with anti-TNF-α agents (first in inflammatory rheumatic diseases, and then in psoriasis and CD) and more rarely with the other biological classes used in the treatment of RA and other conditions. This may be explained by the fact that anti-TNF-α agents were first introduced onto the market, and have a large number of indications. PAEs are not organ-specific, leading to the description of a wide range of conditions, including cutaneous, intestinal, ophthalmological and also vascular adverse events (table 1).
Table 1.
Paradoxical conditions described under biological agents given for immune-mediated diseases
| True PAE |
Borderline PAE |
||
|---|---|---|---|
| AE | Biological agent | AE | Biological agent |
| Psoriasis | Anti-TNF-α Rituximab Tocilizumab Ustekinumab |
Uveitis | Anti-TNF-α (etanercept) |
| Crohn's disease and/or ulcerative colitis | Anti-TNF-α (etanercept) | Scleritis | Anti-TNF-α |
| Hidradenitis suppurativa | Anti-TNF-α (adalimumab) Rituximab |
Sarcoidosis | Anti-TNF-α (etanercept) |
| Granuloma annulare | Anti-TNF-α | ||
| Interstitial granulomatous dermatitis | Anti-TNF-α | ||
| Vasculitis | Anti-TNF-α | ||
| Alopecia areata | Anti-TNF-α | ||
| Vitiligo | Anti-TNF-α ustekinumab | ||
AE adverse events; PAE, paradoxical adverse events; TNF, tumour necrosis factor.
Limited data are available concerning the incidence of PAEs. From January 2002 to September 2009, 57 cases of new onset or aggravation of pre-existing psoriasis were declared to the French national pharmacovigilance database.3 In a single primary referral centre in France, 12 PAEs (psoriasis, acute anterior uveitis and inflammatory bowel disease (IBD)) were reported among 296 patients with SpA treated by infliximab, etanercept or adalimumab, giving an overall frequency of 1.9/100 patient-years.4
‘True’ PAEs
One of the most frequently described cutaneous PAEs is psoriasis.5
Psoriasis
anti-TNF-α agents
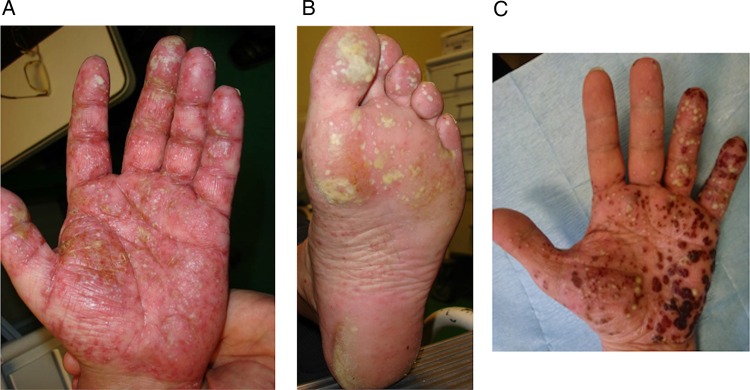
Since an initial report in 2003,6 numerous cases have been described, and two systematic literature reviews have been performed, the most recent analysing 207 cases.7 8 Psoriasis occurring during anti-TNF-α therapy may be induced or exacerbated by biological agents without identified predisposing factors. In particular, co-medication such as methotrexate given for the underlying disease is not a protective factor. Infections are not observed as a triggering event. Paradoxical psoriasis has been observed in men and women, with no apparent age effect. Most of the patients have no previous personal or family history of psoriasis. All the diseases treated by anti-TNF-α agents may develop paradoxical psoriasis, but cases seem to predominate in RA with, in general, good control of the disease by the drug. Paradoxical psoriasis may occur at any time from a few days to several years after drug initiation. The reported cutaneous lesions are plaque, pustular or guttate-type psoriasis. The most frequently affected areas are the scalp and flexures and palmoplantar areas.9 Pustular psoriasis affecting the palm and soles seems to be frequent and was reported in more than 50% of cases (figure 1). The nails are involved less often, but typical changes (onycholysis, discolouration and pitting) have been described.7 8 The rate of new onset/exacerbation of psoriasis in patients receiving anti-TNF-α agents has been calculated from two registries. The incidence rate of psoriasis in an RA population from the UK was estimated at 1.04/1000 person-years (95% CI 0.67 to 1.54), compared to a rate of zero in an RA group without biologics10 (table 2). Interestingly, patients with adalimumab had a significantly increased risk of psoriasis compared to etanercept (incidence rate ratio (IRR) 4.6 (1.7 to 12.1)) and compared to infliximab (IRR 3.5 (1.3 to 9.3)).10 Data from the Spanish biological register (BIOBADASER) found similar results, with a global incident rate of 2.31/1000 patient-years, but no highest incidence was observed with a specific anti-TNF-α agent.11 One may remark that the risk for paradoxical psoriasis in these registries was found to be low and non-significant according to the large CIs. Paradoxical psoriasis has the same pattern of histological abnormalities as conventional psoriasis lesions. In general, the outcome of paradoxical psoriasis is favourable. Most patients were able to continue their treatment with full or partial resolution of the skin lesions. For patients who were switched to another anti-TNF-α agent, half of them experienced a recurrence of their lesions.7 Only a minority of patients reported a severe course with erythrodermic lesions requiring systemic corticosteroids. The treatments given were usually topical steroids and/or vitamin D analogues and keratolytics.
Figure 1.
Palmoplantar pustular psoriasis in a patient with spondyloarthritis under golimumab.
Table 2.
Occurrence of paradoxical adverse events in European and American registries
| Registry Reference |
PAE | Disease | Biological agent | Number of participants | Results |
|---|---|---|---|---|---|
| British society for rheumatology biologics register (BSRBR, UK)10 | Psoriasis | RA | Anti-TNF-α agents | 9826 anti–TNF-α- treated patients 2880 DMARD-treated patients | Incident rate ratio for 1000 person-years (95% CI): Global: 1.04 (0.67–1.54) Adalimumab vs etanercept : 4,6 (1.7–12.1) Adalimumab vs Infliximab: 3.5 (1.3–9.3) |
| Spanish registry for adverse events of biological therapy in rheumatic diseases (BIOBADASER, Spain)11 | Psoriasis | Anti-TNF-α agents | Incident rate/1000 patient-year: 2.31 (1.69–2.35)adalimumab: 3.2 (1.8–5.8) (etanercept:) 2 (1.1–3.6) Infliximab: 2.2 (1.4–3.4) |
||
| Autoimmunity and rituximab registry (AIR, France)14 | Psoriasis | RA | Rituximab | 1927 | Incident rate/1000 person-years (95%CI): New-onset psoriasis: 1.04 (0.13–3.8) Flare of pre-existing psoriasis: 2.6 (0.84–6.1) |
| Registry from the Netherlands, Germany, Finland, Denmark, Italy33 | IBD | JIA | Etanercept | ND | Incidence rate of IBD/100 000 person-years: 362 |
| A WHO adverse event database (Sweden) and National registry of drug-induced ocular side effects (USA)41 | Uveitis | RA, SpA, PsA and CD | Anti-TNF-α\xEF\x80 agents | ND | 43 cases associated with etanercept, 14 with infliximab and 2 with adalimumab. (the higher number of uveitis with etanercept persists after excluding patients whose uveitis may result from the underlying disease) |
| Swedish biologic registry (ARTIS, Sweden)43 | Uveitis | AS | Anti–TNF-α agents | 1385 Adalimumab: 406 Etanercept: 354 Infliximab: 605 |
Flare of uveitis before and after anti-TNF-a agent initiation/100 person-years (95% CI) Adalimumab 12.9 (11.7–14.2) and 7.7 (5.9–9.6) Etanercept: 9.6 (9.4–10.9) and 20.2 (17.5–22.8) Infliximab: 12.7 (11.5–13.8) and 11.7 (10.1–13.3) |
| German Biologics in Pediatric Rheumatology (BIKER, Germany)44 | Uveitis | JIA | Anti-TNF-α agents or MTX | 3467 | Rate of uveitis MTX: 51/2884 Etanercept: 37/1700 Adalimumab: 13/364 Rate of new uveitis: MTX: 3.2/1000 person-years Etanercept: 1.9/1000 person-years Etanercept+MTX: 0.9/1000 person-years |
CD, Crohn's disease; DMARD, disease modifying antirheumatic drugs; IBD, inflammatory bowel disease; JIA, juvenile idiopathic arthritis; PAE, paradoxical adverse event; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, spondyloarthritis.
Other biological agents
Psoriasis has also been reported as a PAE with some other biological agents, albeit with a lower frequency.
Preliminary results from an open-label study suggested that rituximab may be effective in patients with a peripheral form of PsA.12 Three cases of de novo psoriasis have been reported in patients receiving rituximab for seronegative RA or systemic lupus erythematosus.13 In the French AIR registry, seven patients were reported to have experienced new-onset psoriasis or a flare up of pre-existing psoriasis, resulting in incidence rates of 1.04/1000 person-years (0.13 to 3.8) and 2.6/1000 person-years (0.84 to 6.1), respectively (table 2). However, rechallenge of the drug did not induce a recurrence/exacerbation of psoriasis.14
Abatacept was shown to be effective in PsA in a randomised, placebo-controlled trial and improving skin psoriatic lesions.15 A limited number of cases of psoriasis have been reported during abatacept therapy for RA or PsA, but there is no specific signal for this event from pharmacovigilance surveillance programmes.16–19
Tocilizumab was reported to be exceptionally associated with reactivation of psoriasis in two patients.20 Another case of psoriasiform palmoplantar pustulosis was reported in a patient with RA treated by tocilizumab and the skin lesion disappeared after drug discontinuation.21
Finally, a paradoxical flare of psoriasis was described in two patients with psoriasis treated by ustekinumab.22 23
Alternatively, onset of PsA has been reported in a series of 16 patients receiving efalizumab (an anti-CD11a monoclonal antibody that was withdrawn from the market) for severe psoriasis.24 Similarly, ustekinumab was associated with the onset of disabling PsA in a patient with severe plaque psoriasis.25 Finally, cases of paradoxical inflammation of the joints have been described in patients with IBD treated by anti–TNF-α agents.26
Hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a painful chronic skin disease characterised by recurrent inflammatory nodules and abscesses, sinus tract and fistula formation, purulent drainage and subsequent scarring involving the apocrine gland bearing areas.27 Adalimumab has recently been approved as a new indication in the treatment of active moderate to severe HS in patients who do not respond to conventional drugs.28 There are a limited number of reports of HS onset in patients receiving a biological agent29 30 (figure 2). A recent multicentre nationwide retrospective study reported a large series of new-onset HS under biological agents. Twenty-five patients were described who received mainly anti-TNF-α agents, including adalimumab. The underlying diseases were chronic inflammatory disease, CD or psoriasis. Complete resolution of HS was observed after treatment discontinuation or a switch to another biological agent. Rechallenging the drug led to HS relapse in a limited number of patients.31
Figure 2.
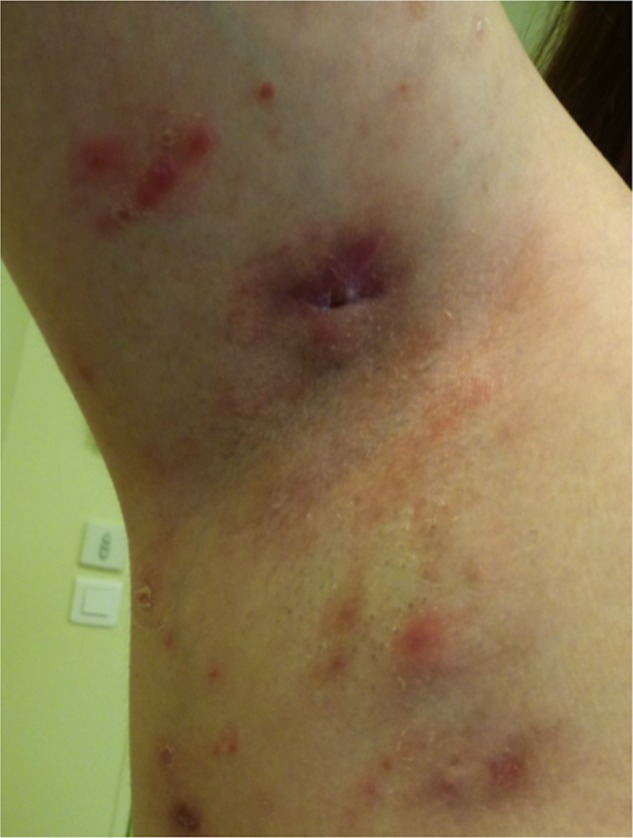
Hidradenitis suppurativa in a 29-year-old woman with Crohn's disease treated with adalimumab.
Crohn's disease and other IBD
We previously reported 16 cases of IBD occurring after anti-TNF-α agent initiation for inflammatory rheumatic disease.32 This series mainly included patients with ankylosing spondylitis (AS) or related SpA. The type of IBD observed under anti-TNF-α therapy was CD in 94%, while UC was infrequent (6%). Most of the patients received etanercept when intestinal symptoms occurred (87.5%). The underlying inflammatory rheumatic disease was generally well controlled by the anti-TNF-α agent given at an appropriate dosage. All the reported patients had a favourable intestinal outcome after discontinuing the anti-TNF-α agent or after switching to a drug that is a monoclonal antibody.
The incidence of IBD in patients with juvenile idiopathic arthritis (JIA) treated by etanercept was calculated from different European registries and found to be 362/100 000 patient-years, a result that was around 43 times higher than in the general population33 (table 2).
‘Borderline’ PAEs
Uveitis
Anti-TNF-α agents
Open-label studies and post hoc analysis of randomised controlled trials in patients with SpA indicate that anti-TNF-α agents may reduce the frequency of uveitis flares.34–37 The results were more salient with infliximab or adalimumab, compared to etanercept.38 On the other hand, anecdotal reports have suggested that uveitis can occur during anti-TNF-α therapy.39 The effect of anti-TNF-α agents on new flares of uveitis in patients with a previous history of uveitis was analysed in a Spanish cohort of patients with SpA.40 The results showed that infliximab reduced uveitis flares, whereas the opposite appeared to be the case with etanercept. An analysis from two drug event databases found similar results, with a greater number of uveitis occurring under etanercept as compared to infliximab or adalimumab.41 A recent study analysed 31 cases of new-onset uveitis in patients under anti-TNF-α therapy.42 The majority of patients had SpA, while the others had RA or JIA. In this series, most cases occurred with etanercept (84%). In the same paper, a systematic analysis of the literature found 121 similar cases. Overall, in these reported cases, etanercept was frequently associated with a greater rate of uveitis than infliximab or adalimumab, while the underlying disease requiring the anti–TNF-α therapy was mainly SpA (72%) followed by JIA (11%) and RA (10%). In general, treatment of uveitis was local, without interrupting the biological agent. Treatment was discontinued in a limited number of cases, and there was recurrence of uveitis when the treatment was reintroduced.42 An analysis of the Swedish biologics registry reported similar findings, namely that etanercept was associated with a higher risk of recurrence of uveitis in adult patients with SpA, while there was a reduction in uveitis rates with adalimumab treatment and a slight reduction with infliximab.43 Conversely, in an analysis from the biologics in paediatric rheumatology registry, only a few patients with JIA developed a first uveitis event while taking etanercept44 (table 2).
Other biological drugs
Rituximab and tocilizumab may improve uveitis in selected patients with refractory eye disease, but these agents have not been specifically evaluated in this indication.45 There is no new onset or flare of uveitis associated with the use of rituximab, tocilizumab or abatacept.
Scleritis
Infliximab has been tested in the treatment of scleritis, with encouraging results.46 Conversely, three cases of severe scleritis were reported during etanercept therapy for RA. Ocular inflammation improved after treatment discontinuation, with no other relapses. Rechallenge of the drug in one case led to the reappearance of ocular symptoms.47 Preliminary results indicate that rituximab may be an effective treatment for non-infectious refractory scleritis, but conversely there is no case of scleritis induced by this agent.48
Sarcoidosis
The use of anti-TNF-α drugs, especially infliximab, has been investigated for the treatment of sarcoidosis, with promising results in selected cases of refractory disease.2 A randomised controlled trial in sarcoidosis with pulmonary involvement was performed, showing a significant effect of infliximab on forced vital capacity, though with a minimal improvement (2.5%).49 In parallel, etanercept failed to demonstrate efficacy in patients with ocular sarcoidosis.50 Owing to these negative results, anti-TNF-α agents are not currently licensed for the treatment of sarcoidosis. In parallel, cases of sarcoidosis and granulomatosis-like diseases occurring under anti-TNF-α therapy have been described.51 Ten cases of sarcoid-like granulomatosis were reported in one series, describing the main clinical and paraclinical characteristics and outcome of these patients.52 A previous literature review of 28 similar cases was available, but additional cases have since been reported.2 Taking these data together, etanercept was found to be the main anti-TNF-α agent associated with the development of such cases (57%), but sarcoidosis may also occur during treatment with an anti-TNF-α monoclonal antibody (infliximab or adalimumab). The underlying disease requiring the anti-TNF-α agent was RA or SpA, and sarcoidosis was started after a mean delay of 20 months after drug initiation. Sarcoidosis under anti-TNF-α agents had no specific features compared to spontaneous sarcoidosis, and therefore usual clinical manifestations were observed involving the skin and lungs (figure 3). In most cases, the anti-TNF-α agent was discontinued and additional treatment with corticosteroids given, leading to a favourable outcome. Rechallenge was not performed, but a limited number of patients switched therapy (in general from etanercept to a monoclonal antibody) without relapse.2
Figure 3.

Sarcoidosis with bilateral hilar lymph node enlargement in a patient with rheumatoid arthritis treated by adalimumab.
Other aseptic granulomatous diseases
Granuloma annulare is a benign skin disease that typically consists of grouped papules in an enlarging ring shape.1 Anecdotal cases suggest that anti-TNF-α therapy may be beneficial in recalcitrant granuloma annulare.53 However, cases of granuloma annulare have also been described during exposure to anti-TNF-α agents.1 One series described the occurrence of nine cases of granuloma annulare among 199 patients with RA.54 The skin lesion appeared after a mean delay of 6 months and adalimumab was the most frequently involved drug. The outcome was good with resolution of the granuloma with topical corticosteroids while maintaining the anti-TNF-α agent in seven cases.54
Interstitial granulomatous dermatitis is a rare disease that presents clinically as a pruritic and painful rash revealing symmetric, erythematous and violaceous plaques over the lateral trunk, buttocks and thighs. This skin disease is reported to be associated with certain systemic autoimmune disease such as RA, systemic lupus erythematosus or vasculitis.1 Infliximab has been proposed as an efficient drug for this disease. Conversely, exceptional cases of interstitial granulomatous dermatitis have been reported under infliximab, etanercept or adalimumab. The lesion can resolve after drug discontinuation or can persist with its maintenance.55
Pulmonary nodulosis
We previously reported 11 cases of pulmonary nodulosis or granulomatous lung disease (defined by aseptic and non-caseating granulomatous inflammation) that developed under anti-TNF-α therapy.56 Biopsy was available for eight patients showing typical rheumatoid nodules in four cases and non-caseating granulomatous lesions in the other cases. Five of the patients in this series had pulmonary clinical symptoms. Six patients were treated by etanercept and the others had infliximab or adalimumab. The outcome was favourable for all the patients after discontinuation or maintenance of anti-TNF-α treatment.
There is no additional report of the appearance or worsening of rheumatoid nodules in patients with RA treated by other biological drugs. However, rituximab was found to be effective in reducing the size and number of pulmonary rheumatoid nodules in a retrospective analysis of 10 patients.57 It has also been observed that tocilizumab may lead to the disappearance of olecranon rheumatoid nodules,58 and this drug was associated with a marked improvement in lung rheumatoid nodules in one patient.59
Vasculitis
Rituximab is an effective drug in the treatment of antineutrophilic cytoplasmic antibody associated vasculitis. Anti-TNF-α agents are not considered to improve vasculitis in patients with RA but they have become the leading culprits in drug-induced vasculitis with over 200 cases worldwide.60 In general, cutaneous small vessel vasculitis was the most common finding, but systemic vasculitis with peripheral nerves or renal involvement was also observed. In a retrospective analysis from the Mayo Clinic, eight cases were described.61 These patients had RA or CD/UC. The mean time lapse between treatment initiation and onset of vasculitis was 34.5 months. The skin was the most affected organ, followed by the peripheral nervous system and the kidney. The most common clinical manifestation was purpura and other cutaneous manifestations included ulceration, blisters and erythematous macules. The majority of patients improved after treatment interruption and rechallenge with anti-TNF-α therapy was not attempted. The largest series of vasculitis appearing during anti-TNF-α therapy involved 39 cases during a nationwide survey conducted in France between 2004 and 2005.62 Most of these cases occurred in patients with RA (87%), and the remainder in those with JIA or SpA. Etanercept was the most frequently incriminated anti-TNF-α drug (54%). Clinical manifestation of vasculitis involved the skin (82%), the peripheral nervous system (28%), the central nervous system (7.7%) and the lung/pleura or pericardium in the other cases. Most patients had a biopsy showing non-necrotising vasculitis (31%), necrotising vasculitis (18%) and various changes in the other cases. Outcome was favourable after cessation of therapy and high-dose corticosteroids or immunosuppressive drugs. Another series from the adverse events reporting system of the US Food and Drug Administration showed similar overall findings.63
Vitiligo
TNF-α has been identified as a potential cytokine involved in the pathogenesis of vitiligo.64 Indeed, TNF-α inhibits melanocyte differentiation from stem cells as well as melanocyte function; it also destroys melanocytes by inducing apoptosis. Thus, anti-TNF-α agents have been tested in the treatment of vitiligo, giving mixed results.65 66 There are accumulating cases reporting the development of vitiligo under anti-TNF-α therapy.67–69 In a multicentre nationwide retrospective study, we described a large series of 18 patients who developed new-onset vitiligo under a biological agent. These patients had mainly psoriasis or chronic inflammatory rheumatic diseases. Anti-TNF-α agents were the most frequently involved biological agent (72%), while ustekinumab (22.2%) was less represented. The outcome was favourable in general, while maintaining the biological agent.70
Other cutaneous PAE
Alopecia areata (AA) has been described under anti-TNF-α therapy (figure 4). The largest series described 29 patients.71 The underlying disease requiring anti-TNF-α therapy was psoriasis in 40%, chronic inflammatory rheumatic disease in 38% and IBD in 24%. The patients predominantly developed patchy AA. Interestingly, seven patients had another PAE/autoimmune disease such as vitiligo, psoriasiform eruption or Hashimoto thyroiditis. Infliximab, etanercept and adalimumab were associated with the development of AA in equal proportions. The anti-TNF-α agent was discontinued in half of the patients, and maintained in the other half, with a favourable outcome in 76% of cases, regardless of anti-TNF-α interruption.71
Figure 4.

Alopecia areata in a patient with psoriasis treated with adalimumab.
Mechanisms involved in PAE
In general, PAEs are described as isolated events and they are mainly reported with anti-TNF-α agents. This may be explained by the long-term use of anti-TNF-α agents compared to more recently introduced biological drugs. Certain PAEs such as uveitis, CD or sarcoidosis occur more frequently with the TNF-α soluble receptor (namely etanercept) as compared to monoclonal antibodies, suggesting the involvement of the differential immunological properties of these two classes of anti-TNF-α agents. Conversely, etanercept is used for more than 15 years compared to the more recently available anti-TNF-α monoclonal antibodies. The pre-existing condition that requires TNF-α inhibition is in general well controlled, indicating that the anti-TNF-α agent is given at an adequate dosage or interval. However, some PAEs correspond to conditions that usually require a high dose regimen compared to the standard dose given for inflammatory rheumatic disease. For instance, adalimumab treatment in CD and/or HS requires a loading dose at initiation. This could potentially explain certain PAEs observed with standard dose anti-TNF-α. The hypothesis of an imbalance in the cytokine milieu is advanced for most PAEs, especially for psoriasis, as well as a shift towards a Th1 cytokine profile or unopposed production of IFN-α.
Psoriasis
Three main hypotheses have been proposed for psoriasis induced by biological agents. The most popular is a disequilibrium in cytokine balance under the pressure of TNF-α inhibition. Indeed, psoriasis is an autoimmune disease that involves three major cytokines, that is, TNF-α, IFN type I and the IL-23/Th17 axis. These cytokines are interwoven in the pathogenesis of psoriasis and are linked in a triangular interplay. Thus, targeting one of these cytokines may have consequences on the other two.72 In this sense, it is well known that TNF-α suppresses the development of plasmacytoid dendritic cells (pDCs), a major cellular component for the production of IFN type I such as IFN-α 73 (figure 5). Experimental data demonstrated that TNF-α regulates IFN-α production by inhibiting the generation of pDCs from CD34+haematopoietic progenitors and that neutralisation of endogenous TNF-α sustains IFN-α secretion by pDCs. In addition, patients with systemic JIA receiving anti–TNF-α treatment display overexpression of IFN-α regulated genes in their peripheral blood leucocytes.74 Plasmacytoid dendritic cells are expressed in the skin of patients with psoriasis and IFN-α production favours the development of psoriasis by stimulating and activating pathogenic T cells, which in turn produce TNF-α. 75 IFN-α is an inducer of certain chemokine receptors on T cells (CXCR3) that induce T-cell migration to the skin. It was also demonstrated that serum IFN-α activity increased in patients with Sjögren's syndrome or inflammatory myopathy while receiving etanercept or infliximab, a mechanism that was proposed to explain the lack of efficacy of these agents in these conditions.76 77 In addition, in chronic inflammatory diseases such as RA, TNF-α inhibition may induce a shift towards Th1 lymphocytes in the peripheral circulation. In this setting, there is decreased traffic towards the inflammatory site such as the synovium, a context that may subsequently favour cell homing to other pathogenic sites such as the skin.7 Polymorphisms of genes involved in cytokine production, such as IL-23-R, are also probably involved. In this sense, psoriasis-like lesions are described in patients with SpA or CD, two conditions linked to the IL-23-R polymorphism, arguing for a common genetic susceptibility trait.
Figure 5.
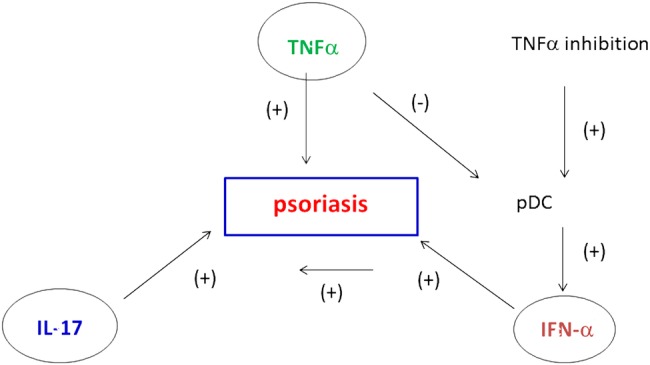
Pathogenic mechanisms involved in psoriasiform skin lesions during anti-TNF-α therapy. TNF-α, IL-17 and IFN-α are the main cytokines that contribute to the development of psoriatic lesions. TNF-α inhibits the activity of plasmacytoid dendritic cells (pDC), which are key producers of IFN-α. During anti-TNF-α treatment, there is an unopposed IFN-α production by pDC. In parallel, IFN-α leads to the expression of chemokines such as CXCR3 on T cells, favouring T cell homing to the skin. IFN-α also stimulates and activates T cells to produce TNF-α and IL-17, sustaining inflammatory mechanisms for psoriasis lesions. IFN-α, interferon α; IL-17, interleukin 17; TNF-α, tumour necrosis factor α.
Hidradenitis suppurativa
To explain the occurrence of HS as a PAE, local modification of cytokine balance and activation of alternate pathways such as IFN type I or IL-1β have been advanced.28 Alternatively, the biological agent may favour occult infection, which is a well-known trigger for HS.
Crohn's disease
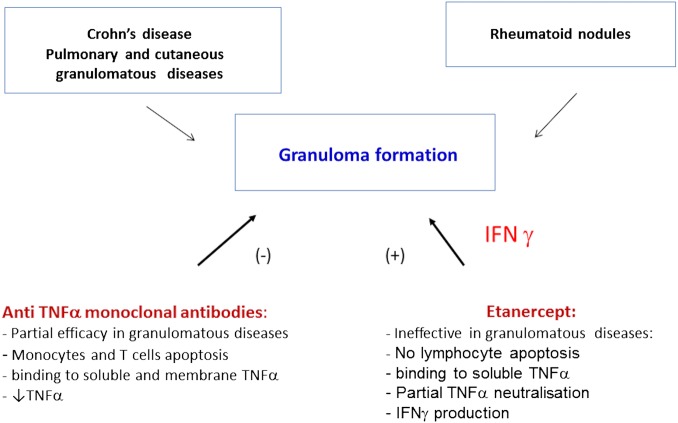
Cases of CD have been reported to occur mainly with etanercept in patients with various inflammatory rheumatic diseases, predominantly SpA. A temporal relationship has been observed between IBD onset and anti-TNF-α therapy exposure. However, the development of CD during anti-TNF-α therapy may be coincidental and related to the underlying disease. In this sense, it is well known that 40–60% of patients with SpA have subclinical gut involvement with abnormal endoscopic lesions similar to CD. In SpA, the frequency of flares or new-onset IBD has been estimated from placebo-controlled and open label extension trials at 2.2/100 patient-years with etanercept, and to 0.2/100 patient-years with infliximab.78 It is thus probable that etanercept is directly linked to the development of paradoxical IBD. Indeed, infliximab can induce apoptosis of peripheral blood cells and lamina propria T cells in CD, but not etanercept. In addition, etanercept may induce the production of TNF-α and IFN-γ, favouring inflammation in the bowel mucosa, and IFN-γ is thought to contribute to granuloma formation32 (figure 6).
Figure 6.
Mechanisms involved in aseptic granulomatous diseases occurring with anti–TNF-α therapy. Anti–TNF-α monoclonal antibodies and the p75 soluble TNF-α receptor have differential immunological properties that may be associated with granuloma formation and thus with the development of granulomatous diseases. Indeed, anti TNF-α monoclonal antibodies induce profound TNF-α inhibition, whereas etanercept partially respects the production of this cytokine. Etanercept is associated with IFN-α production, which contributes to granuloma formation, while anti–TNF-α monoclonal antibodies inhibit IFN-γ release. IFN-α, interferon α; TNF-α, tumour necrosis factor α.
Uveitis
New-onset uveitis is mainly described with the use of etanercept in patients with different immune-mediated diseases, primarily SpA. Uveitis is a frequent extra-articular manifestation of SpA, and thus new-onset uveitis under anti-TNF-α therapy may be coincidental. However, uveitis was also described in patients with diseases other than SpA (eg, RA). Collectively, these data suggest that new-onset uveitis is probably a PAE in patients predisposed to this type of eye disease.42 In this setting, the administration of etanercept is not (sufficiently) effective for the control of eye inflammation.
Sarcoidosis
Cases of sarcoidosis as PAEs are mainly described with etanercept in patients with inflammatory rheumatic diseases. TNF-α play a central role in the formation/maintenance of granuloma, but the different anti-TNF-α agents do not have the same effects on this phenomenon. Indeed, anti-TNF-α monoclonal antibodies bind to soluble and membrane TNF-α, and are associated with monocyte and T-cell apoptosis and decreased circulating TNF-α. Conversely, the p75 soluble receptor etanercept binds only to soluble TNF-α and does not induce lymphocyte apoptosis. This agent is consequently associated with partial TNF-α neutralisation that may preserve granuloma formation, at least to some degree. In addition, etanercept can enhance T cell production of IFN-γ while infliximab inhibits it, and IFN-γ is a key player in granuloma formation51 52 (figure 6).
Pulmonary nodulosis
The mechanisms that may explain such lung nodular reactions are unclear, but closely related to those involved in granulomatous PAEs. In fact, rheumatoid nodules are characterised by low levels of apoptosis, and etanercept, the most frequently involved drug, does not induce apoptosis, unlike anti-TNF-α monoclonal antibodies. Vasculitis is involved in rheumatoid nodule formation and anti-TNF-α therapy may paradoxically induce vasculitis.56 Finally, anti-TNF-α reduces cell trafficking to the inflamed joints, and thus these agents may favour cellular infiltration of other inflamed tissue such as the skin, promoting nodule formation.
Vasculitis
In cases of vasculitis under anti-TNF-α agents, it is difficult to distinguish between rheumatoid vasculitis and drug-induced reactions. Vasculitis has been described in conditions other than RA, such as SpA. Other arguments for a causal relationship include the short delay of onset of vasculitis after anti-TNF-α initiation in certain cases, the improvement after drug withdrawal and also the symptom recurrence on re-initiation of anti-TNF-α therapy. Immune complexes containing the drug may deposit on small vessels and induce local complement activation. The cytokine imbalance that is secondary to TNF-α inhibition may also play a role, with a shift from a Th1 to a Th2 profile, which can upregulate antibody production.62
Vitiligo
The underlying mechanisms to explain vitiligo as a PAE are that the biological agent may lead to local modification of the cytokine balance and/or activation of alternative pathways, such as type I interferon.67 Inhibiting TNF-α can also be associated with a decrease in Treg production and activation, and reduced Treg skin homing allows T cell autoreactivity against melanocytes.79 IFN-γ is a cytokine that plays a central role in vitiligo by suppressing Treg function and inducing melanocyte apoptosis. A dual role of TNF-α inhibitors on Th1/Th2 cytokine balance has been reported, especially in SpA, reducing (infliximab) or enhancing (etanercept) IFN-γ production.80 81 Conversely, the co-occurrence of vitiligo with various inflammatory diseases (including psoriasis, IBD, RA or SpA) is well described, and thus the development or worsening of skin depigmentation may be coincidental and related to the underlying disease.
Approaches for the specific management of PAEs
Whether the biological agent associated with PAE onset may be maintained or not is a challenging issue that depends on a number of factors, namely: the type of PAE and its severity, the pre-existing condition that initially required the biological agent, and the existence of alternative therapeutic options for the underlying disease.
Specific recommendations for the management of psoriasis-induced lesions have been reported.7 First, the diagnosis of skin psoriasis must be confirmed by a dermatologist. A skin biopsy may be required in some cases to eliminate differential diagnosis for psoriasis mimickers. A triggering event including infection or a stressful life event must be systematically investigated. Intake of specific drugs that can induce psoriasiform-lesions must also be identified. After diagnostic confirmation, the severity of psoriasis should be evaluated by specific skin assessment. Most of the time, psoriasis induced during anti-TNF-α therapy is compatible with the maintenance of the drug. Alternatively, if the patient develops severe skin disease or does not wish to continue anti-TNF-α therapy, the drug should be discontinued. In this situation, topical treatment, phototherapy or methotrexate may be added to treat the psoriasis lesions. Switching to another anti-TNF-α agent or to a different class of biological agent is also an option. In patients with PsA who worsen their skin disease, ustekinumab is a valid alternative. It is also necessary to evaluate disease activity of the pre-existing condition in parallel. If an anti-TNF-α agent is strongly required for the patient, switching to another anti-TNF-α agent must be discussed. In patients with RA, changing the TNF-α blocking agent to another class of biologics is feasible. For patients with CD who develop psoriasis and in severe cases, ustekinumab (still not approved for CD), certolizumab or vedolizumab are valuable biological alternatives.
For CD and other IBD that appears under anti-TNF-α therapy, the patient must be managed with the gastroenterologist for diagnostic purposes and assessment of severity. In most reported cases, the causal anti-TNF-α (mainly etanercept) was stopped with improvement of symptoms.32 Corticosteroids and immunosuppressive drugs for the IBD are rarely required. Since the cases were mainly described in patients with SpA, switching from etanercept to a monoclonal antibody is the ideal strategy. If a patient develops IBD under infliximab or adalimumab, this drug must be stopped and the patient switched to another anti-TNF-α monoclonal antibody. Vedolizumab is another option.
Similarly, cases of uveitis have mainly been described in patients with SpA and receiving etanercept. Diagnosis and treatment must be managed together with the ophthalmologist. In general, etanercept is maintained and uveitis treated by local instillation of corticosteroids. In some cases, cessation of the drug is mandatory due to persisting and/or recurrent eye disease. If the underlying disease requires a biological agent, switching to an anti-TNF-α monoclonal antibody may be proposed.40
In case of vasculitis occurring under anti-TNF-α therapy, a tissue biopsy is required for histological analysis and diagnostic confirmation. In most cases, discontinuation of the offending agent is the key management step to be taken. In selected cases with mild symptoms such as skin involvement, the biological agent may be maintained but with close dermatological monitoring. In case of severe organ involvement, anti-TNF-α therapy must be unquestionably discontinued. Cessation of the drug in general leads to control and/or resolution of vasculitis-related manifestations, but on the other hand corticosteroids (high-dose methylprednisolone) or immunosuppressive drugs (cyclophosphamide) may be necessary in cases with serious involvement.62 Rituximab is a therapeutic option for the underlying disease, especially when vasculitis occurs in a patient with RA.
In case of sarcoidosis induced by anti-TNF-α agents, recommended management is to perform tissue biopsy to confirm diagnosis. When the diagnosis of sarcoidosis is confirmed, clinical assessment and investigations for multiorgan involvement (lung, skin, lymphadenopathies) are required. In general, cessation of therapy is sufficient to induce remission. Since paradoxical sarcoidosis has mainly been described with etanercept, when the underlying disease requires restarting and/or continuing anti-TNF-α therapy, the best option is to choose a monoclonal antibody. When sarcoidosis occurs with a monoclonal antibody, switching to another anti-TNF-α excluding etanercept may be proposed.
When HS occurs during biological therapy, the patient must be referred to a dermatologist. After diagnostic confirmation, specific HS treatment should be proposed. In case of HS with mild symptoms, the biological agent may be maintained. For patients with moderate to severe disease, the physician should consider interrupting the biological agent and/or switching to an alternative, including another drug from the same class. If the patient had pre-existing disease that may benefit from anti-TNF-α therapy, the choice may be adalimumab with an adapted dosage to control HS. If HS occurs under adalimumab, another anti–TNF-α must be chosen.31
In cases of vitiligo or alopecia onset under a biological agent, the drug is usually maintained with a favourable outcome. Treatment withdrawal may be exceptionally required with a switch to another anti-TNF-α agent or to another class of biological agent.70
Conclusion
A wide range of PAEs have been described with the introduction of biological drugs, and especially anti-TNF-α agents. Some are potentially strongly linked to the biological drug (psoriasis, CD), while for others the link with these agents seems more hypothetical (vasculitis, vitiligo). The leading class of drugs associated with these unexpected reactions is anti-TNF-α agents, raising questions about the mechanisms involved. Among the different theories that have been proposed to explain PAEs, a disequilibrium in cytokine balance is probably the most realistic hypothesis. Biological agents modify the cytokine milieu and create a favourable immunological context, driving and/or redirecting new pathological pathways that lead to PAE. Since chronic immune-mediated diseases are complex, with the involvement of multiple immunological pathways, the onset of PAEs is not surprising per se. In this sense, close surveillance of newly available biological drugs (anti-IL-17/23, anti-integrin) is necessary in order to detect the occurrence of new and/or undescribed PAEs.
Footnotes
Contributors: ET is the main author of the paper and drafts all the manuscript. FA critically review and complete the paper. FA provides figures of dermatological cases.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Viguier M, Richette P, Bachelez H et al. Paradoxical adverse effects of anti-TNF-alpha treatment: onset or exacerbation of cutaneous disorders. Expert Rev Clin Immunol 2009;5:421–31. 10.1586/eci.09.18 [DOI] [PubMed] [Google Scholar]
- 2.Toussirot E, Pertuiset E. TNFα blocking agents and sarcoidosis: an update. Rev Med Interne 2010;31:828–37. 10.1016/j.revmed.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 3.Joyau C, Veyrac G, Dixneuf V et al. Anti-tumour necrosis factor alpha therapy and increased risk of de novo psoriasis: is it really a paradoxical side effect? Clin Exp Rheumatol 2012;30:700–6. [PubMed] [Google Scholar]
- 4.Fouache D, Goëb V, Massy-Guillemant N et al. Paradoxical adverse events of anti-tumour necrosis factor therapy for spondyloarthropathies: a retrospective study. Rheumatology (Oxford) 2009;48:761–4. 10.1093/rheumatology/kep083 [DOI] [PubMed] [Google Scholar]
- 5.Flendrie M, Vissers WH, Creemers MC et al. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther 2005;7:R666–76. 10.1186/ar1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten D, Kruithof E, Van den Bosch F et al. Systematic safety follow-up in a cohort of 107 patients with spondyloarthropathy treated with infliximab: a new perspective on the role of host defence in the pathogenesis of the disease? Ann Rheum Dis 2003;62:829–34. 10.1136/ard.62.9.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collamer AN, Guerrero KT, Henning JS et al. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum 2008;59:996–1001. 10.1002/art.23835 [DOI] [PubMed] [Google Scholar]
- 8.Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum 2010;40:233–40. 10.1016/j.semarthrit.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Rahier JF, Buche S, Peyrin-Biroulet L et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol 2010;8:1048–55. 10.1016/j.cgh.2010.07.022 [DOI] [PubMed] [Google Scholar]
- 10.Harrison MJ, Dixon WG, Watson KD et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti-tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009;68:209–15. 10.1136/ard.2007.087288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez MV, Sanmarti R, Canete JD et al. Cutaneous adverse events during treatment of chronic inflammatory rheumatic conditions with tumor necrosis factor antagonists: study using the Spanish registry of adverse events of biological therapies in rheumatic diseases. Arthritis Care Res 2013;65:2024–31. 10.1002/acr.22096 [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Boj E, Stamm TA, Sadlonova M et al. Rituximab in psoriatic arthritis: an exploratory evaluation. Ann Rheum Dis 2012;71:1868–71. 10.1136/annrheumdis-2012-201897 [DOI] [PubMed] [Google Scholar]
- 13.Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum 2007;56:2715–18. 10.1002/art.22811 [DOI] [PubMed] [Google Scholar]
- 14.Thomas L, Canoui-Poitrine F, Gottenberg JE et al. Incidence of new-onset and flare of preexisting psoriasis during rituximab therapy for rheumatoid arthritis: data from the French AIR registry. J Rheumatol 2012;39:893–8. 10.3899/jrheum.111347 [DOI] [PubMed] [Google Scholar]
- 15.Mease P, Genovese MC, Gladstein G et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum 2011;63:939–48. 10.1002/art.30176 [DOI] [PubMed] [Google Scholar]
- 16.Jost C, Hermann J, Caelen Lel-S et al. New onset psoriasis in a patient receiving abatacept for rheumatoid arthritis. BMJ Case Rep 2009;2009:pii:bcr09.2008.0845 10.1136/bcr.09.2008.0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato K, Satoh T, Nishizawa A et al. Psoriasiform drug eruption due to abatacept. Acta Derm Venereol 2011;91:362–3. 10.2340/00015555-1042 [DOI] [PubMed] [Google Scholar]
- 18.Florent A, Albert C, Giacchero D et al. Reactivation of cutaneous psoriasis during abatacept therapy for spondyloarthropathy. Joint Bone Spine 2010;77:626–7. 10.1016/j.jbspin.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 19.Brunasso AM, Laimer M, Massone C. Paradoxical reactions to targeted biological treatments: a way to treat and trigger? Acta Derm Venereol 2010;90:183–5. 10.2340/00015555-0777 [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Le Parc JM, Clérici T et al. Onset of psoriasis following treatment with tocilizumab. Br J Dermatol 2010;163:1364–5. 10.1111/j.1365-2133.2010.10005.x [DOI] [PubMed] [Google Scholar]
- 21.Palmou-Fontana N, Sánchez Gaviño JA, McGonagle D et al. Tocilizumab-induced psoriasiform rash in rheumatoid arthritis. Dermatology (Basel) 2014;228:311–13. 10.1159/000362266 [DOI] [PubMed] [Google Scholar]
- 22.Puig L, Morales-Múnera CE, López-Ferrer A et al. Ustekinumab treatment of TNF antagonist-induced paradoxical psoriasis flare in a patient with psoriatic arthritis: case report and review. Dermatology (Basel) 2012;225:14–17. 10.1159/000339864 [DOI] [PubMed] [Google Scholar]
- 23.Hay RA, Pan JY. Paradoxical flare of pustular psoriasis triggered by ustekinumab, which responded to adalimumab therapy. Clin Exp Dermatol 2014;39:751–2. 10.1111/ced.12392 [DOI] [PubMed] [Google Scholar]
- 24.Viguier M, Richette P, Aubin F et al. Onset of psoriatic arthritis in patients treated with efalizumab for moderate to severe psoriasis. Arthritis Rheum 2008;58:1796–802. 10.1002/art.23507 [DOI] [PubMed] [Google Scholar]
- 25.Čarija A, Ivić I, Marasović-Krstulović D et al. Paradoxical psoriatic arthritis in a patient with psoriasis treated with ustekinumab. Rheumatology (Oxford) 2015;54:2114–16. 10.1093/rheumatology/kev263 [DOI] [PubMed] [Google Scholar]
- 26.Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012;9:496–503. 10.1038/nrgastro.2012.125 [DOI] [PubMed] [Google Scholar]
- 27.Kelly G, Sweeney CM, Tobin AM et al. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol 2014;53:1186–96. 10.1111/ijd.12550 [DOI] [PubMed] [Google Scholar]
- 28.Kimball AB, Kerdel F, Adams D et al. Adalimumab for the treatment of moderate to severe Hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012;157:846–55. 10.7326/0003-4819-157-12-201212180-00004 [DOI] [PubMed] [Google Scholar]
- 29.Pellegrino M, Taddeucci P, Peccianti C et al. Etanercept induced hidradenitis suppurativa. G Ital Dermatol Venereol 2011;146:503–4. [PubMed] [Google Scholar]
- 30.Delobeau M, Abdou A, Puzenat E et al. Observational case series on adalimumab-induced paradoxical hidradenitis suppurativa. J Dermatolog Treat 2016;27:251–3. 10.3109/09546634.2015.1094179 [DOI] [PubMed] [Google Scholar]
- 31.Faivre C, Villani AP, Aubin F et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biological agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol 2016;74:1153–9. 10.1016/j.jaad.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 32.Toussirot É, Houvenagel É, Goëb V et al. Development of inflammatory bowel disease during anti-TNF-α therapy for inflammatory rheumatic disease: a nationwide series. Joint Bone Spine 2012;79:457–63. 10.1016/j.jbspin.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 33.van Dijken TD, Vastert SJ, Gerloni VM et al. Development of inflammatory bowel disease in patients with juvenile idiopathic arthritis treated with etanercept. J Rheumatol 2011;38:1441–6. 10.3899/jrheum.100809 [DOI] [PubMed] [Google Scholar]
- 34.Suhler EB, Smith JR, Giles TR et al. Infliximab therapy for refractory uveitis: 2-year results of a prospective trial. Arch Ophthalmol 2009;127:819–22. 10.1001/archophthalmol.2009.141 [DOI] [PubMed] [Google Scholar]
- 35.Galor A, Perez VL, Hammel JP et al. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 2006;113:2317–23. 10.1016/j.ophtha.2006.04.038 [DOI] [PubMed] [Google Scholar]
- 36.Guignard S, Gossec L, Salliot C et al. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis 2006;65:1631–4. 10.1136/ard.2006.052092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudwaleit M, Rødevand E, Holck P et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 2009;68:696–701. 10.1136/ard.2008.092585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun J, Baraliakos X, Listing J et al. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 2005;52:2447–51. 10.1002/art.21197 [DOI] [PubMed] [Google Scholar]
- 39.Le Garrec J, Marcelli C, Mouriaux F. Can tumor necrosis factor inhibitors induce sclero-uveitis? J Fr Ophtalmol 2009;32:511.e1–6. 10.1016/j.jfo.2009.04.029 [DOI] [PubMed] [Google Scholar]
- 40.Cobo-Ibáñez T, del Carmen Ordóñez M, Muñoz-Fernández S et al. Do TNF-blockers reduce or induce uveitis? Rheumatology (Oxford) 2008;47:731–2. 10.1093/rheumatology/ken091 [DOI] [PubMed] [Google Scholar]
- 41.Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum 2007;56:3248–52. 10.1002/art.22918 [DOI] [PubMed] [Google Scholar]
- 42.Wendling D, Paccou J, Berthelot JM et al. New onset of uveitis during anti-tumor necrosis factor treatment for rheumatic diseases. Semin Arthritis Rheum 2011;41:503–10. 10.1016/j.semarthrit.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 43.Lie E, Lindström U, Zverkova-Sandström T et al. The Effect of TNF inhibitor treatment on occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Arthritis Rheum 2015;67:1271–2. [DOI] [PubMed] [Google Scholar]
- 44.Foeldvari I, Becker I, Horneff G. Uveitis events during adalimumab, etanercept, and methotrexate therapy in Juvenile idiopathic arthritis: data from the biologics in pediatric rheumatology registry. Arthritis Care Res (Hoboken) 2015;67:1529–35. 10.1002/acr.22613 [DOI] [PubMed] [Google Scholar]
- 45.Heiligenhaus A, Miserocchi E, Heinz C et al. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology (Oxford) 2011;50:1390–4. 10.1093/rheumatology/ker107 [DOI] [PubMed] [Google Scholar]
- 46.Sen HN, Sangave A, Hammel K et al. Infliximab for the treatment of active scleritis. Can J Ophthalmol 2009;44:e9–e12. 10.3129/i09-061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaujoux-Viala C, Giampietro C, Gaujoux T et al. Scleritis: a paradoxical effect of etanercept? Etanercept-associated inflammatory eye disease. J Rheumatol 2012;39:233–9. 10.3899/jrheum.110865 [DOI] [PubMed] [Google Scholar]
- 48.Suhler EB, Lim LL, Beardsley RM et al. Rituximab therapy for refractory scleritis: results of a phase I/II dose-ranging, randomized, clinical trial. Ophthalmology 2014;121:1885–91. 10.1016/j.ophtha.2014.04.044 [DOI] [PubMed] [Google Scholar]
- 49.Baughman RP, Drent M, Kavuru M et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. 10.1164/rccm.200603-402OC [DOI] [PubMed] [Google Scholar]
- 50.Baughman RP, Lower EE, Bradley DA et al. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest 2005;128:1062–47. 10.1378/chest.128.2.1062 [DOI] [PubMed] [Google Scholar]
- 51.Toussirot E, Pertuiset E, Kantelip B et al. Sarcoidosis occuring during anti-TNF-alpha treatment for inflammatory rheumatic diseases: report of two cases. Clin Exp Rheumatol 2008;26:471–5. [PubMed] [Google Scholar]
- 52.Daïen CI, Monnier A, Claudepierre P et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford) 2009;48:883–6. 10.1093/rheumatology/kep046 [DOI] [PubMed] [Google Scholar]
- 53.Min MS, Lebwohl M. Treatment of recalcitrant granuloma annulare (GA) with adalimumab: a single-center, observational study. J Am Acad Dermatol 2016;74:127–3.. 10.1016/j.jaad.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 54.Voulgari PV, Markatseli TE, Exarchou SA et al. Granuloma annulare induced by anti-tumour necrosis factor therapy. Ann Rheum Dis 2008;67:567–70. 10.1136/ard.2007.075663 [DOI] [PubMed] [Google Scholar]
- 55.Deng A, Harvey V, Sina B et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol 2006;142:198–202. 10.1001/archderm.142.2.198 [DOI] [PubMed] [Google Scholar]
- 56.Toussirot E, Berthelot JM, Pertuiset E et al. Pulmonary nodulosis and aseptic granulomatous lung disease occurring in patients with rheumatoid arthritis receiving tumor necrosis factor-alpha-blocking agent: a case series. J Rheumatol 2009;36:2421–7. 10.3899/jrheum.090030 [DOI] [PubMed] [Google Scholar]
- 57.Glace B, Gottenberg JE, Mariette X et al. Efficacy of rituximab in the treatment of pulmonary rheumatoid nodules: findings in 10 patients from the French AutoImmunity and Rituximab/Rheumatoid Arthritis registry (AIR/PR registry). Ann Rheum Dis 2012;71:1429–31. 10.1136/annrheumdis-2011-200915 [DOI] [PubMed] [Google Scholar]
- 58.Al Attia HM, Abushawish M. Treatment with tocilizumab leads to the disappearance of olecranon rheumatoid nodules. Int J Dermatol 2012;51:197–8. 10.1111/j.1365-4632.2011.04966.x [DOI] [PubMed] [Google Scholar]
- 59.Andres M, Vela P, Romera C. Marked improvement of lung rheumatoid nodules after treatment with tocilizumab. Rheumatology (Oxford) 2012;51:1132–4. 10.1093/rheumatology/ker455 [DOI] [PubMed] [Google Scholar]
- 60.Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep 2015;17:71 10.1007/s11926-015-0545-9 [DOI] [PubMed] [Google Scholar]
- 61.Sokumbi O, Wetter DA, Makol A et al. Vasculitis associated with tumor necrosis factor-α inhibitors. Mayo Clin Proc 2012;87:739–45. 10.1016/j.mayocp.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saint Marcoux B, De Bandt M. Vasculitides induced by TNFalpha antagonists: a study in 39 patients in France. Joint Bone Spine 2006;73:710–13. 10.1016/j.jbspin.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 63.Mohan N, Edwards ET, Cupps TR et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol 2004;31:1955–8. [PubMed] [Google Scholar]
- 64.Alikhan A, Felsten LM, Daly M et al. Vitiligo: a comprehensive overview. Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol 2011;65:473–91. 10.1016/j.jaad.2010.11.061 [DOI] [PubMed] [Google Scholar]
- 65.Lv Y, Li Q, Wang L et al. Use of anti-tumor necrosis factor agents: a possible therapy for vitiligo. Med Hypotheses 2009;72:546–7. 10.1016/j.mehy.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 66.Simón JA, Burgos-Vargas R. Vitiligo improvement in a patient with ankylosing spondylitis treated with infliximab. Dermatology 2008;216:234–5. 10.1159/000112932 [DOI] [PubMed] [Google Scholar]
- 67.Ramírez-Hernández M, Marras C, Martínez-Escribano JA. Infliximab-induced vitiligo. Dermatology 2005;210:79–80. 10.1159/000081494 [DOI] [PubMed] [Google Scholar]
- 68.Toussirot E, Salard D, Algros MP et al. Occurrence of vitiligo in a patient with ankylosing spondylitis receiving adalimumab. Ann Dermatol Venereol 2013;140:801–2. 10.1016/j.annder.2013.09.158 [DOI] [PubMed] [Google Scholar]
- 69.Posada C, Flórez A, Batalla A et al. Vitiligo during treatment of Crohn's disease with adalimumab: adverse Effect or co-occurrence? Case Rep Dermatol 2011;3:28–31. 10.1159/000324619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mery-Bossard L, Bagny K, Charby G et al. New onset vitiligo and progression of pre-existing vitilligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol 2016; [Epub ahead of Print 13 Jun 2016] 10.1111/jdv.13759 [DOI] [PubMed] [Google Scholar]
- 71.Tauber M, Buche S, Reygagne P et al. Alopecia areata occurring during anti-TNF therapy: a national multicenter prospective study. J Am Acad Dermatol 2014;70:1146–9. 10.1016/j.jaad.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 72.Grine L, Dejager L, Libert C et al. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev 2015;26:25–33. 10.1016/j.cytogfr.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 73.Seneschal J, Milpied B, Vergier B et al. Cytokine imbalance with increased production of interferon-alpha in psoriasiform eruptions associated with antitumour necrosis factor-alpha treatments. Br J Dermatol 2009;161:1081–8. 10.1111/j.1365-2133.2009.09329.x [DOI] [PubMed] [Google Scholar]
- 74.Palucka AK, Blanck JP, Bennett L et al. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proc Natl Acad Sci USA 2005;102:3372–7. 10.1073/pnas.0408506102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blanco P, Palucka AK, Pascual V et al. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 2008;19:41–52. 10.1016/j.cytogfr.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mavragani CP, Niewold TB, Moutsopoulos NM et al. Augmented interferon-alpha pathway activation in patients with Sjögren's syndrome treated with etanercept. Arthritis Rheum 2007;56:3995–4004. 10.1002/art.23062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dastmalchi M, Grundtman C, Alexanderson H et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis 2008;67:1670–7. 10.1136/ard.2007.077974 [DOI] [PubMed] [Google Scholar]
- 78.Braun J, Baraliakos X, Listing J et al. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor—a agents. Arthritis Rheum 2007;57:639–47. 10.1002/art.22669 [DOI] [PubMed] [Google Scholar]
- 79.Chatterjee S, Eby JM, Al-Khami AA. et al. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol 2014;134:1285–94. 10.1038/jid.2013.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baeten D, Van Damme N, Van den Bosch F et al. Impaired Th1 cytokine production in spondyloarthropathy is restored by anti-TNFalpha. Ann Rheum Dis 2001;60:750–5. 10.1136/ard.60.8.750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou J, Rudwaleit M, Brandt J et al. Upregulation of the production of tumour necrosis factor alpha and interferon gamma by T cells in ankylosing spondylitis during treatment with etanercept. Ann Rheum Dis 2003;62:561–4. 10.1136/ard.62.6.561 [DOI] [PMC free article] [PubMed] [Google Scholar]