Abstract
A 19-year-old patient presented with slowly enlarging, painless, left-sided cervical mass. She had a background of multiple endocrine neoplasia 2B and had undergone a total thyroidectomy for medullary thyroid carcinoma during childhood. A cervical recurrence was therefore suspected. Ultrasonographic and MRI examination revealed a well-defined lesion within the left sternocleidomastoid muscle. Further evaluation with sestamibi and single-photon emission CT revealed elevated tracer uptake within the lesion. Cytological analysis, following ultrasound-guided sampling, revealed absent staining for calcitonin and blood samples confirmed a normal serum calcitonin level; however, the serum parathyroid hormone level was elevated. Overall, summative findings were consistent with a diagnosis of a parathyroid adenoma arising within the left sternocleidomastoid muscle. Given that this is not a location for a physiological parathyroid tissue, the adenoma might have arisen within the autotransplanted parathyroid tissue, injected into the muscular sheath during thyroidectomy. The clinical, radiological and pathological features are considered in this article.
Background
Adenomatous transformation of an autotransplanted parathyroid tissue, although rare, is an important diagnosis to consider when evaluating the post-total thyroidectomy neck. It is also important to differentiate this diagnosis from alternatives, including metastatic nodal disease or an inadvertently autoimplanted malignant tissue. We present the clinical, pathological and radiological work-up. To the authors' knowledge, such a case has not been previously described in the literature, in the setting of total thyroidectomy for multiple endocrine neoplasia (MEN) 2B.
Case presentation
A 19-year-old patient of African descent was referred for ultrasonographic evaluation of her neck, following the discovery of a painless, left-sided, cervical mass on self-palpation. Her medical history included a background of MEN2B, for which she had undergone a prophylactic total thyroidectomy with autotransplantation of the parathyroid tissue into the left sternocleidomastoid muscle during childhood. Postoperative histology at the time had revealed the presence of non-invasive medullary thyroid carcinoma within the resected gland. Given the history of medullary carcinoma, the presence of a new, left-sided neck mass led the clinical team to suspect a nodal metastasis from recurrent medullary thyroid carcinoma.
Investigations
Ultrasonographic evaluation of the left-sided mass revealed a well-defined, 12×6×16 mm, ovoid lesion of homogeneous isoechogenicity (with respect to the skeletal muscle), within the medial aspect of the left sternocleidomastoid muscle at the level of the mid deep cervical chain (level III; figure 1). There was no cervical lymphadenopathy or residual tissue within the thyroid bed.
Figure 1.
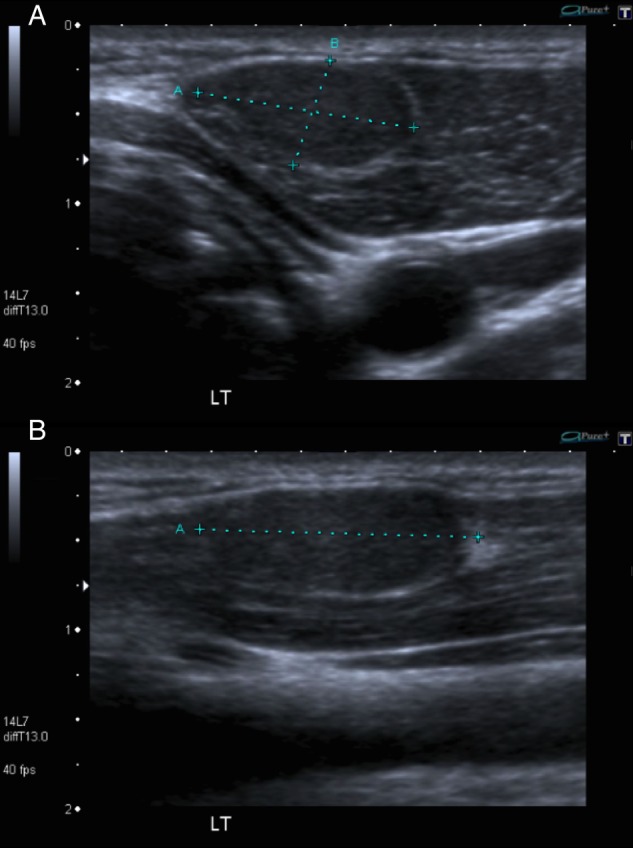
Axial (A) and longitudinal (B) axis images of the left side of the neck demonstrating a 12×6×16 mm, isoechoic lesion embedded within the left sternocleidomastoid muscle.
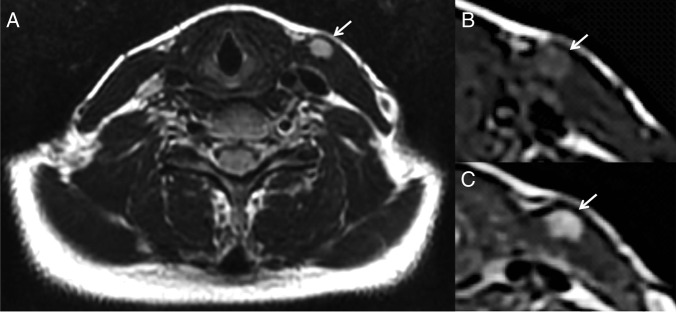
As the intramuscular location of the lesion was atypical for a metastasis, further evaluation with MRI was carried out. The lesion enhanced avidly following intravenous gadolinium administration and demonstrated high signal on T2 and intermediate signal on T1-weighted sequences (figure 2). Given the site of the lesion, within the sternocleidomastoid, lymphadenopathy could be excluded.
Figure 2.
Axial MRIs through the neck, demonstrating a well-defined lesion within the left sternocleidomastoid muscle (arrowed), which is of high signal on T2 (A), intermediate signal on unenhanced T1 (B) and enhances following intravenous gadolinium injection on T1-weighted imaging (C).
Biochemical evaluation of the patient's blood revealed that the calcitonin level was within normal limits: 1.8 ng/L (local reference range 0–4.8 ng/L), further lending credence to the suspicion that the lesion did not represent a nodal focus of recurrent disease. Furthermore, carcinoembryonic antigen (a non-specific biomarker for medullary thyroid carcinoma) was not elevated: 1 μg/L (local reference range 0–5 μg/L). The parathyroid hormone levels were also measured and were confirmed to be elevated: 12.2 pmol/L (local reference range 1.1–6.9 pmol/L). The patient was, however, eucalcaemic, with an adjusted calcium level of 2.35 mmol/L (local reference range 2.2–2.6 mmol/L). Serum phosphate was also within normal limits: 1.02 mmol/L (local reference range 0.8–1.5 mmol/L).
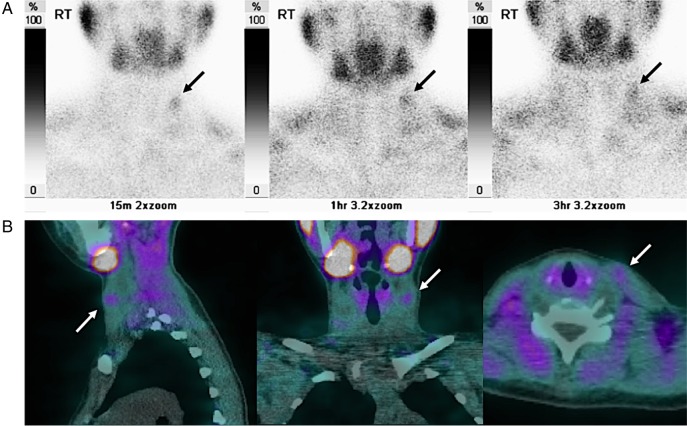
Given the elevated parathyroid hormone levels, a hyperfunctioning parathyroid adenoma was suspected. Further evaluation of the neck was carried out with technitium-99 m (Tc-99 m)-labelled sestamibi and single-photon emission CT (SPECT; figure 3). This confirmed the absence of uptake within the thyroidectomy bed, but a focus of increased tracer uptake was demonstrated at the level of the mid deep cervical chain (level III), which appeared to be related to the left sternocleidomastoid muscle on SPECT. This location corresponded to the lesion seen on ultrasound scanning and MRI. Uptake remained present on delayed (3-hour), planar sestamibi images (a characteristic finding for parathyroid adenomata, which retain Tc-99 m-labelled sestamibi for longer than the adjacent thyroid tissue).
Figure 3.
Selected planar images from technitium-99 m–sestamibi (A) and sagittal, coronal and axial reconstructions from single-photon emission CT (B) studies demonstrate focally elevated radiotracer uptake within the left sternocleidomastoid muscle, which persists on delayed imaging (row A, far right). This corresponded to the lesion seen on ultrasound and MRI.
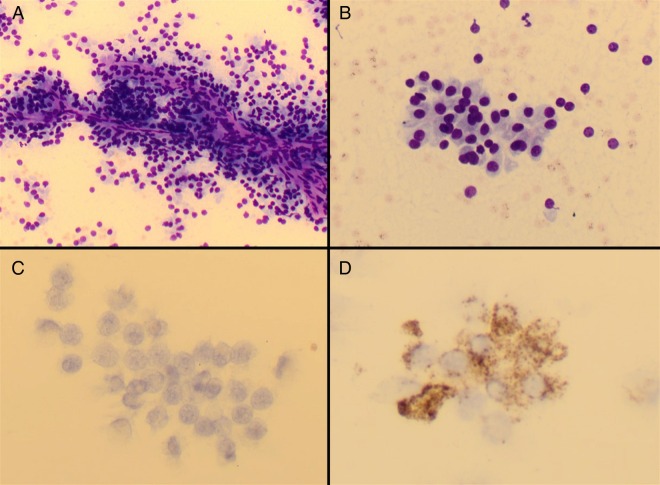
During workup, the patient also underwent ultrasound-guided fine-needle aspiration of the lesion within the left sternocleidomastoid muscle. A sample was sent for cytological analysis (figure 4). The preparations showed groups of cells, which predominantly displayed papillaroid architecture with focal areas of microacinar formation. The cells exhibited round nuclei with occasional anisonucleosis, a speckled chromatin pattern, inconspicuous nucleoli and abundant granular cytoplasm. Immunocytochemistry for chromogranin showed patchy positivity; however, the cells were negative for CD56 (a marker of neuroendocrine differentiation) and calcitonin (a specific marker for medullary thyroid carcinoma).
Figure 4.
Cytopathology specimen obtained by fine-needle aspiration. May-Grünwald-Giemsa-stained direct spread preparations show cohesive, papillaroid groups of cells with round to ovoid nuclei, inconspicuous nucleoli and abundant granular cytoplasm ((A) ×20 magnification and (B) ×40 magnification). On immunohistochemistry, the cells are negative for calcitonin ((C) ×40 magnification) and show patchy positivity for chromogranin A ((D) ×40 magnification).
Subsequent obtainment of the operative report from the institution where the operation during her childhood had taken place confirmed that intraoperative autoimplantation of both left-sided parathyroid glands had occurred at the time of total thyroidectomy. The right-sided parathyroid glands had been left in situ.
Differential diagnosis
Differential diagnosis in the setting of a new cervical lesion in a patient who has previously suffered medullary thyroid carcinoma includes a local recurrence (nodal metastasis). Other differential diagnoses to consider are branchial cleft cyst and intramuscular lipoma. Parathyroid adenoma within an autotransplanted tissue represents an unusual differential. In order to confirm the diagnosis radiologically, multimodal imaging was employed, which serves to improve the sensitivity, specificity and diagnostic confidence. Initial clinical suspicions were of recurrent nodal disease, but the location within the sternocleidomastoid muscle, combined with appearances on ultrasonography demonstrating a lack of typical nodal appearance made this differential unlikely. Furthermore, Tc-99m–sestamibi scanning demonstrated findings consistent with a parathyroid adenoma, with focal, persistent and delayed uptake within the left sternocleidomastoid muscle. This test can be positive in the setting of metastatic medullary carcinoma, but it is only typically positive in this context when calcitonin levels are very high.1 Parathyroid adenomata may arise within ectopic native glands, with locations ranging from the carotid sheath, thyroid, retro-oesophageal region, thymus, mediastinum, aortopulmonary window and pericardium.2 An intramuscular location is not embryologically compatible with an aberrant native gland. Furthermore, it is the usual site of autotransplantation during total thyroidectomy. The MRI findings are also in keeping with a parathyroid adenoma, which is typically hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging and enhances avidly following contrast administration.3 Although these findings are non-specific, nodal disease would not present intramuscularly. Branchial cleft cysts are usually centred more superiorly in the neck at the level of the upper deep cervical chain (level II); they are typically anechoic on ultrasound scanning (unless infected) and do not demonstrate any intrinsic enhancement. Intramuscular lipomata are hyperechoic on ultrasound scanning in comparison to the surrounding musculature and would demonstrate a high signal on T1-weighted MRI.
Treatment
Following collation of the clinical, radiological, biochemical and cytological results, the case was discussed in a multidisciplinary team meeting. The resultant consensus was that the lesion identified represented a hyperfunctioning parathyroid adenoma within the left sternocleidomastoid muscle. The reason for its ectopic location was iatrogenic autotransplantation of the salvaged parathyroid tissue during the patient's original total thyroidectomy.
Outcome and follow-up
The patient is clinically well and currently eucalcaemic. In view of this, the patient continues to be managed conservatively with close endocrinological review. Surgical excision may be considered in future in the event of hypercalcaemia.
Discussion
MEN type 2B results from a germline mutation in the rearranged during transfection (RET) proto-oncogene, which is inherited in an autosomal dominant fashion. It is characterised by a marfanoid habitus, mucosal neuromas, phaeochromocytoma, ganglioneuromatosis of the gastrointestinal tract and medullary thyroid carcinoma.4 The medullary carcinoma is typically highly aggressive and, untreated, patients rarely survive beyond adolescence.5 Therefore, as in this case, prophylactic total thyroidectomy is often performed.6 However, one of the principle complications of such surgery is hypoparathyroidism, secondary to inadvertent injury to the parathyroid glands.
Autotransplantation of the parathyroid tissue is an established procedure for the prevention of hypocalcaemia, which may complicate total thyroidectomy and other forms of anterior neck surgery. Parathyroid glands may be excised or devascularised intraoperatively, particularly during thyroid capsular dissection.7 If all the native parathyroid tissue is lost or rendered non-functional, the patient will develop severe hypoparathyroidism and consequent hypocalcaemia with potentially fatal sequelae. Although hypocalcaemia may be managed with oral supplements, additional complications may arise from the lack of physiological parathyroid hormone production. Autotransplantation seeks to circumvent this problem and may be achieved by excision, fragmentation and intramuscular injection of a non-viable parathyroid gland.7 The typical site for transplantation is the substance of the sternocleidomastoid muscle. Autotransplantation into the non-dominant forearm may also be carried out if future re-exploration is likely, such as in MEN2A or secondary hyperparathyroidism.8
Autotransplantation is generally considered a safe procedure, with the primary risk being graft failure and consequent hypocalcaemia. Other complications are extremely rare. These include recurrent thyroid malignancy (arising within inadvertently transplanted malignant tissue),9 graft hyperfunction and parathyroid adenoma.10 11 The latter is rare and a recent case report and review of the literature highlighting the presence of an adenoma arising from autotransplanted parathyroid tissue in the setting of secondary hyperparathyroidism identified fewer than 20 previous cases in the literature.12 However, the risk of hyperfunctioning glandular tissue in this group is considerably higher, owing to autotransplantation of abnormal glands (this also occurs in MEN1).13 14
The development of a parathyroid adenoma in an autotransplanted tissue following thyroidectomy is extremely rare, with minimal information in the available literature. To the authors' knowledge, such an occurrence, in the setting of total thyroidectomy for MEN2B, has not been previously reported.
Learning points.
Thorough clinical evaluation of the neck is imperative in patients at high risk of recurrent malignancy, such as in the setting of previous medullary carcinoma in multiple endocrine neoplasia (MEN) 2B.
A multidisciplinary approach with appropriate use of multimodality imaging (ultrasound, MRI and nuclear medicine) is essential in reaching an appropriate diagnosis in complex cases.
Autotransplantation of parathyroid tissue is a well-established technique employed to prevent long-term hypocalcaemia in the setting of total thyroidectomy and clinicians, radiologists and pathologists should be aware of its use and possible complications.
Acknowledgments
The authors would like to thank Dr EJ Adam and our colleagues in the departments of radiology, pathology, endocrinology and otolaryngology at St. George's Hospital.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Learoyd DL, Roach PJ, Briggs GM et al. . Technetium-99m-sestamibi scanning in recurrent medullary thyroid carcinoma. J Nucl Med 1997;38:227–30. [PubMed] [Google Scholar]
- 2.Nguyen BD. Parathyroid imaging with Tc-99 m sestamibi planar and SPECT scintigraphy. Radiographics 1999;19:601–14; discussion 615–6 10.1148/radiographics.19.3.g99ma10601 [DOI] [PubMed] [Google Scholar]
- 3.Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000;38:1105–29. 10.1016/S0033-8389(05)70224-4 [DOI] [PubMed] [Google Scholar]
- 4.Moline J, Eng C. Multiple endocrine neoplasia type 2: an overview. Genet Med 2011;13:755–64. 10.1097/GIM.0b013e318216cc6d [DOI] [PubMed] [Google Scholar]
- 5.Pinchera A, Elisei R. Medullary thyroid cancer: diagnosis and management. Practical management of thyroid cancer. Springer, 2006;Ch 21:255–79. [Google Scholar]
- 6.Wells SA Jr, Asa SL, Dralle H et al. . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. 10.1089/thy.2014.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis I, Constantinides VA, Tolley N et al. . Parathyroid autotransplantation during thyroid surgery. World J Endocr Surg 2012;4:115–17. 10.5005/jp-journals-10002-1112 [DOI] [Google Scholar]
- 8.Olson JA Jr, DeBenedetti MK, Baumann DS et al. . Parathyroid autotransplantation during thyroidectomy. Results of long-term follow-up. Ann Surg 1996;223:472–8; discussion 478–80 10.1097/00000658-199605000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo CY, Lam KY. Parathyroid autotransplantation during thyroidectomy: is frozen section necessary? Arch Surg 1999;134:258–60. 10.1001/archsurg.134.3.258 [DOI] [PubMed] [Google Scholar]
- 10.Shepet K, Alhefdhi A, Usedom R et al. . Parathyroid cryopreservation after parathyroidectomy: a worthwhile practice? Ann Surg Oncol 2013;20:2256–60. 10.1245/s10434-013-2941-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lando MJ, Hoover LA, Zuckerbraun L et al. . Autotransplantation of parathyroid tissue into sternocleidomastoid muscle. Arch Otolaryngol Head Neck Surg 1988;114:557–60. 10.1001/archotol.1988.01860170087025 [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Ondracek TC, Chen F. Parathyroid adenoma arising from autotransplanted parathyroid tissue in sternocleidomastoid muscle: a case report with review of the literature. N A J Med Sci 2015;8:89–91. [Google Scholar]
- 13.Chou FF, Lee CH, Chen HY et al. . Persistent and recurrent hyperparathyroidism after total parathyroidectomy with autotransplantation. Ann Surg 2002;235:99–104. 10.1097/00000658-200201000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonelli F, Giudici F, Cavalli T et al. . Surgical approach in patients with hyperparathyroidism in multiple endocrine neoplasia type 1: total versus partial parathyroidectomy. Clinics (Sao Paulo) 2012;67(Suppl 1):155–60. 10.6061/clinics/2012(Sup01)26 [DOI] [PMC free article] [PubMed] [Google Scholar]