Abstract
Gastric perforation secondary to metastasis from breast cancer occurs infrequently. We present the case of a 72-year-old postmenopausal female patient with a known history of lobular carcinoma of the breast who presented to a district general hospital with a clinical diagnosis of an acute abdomen. Further contrast-enhanced CT scan demonstrated free gas and fluid in the abdomen. She underwent emergency exploratory laparotomy and onlay Graham's omentopexy patch due to 1×1 cm prepyloric gastric perforation. Final histopathology proved the presence of metastatic malignant cells in the breast origin. We discuss the issues involved in postoperative investigation and management.
Background
We report a rare disease, and this unusual clinical presentation should be of interest to readers.
Case presentation
A 72-year-old female patient presented to emergency department with a 4-day history of sudden onset of upper abdominal pain associated with nausea and vomiting. There were no urinary and bowel symptoms, and no history of recent travel and previous history of peptic ulcer disease. On physical examination, her temperature was 37.0°C, pulse rate 95 bpm, blood pressure 130/80 mm Hg and respiratory rate 20 bpm. Her abdomen was not distended, but rebound tenderness and generalised peritonitis were elicited on examination.
Her previous medical and surgical comorbidities included seropositive rheumatoid arthritis (on sulfasalazine) and a previously diagnosed right breast carcinoma (subtype invasive lobular carcinoma (ILC), grade 2) for which she underwent wide local excision and sentinel lymph node biopsy in 2012. The tumour staging was pT2, N0, M0, estrogen receptor (ER) and progesterone receptor (PR) positive and HER2 negative. She completed adjuvant radiotherapy in 2013 and is currently taking letrozole 2.5 mg/day (a non-steroidal aromatase inhibitor (AI)). She had previously been on tamoxifen 20 mg daily. Other medications include aspirin and glucosamine.
Investigations
Full blood count showed elevated white cell count 11.8×109/L, haemoglobin 13.0 g/dL, platelet 285 109/L, sodium 140 mmol/L, potassium 4.3 mmol/L, urea 7.5 mmol/L, creatinine 66 mmol/L, amylase 57 IU/L, calcium 2.26 mmol/L and lactate 1.5 mmol/L. Urine microscopy—negative for urinary tract infection, leucocytes <10/L and red cells <10/L.
Chest X-ray revealed free gas under the diaphragms consistent with perforation (figure 1) and the plain film of abdomen was unremarkable (figure 2). Diagnosis of perforated viscus was made clinically. A CT of abdomen and pelvis with contrast (figure 3A, B) revealed intra-abdominal free gas, and air locules were seen within the lateral wall of the duodenum that suggest the likelihood of it being the site of perforation.
Figure 1.
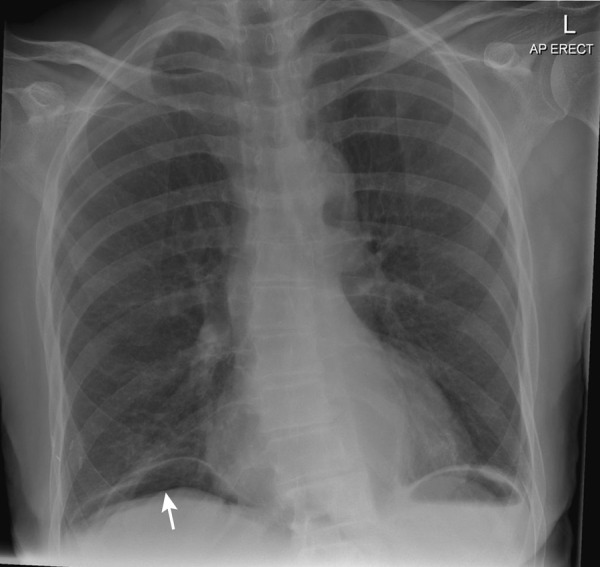
Erect chest X-ray. Free gas under the diaphragm consistent with perforation (white arrow).
Figure 2.
Normal plain film of the abdomen.
Figure 3.
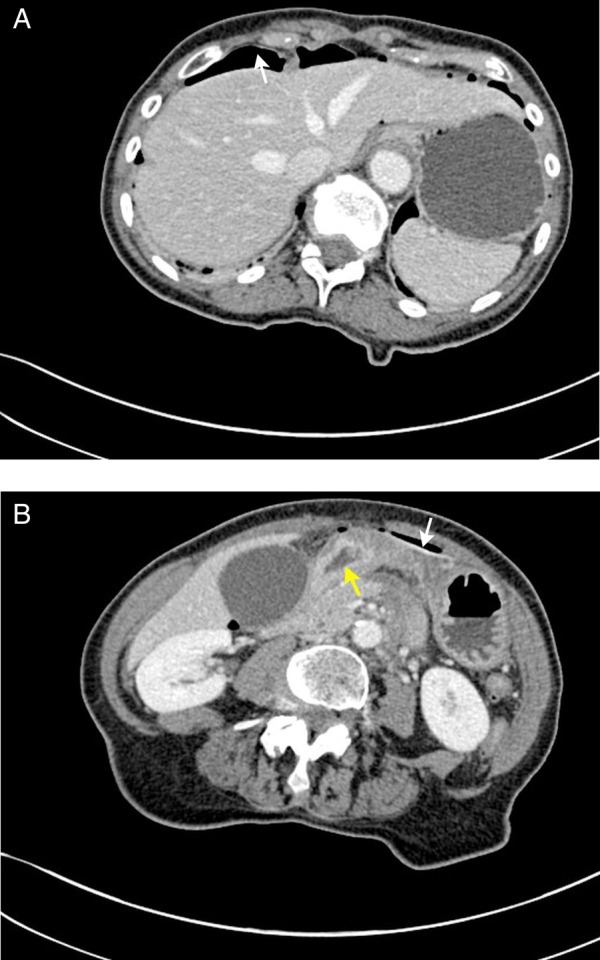
CT abdomen and pelvis (A) and (B). Intra-abdominal free air (labelled as white arrow). Wall defect seen on the lateral wall of the duodenum suggestive of point of perforation (labelled as yellow arrow).
Treatment
Intravenous fluid resuscitation, analgesia, intravenous proton pump inhibitor therapy and broad spectrum intravenous antibiotics were promptly initiated. Nasogastric tube and indwelling urinary catheter were inserted to accurately measure fluid balance.
Operation: intravenous antibiotics were started preoperatively. Upper midline laparotomy was made. There was a 1 cm prepyloric perforated gastric ulcer seen. Wedge biopsy from the edge of the gastric ulcer was taken and sent for histology. The defect was closed with 2/0 polydioxanone (PDS) and buttressed with omental patch (2/0 PDS). A thorough washout with warm saline was carried out, and a subhepatic tube drain was inserted. The abdominal incision was closed by a mass closure technique using loop PDS 1/0 with staples to skin.
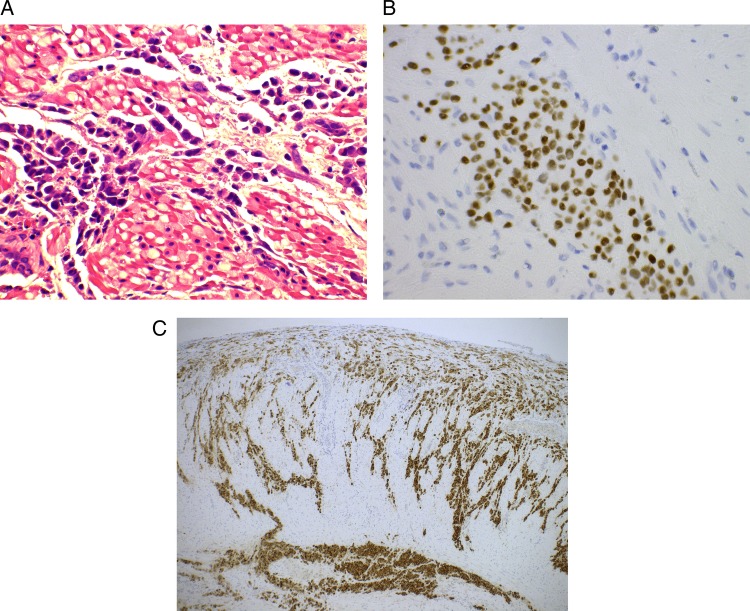
Postoperation: the patient had an uneventful postoperative recovery. Histology (figure 4A–C) showed manifestations are of a lobular carcinoma that are likely to be metastatic in origin. A surveillance CT of the thorax, abdomen and pelvis postoperation, and nuclear medicine whole body bone scan revealed no evidence of lung and bony metastases, respectively. The drain was removed on postoperative day 5, and patient was discharged on postoperative day 10 with prophylaxis medication for Helicobacter pylori eradication (2-week dual-therapy regimens using a proton pump inhibitor and clarithromycin). Hormonal therapy was changed from letrozole (non-steroidal AI) to exemestane (steroidal AI). She remains well after completion of 12 weekly cycles of paclitaxel.
Figure 4.
(A–C) Microscopic view around the perforated prepyloric of the stomach showing numerous malignant cells consistent with gastric metastasis of breast carcinoma invasive lobular carcinoma (A) H&E staining (original magnification ×400) with positive staining for (B) oestrogen (original magnification ×400) and (C) mammoglobin (original magnification ×50).
Discussion
Common sites of metastasis for breast cancer are bone, liver, lung, brain and regional lymph nodes,1 with bone being the commonest site.2 Gastric metastasis is relatively rare in patients with breast carcinoma, but has been reported in the literature. The incidence reported varies between 0.2% and 0.7%3 4 in clinical settings, and from 6% to 18% in an autopsy series.5–8 Metastatic gastric cancer from malignant melanoma, lymphoma and leukaemia have also been reported.9
Interestingly, ILC of the breast has a distinct systemic metastatic pattern, and shows higher incidence of metastatic spread to the intestines, gynaecological organs or peritoneum.10–12 The loss of expression of epithelial-cadherin in ILC is a probable explanation for this peculiar metastatic pattern compared with invasive ductal carcinoma.13 The metastasis to the gastrointestinal (GI) tract generally occurs several years after the diagnosis of the primary breast lesion.14–16 A retrospective review conducted by McLemore et al16 reported a median interval of 7 years between the primary diagnosis of breast cancer and GI metastatic presentation.
It is important to differentiate gastric metastasis of breast cancer from potential surgically resectable primary gastric cancer because surgical approaches in the management of gastric cancer (endoscopic resection, subtotal or partial gastrectomy and total gastrectomy) would have been different depending on the staging of the disease. The histological features of a metastatic breast lobular carcinoma to the stomach consist of the infiltration of the gastric tissue by non-cohesive small tumour cells with an occasional intracytoplasmic lumen arranged in linear cords between the normal gastric glands,17 as shown in our case. Immunohistochemistry staining can be helpful in differentiating primary gastric cancer from the secondary lesions. Metastatic breast carcinomas are usually positive for cytokeratin 7, ERs, PRs and gross cystic disease fluid protein-15, and are negative for cytokeratin 20.14 18
Management of metastatic breast cancer depends on the location of the tumour cells spread and this may include surgery, radiation, chemotherapy, biological and hormonal therapy. In our case, patient developed gastric metastatic disease during the course of adjuvant hormonal therapy (AI) for previous primary breast cancer (ILC). In an acute setting, surgical intervention is required when metastases to the GI produce symptoms due to bleeding, obstruction or perforation. Once perforation has occurred, surgery is the only option, which is similar to the treatment and management in other emergency situations of acute abdomen. Emergent operation and closure with a piece of omentum is the standard of care. Unlike duodenal perforation, biopsy should be routinely taken from the site of gastric perforation to rule out potential primary carcinoma and distant metastatic disease. Duodenal ulcers are extremely unlikely to be malignant, and routine biopsy of these ulcers is not recommended.19
The choice of adjuvant treatment (endocrine or hormonal therapy, chemotherapy or biological radiotherapy or a combination of these) is based on patient's symptoms, age, performance and hormonal status, disease severity and previous systemic treatments. Generally, tamoxifen should be considered for premenopausal and perimenopausal women with hormone-receptor-positive breast cancer while AI (either non-steroidal (letrozole and anastrozole) or steroidal (exemestane)) is recommended for postmenopausal women with hormone-receptor-positive breast cancer. Letrozole is most commonly used as an option for patients with ER positive metastatic breast cancer who have previously received adjuvant tamoxifen. In this vignette, exemestane 25 mg/day would be an adjuvant treatment of choice in which first-line endocrine therapy (letrozole) has failed. AI act predominantly by blocking the conversion of androgens to oestrogens in the peripheral tissues. The use of bisphosphonates, particularly in patients with bony disease, should also be considered to reduce pain and prevent AI-induced osteoporosis.
Trastuzumab (a monoclonal antibody to HER2/neu receptor) should be initiated by a specialist for metastatic breast cancer in postmenopausal patients with HER2-positive tumours, and cytotoxic chemotherapy should be considered for HER2-negative tumours. Systemic chemotherapy should also be considered for fit patients with life-threatening metastatic disease or for patients with symptomatic, recurrent or metastatic disease that does not respond to hormone treatment. The chemotherapy regimens used for metastatic breast cancer are similar to those used for adjuvant and primary systemic treatment. Conventionally, taxanes (paclitaxel and docetaxel) are used for diseases resistant to anthracyclines and for metastatic breast cancer, or in the adjuvant setting.
Proton pump inhibitors should be continued to reduce the risk of stress-induced and non-steroidal anti-inflammatory drug-associated ulcers. A follow-up upper endoscopy can be arranged for most patients after 4–6 weeks to establish healing or exclude other diagnoses. There is no current guideline or recommendation on the role of routine endoscopic surveillance for gastric perforation secondary to either peptic ulcer19 or metastasis disease.20
Conclusion
Gastric perforation secondary to metastatic breast cancer is a rare occurrence. In patients with a history of breast cancer, a high index of suspicion for gastric metastasis should be suspected.
Learning points.
Rare presentation of gastric perforation secondary to invasive lobular carcinoma (breast cancer).
This case highlights the importance of early gastric spread of invasive lobular breast cancer despite being on hormonal treatment.
Acute management of gastric perforation, and further attempts of a curative and follow-up plan for a similar situation.
Acknowledgments
The authors would like to acknowledge Dr Weerasinghe (histopathologist, BVH) for providing histology images and Dr Varia (radiologist, BVH) for his advice on radiology images.
Footnotes
Contributors: CSW and AG wrote the article. PK and AB reviewed and approved the article for publication.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kamby C, Sengelov L. Pattern of dissemination and survival following isolated locoregional recurrence of breast cancer. A prospective study with more than 10years of follow up. Breast Cancer Res Treat 1997;45:181–92. 10.1023/A:1005845100512 [DOI] [PubMed] [Google Scholar]
- 2.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol 1983;23:175–80. 10.1002/jso.2930230311 [DOI] [PubMed] [Google Scholar]
- 3.De Palma GD, Masone S, Rega M et al. Metastatic tumors to the stomach: clinical and endoscopic features. World J Gastroenterol 2006;12:7326–8. 10.3748/wjg.v12.i45.7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi O, Murakami H, Yoshida T et al. Clinical diagnosis of metastatic gastric tumors: clinicopathologic findings and prognosis of nine patients in a single cancer center. World J Surg 2004;28:548–51. 10.1007/s00268-004-7216-8 [DOI] [PubMed] [Google Scholar]
- 5.Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis 1975;20:903–13. 10.1007/BF01070875 [DOI] [PubMed] [Google Scholar]
- 6.Davis HL Jr, Murray RK, Korbitz BC. Breast carcinoma metastatic to the stomach. Report of a case in a male and review of an autopsy series. Am J Dig Dis 1968;13:868–73. 10.1007/BF02237571 [DOI] [PubMed] [Google Scholar]
- 7.Hartmann WH, Sherlock P. Gastroduodenal metastases from carcinoma of the breast. An adrenal steroid-induced phenomenon. Cancer 1961;14:426–31. [DOI] [PubMed] [Google Scholar]
- 8.Cummings MC, Simpson PT, Reid LE et al. Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol 2014;232:23–31. 10.1002/path.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namikawa T, Hanazaki K. Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surg Today 2014;44:1392–9. [DOI] [PubMed] [Google Scholar]
- 10.Fondrinier E, Guérin O, Lorimier G. [A comparative study of metastatic patterns of ductal and lobular carcinoma of the breast from two matched series (376 patients)]. Bull Cancer 1997;84:1101–7. [PubMed] [Google Scholar]
- 11.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637–41; discussion 41–2. [PubMed] [Google Scholar]
- 12.Taal BG, den Hartog Jager FC, Steinmetz R et al. The spectrum of gastrointestinal metastases of breast carcinoma: I. Stomach. Gastrointest Endosc 1992;38:130–5. 10.1016/S0016-5107(92)70377-0 [DOI] [PubMed] [Google Scholar]
- 13.Sastre-Garau X, Jouve M, Asselain B et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 1996;77:113–20. [DOI] [PubMed] [Google Scholar]
- 14.Ayantunde AA, Agrawal A, Parsons SL et al. Esophagogastric cancers secondary to a breast primary tumor do not require resection. World J Surg 2007;31:1597–601. 10.1007/s00268-007-9099-y [DOI] [PubMed] [Google Scholar]
- 15.Taal BG, Boot H, van Heerde P et al. Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept. Gut 1996;39:556–61. 10.1136/gut.39.4.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLemore EC, Pockaj BA, Reynolds C et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol 2005;12:886–94. [DOI] [PubMed] [Google Scholar]
- 17.Almubarak MM, Laé M, Cacheux W et al. Gastric metastasis of breast cancer: a single centre retrospective study. Dig Liver Dis 2011;43:823–7. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann O, Deidesheimer T, Muehlenberg M et al. Immunohistochemical differentiation of metastatic breast carcinomas from metastatic adenocarcinomas of other common primary sites. Histopathology 1996;29:233–40. 10.1111/j.1365-2559.1996.tb01396.x [DOI] [PubMed] [Google Scholar]
- 19.Committee ASoP Banerjee S, Cash BD, Dominitz JA et al. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest Endosc 2010;71:663–8. [DOI] [PubMed] [Google Scholar]
- 20.Committee ASoP Evans JA, Chandrasekhara V, Chathadi KV et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]