Abstract
Morel-Lavallee seroma (MLS) or post-traumatic pseudocyst is a soft tissue seroma developing due to closed degloving injury by a shearing force that causes separation of subcutaneous fatty layer from the deeper muscular fascia resulting in collection of fluid in the created space. Presentation is usually fluctuant swelling following history of injury. More frequently described in orthopaedic literature, it occurs more commonly over gluteal and trochanteric regions, knee and flanks with occurrence over back, thorax being a rare entity. Despite mimicking several other similar presenting conditions, diagnosis of MLS can be made by meticulous history and physical examination with classical findings on ultrasonography, CT scan and MRI. Treatment modality may vary from conservative management to open surgical debridement of the wound with percutaneous aspiration and sclerodhesis forming useful adjuncts to conservative management.
Background
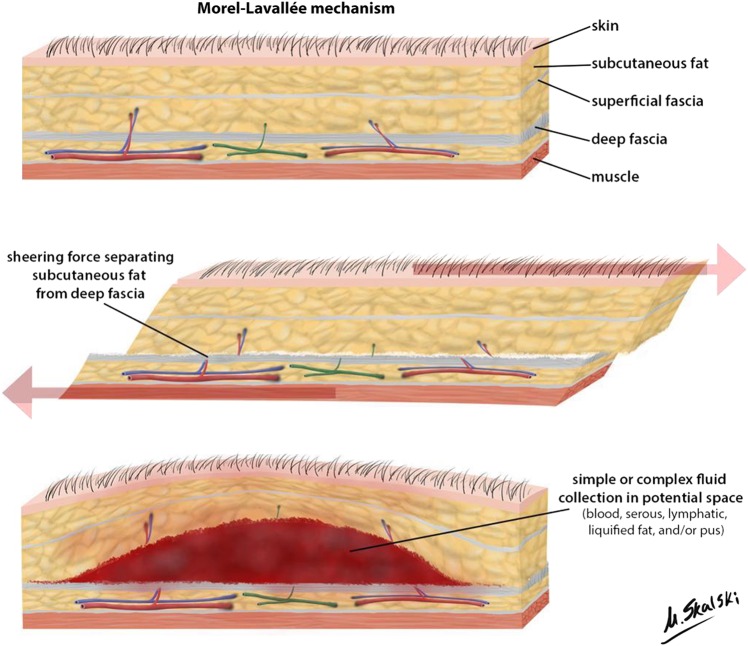
Morel-Lavallee seroma (MLS) or post-traumatic pseudocyst signifies a closed degloving injury often a result of a severe, traumatic, shearing injury, causing separation of the skin and subcutaneous tissue from the underlying deep fascia (figure 1). Though well described in the orthopaedic trauma literature, this lesion is not well recognised by the general surgeons in a polytrauma setting. Presently this injury has been well-described for trochanteric, gluteal and knee1–4 but lesion involving sacral, lumbar and thoracic area of back and bilateral flanks has to best of our knowledge not been mentioned in literature. We describe a case of 18-year-old boy who was referred to general surgery department of King George's Medical University India for back swelling following motor vehicle accident 2 weeks prior. Diagnosis and management of this lesion is challenging and needs to be addressed.
Figure 1.
Mechanism of Morel-Lavallee seroma lesion (case courtesy of Dr Matt Skalski, Radiopaedia.org, rID: 22762).
Case presentation
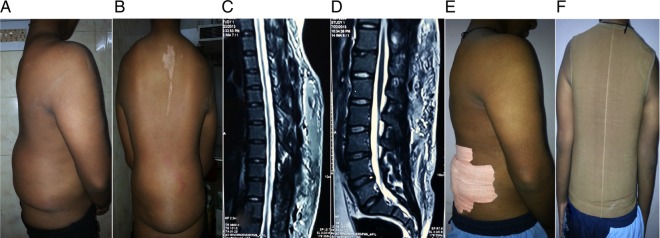
Eighteen years male weighing 65 kgs was referred to general surgery for symptom of large back swelling following motor vehicle accident. On interviewing, the patient developed a tender swelling in lower back immediately after the accident in which there was shear injury to the back (figure 2 A, B) following fall along large tyre of vehicle, for which patient was admitted to another hospital for few days and improved on analgesics and antibiotics and subsequently discharged. Two weeks later patient noticed large non-tender swelling in back for which needle aspiration was performed in a private hospital after which he was referred to our tertiary care facility.
Figure 2.
(A and B) Clinical presentation of patient postinjury with fluctuant swelling over back and flanks; (C and D) MRI studies (upper limit in (C); lower limit in (D)) show homogeneous hyperintensity on both T1-weighted and T2-weighted MRI sequences and appear surrounded by a hypointense peripheral ring representing a fibrous capsule; (D and E) Postsclerodhesis patient was adviced to wear compression garments for 6–8 weeks.
Investigations
After initial work up of the patient including physical examination, coagulation profile (within normal limits), serum and fluid albumin and protein levels (serum albumin=3.8 gm/dL, serum protein=6.5 gm/dL; fluid biochemistry: fluid albumin=2.8 gm/dL, protein=4.1 gm/dL), high-resolution ultrasonography (USG) of back was performed which was suggestive of collection in subcutaneous tissue in superficial plane in almost entire extent of back extending from nape of neck to lower back on both sides of midline. Lymphangiography was performed to rule out lymphocele and abnormal vascular connections. MRI back was suggestive of subcutaneous collection with septations extending in thoracic, lumbar and sacral region (hyperintense collection with septations) (figure 2 C, D).
Treatment
Percutaneous aspiration was performed and 2 L of serous fluid was aspirated followed by repeat aspiration after 4 days. Since the patient was developing recurrent collection, percutaneous USG-guided pigtail catheter was placed in back and compression garments applied. Gradually the daily output decreased to 200–300 mL, the patient was kept on high protein diet ensuring daily supplementation of at least 150 g protein.
Outcome and follow-up
On follow-up after 1 month since the output was 100 mL/day, patient was taken up for sclerodhesis. After ensuring collapse of cavity pigtail catheter removed, sclerodhesis was performed by instilling autoclaved 30 mL doxycycline solution. The patient was discharged with advice to wear compression garments until 6–8 weeks. In follow-up after 1 year, patient had no recurrence.
Discussion
First described by French surgeon Victor Auguste Francois Morel-Lavallee in the mid-19th century, MLS is defined as a closed soft tissue injury leading to a fluctuant, subcutaneous cystic lesion often lined by a fibrous capsule filled with sterile hemolymphatic or serohematic content.
The cause of this lesion is usually a force vector besides a compression and shearing force in a cylindrical structure causing a severe traumatic separation of panniculus adiposus beneath the dermis from underlying deep fascia. This shear injury disrupts perforating vessels and produces a potential space that fills with blood, lymph, serosanguinous fluid or necrotic fat.2 The collection may later lead to the formation of pseudocapsule which may then lead to its progressive enlargement.2 Clinical features depend on the amount of blood and lymphatic fluid collected and the time elapsed since the injury. It may present as a soft, fluctuant, gradually enlarging mass usually weeks to months after inciting event or as an acute painful or painless tender or non-tender swelling or as a chronic, gradually increasing, non-tender swelling. Sometimes skin hypermobility, contour deformity or just decreased cutaneous sensations4–6 might be the only presenting symptoms. Mobility of skin, subcutaneous fluctuation, decreased cutaneous sensation, tire marks or marks of injuries causing tangential force to the skin, and friction burns are useful clinical signs that may help distinguish closed degloving injuries from contusions.4–9 However, the presence of a soft fluctuant area due to fluid collection is the hallmark of its physical findings.10 11
In MLS there is abnormal laxity of skin over the swelling as is seen in our case. This may be a predisposing factor for development of MLS. This lesion should be differentiated from adipose tissue necrosis or haematomas associated with coagulation abnormality and soft tissue tumours, which simulate chronic slow growing lesions. Imaging including USG and MRI help differentiate them. On USG, it is characterised by hyperechoic (blood-predominant) or anechoic (lymph-predominant) fluid collection depending on the age of the lesion and its predominant content. Acute and subacute lesions <1 month old show a heterogeneous appearance with irregular margins and lobular shape. On the other hand, lesions older than 18 months or chronic lesions have well-defined margins with homogenous appearance and a flat or fusiform shape.7 8 On CT and MRI, the lesion is visualised as well defined encapsulated fluid collection with fluid–fluid levels. The lesion is better visualised on MRI scans with soft tissue contrast enhancement. Therefore, MRI is a more effective imaging modality than CT in making a diagnosis of MLS.7 9
MLS should be distinguished from lymphocele, which can also occur following closed trauma. This can be performed by getting biochemistry of serous fluid for protein content and cells. Analysis of the fluid demonstrates high triglyceride levels, chylomicrons and the same level of proteins, urea nitrogen, creatinine, electrolytes, and lipids as serum has. Fluid contains erythrocytes, lymphocytes and scant polymorphs. Among the white cell counts (WCCs) there is a predominance of lymphocytes and a 70% lymphocytes of all WCC is characteristic of lymph. When in doubt, dye study clearly delineates nature of swelling and rules out any vascular connection of serous collection which is also important when patient is planned for sclerodesis to prevent fatal complication.
In management of such lesions one should remember that response to treatment is slow and swelling subsides gradually and patient counselling forms an integral part of treatment. Although various strategies for the treatment of MLS have been reported, including the application of compression bandages, percutaneous aspiration and drainage, open debridement and sclerodhesis, still there are no established treatment modalities.3 7 11 12–29 In acute lesions which are small in size, conservative management is considered with the application of compression bandages, non-steroidal anti-inflammatory drug medications, physiotherapy and absolute bed rest. Compression bandage may also be used as a supplement to other conservative measures.3 7 11 12 16 18 24 Larger acute lesions which do not resolve by compression alone may require percutaneous drainage. Percutaneous drainage when combined with sclerotherapy may also be used as a first-line treatment modality for chronic lesions13 20 22 27 because percutaneous drainage alone may lead to recurrent postoperative haematoma and secondary infection.26 Sclerotherapy may also be used in acute lesions which are refractory to conservative management.14 19 21 In patients with acute lesions with underlying open fractures or in chronic lesions with secondary infection or evidence of tissue necrosis, open debridement can be attempted as the first-line treatment modality. It is also the final management option in patients who are refractory to percutaneous drainage combined with sclerotherapy15 17 23 25 26 28 29 and in long-standing cases of MLS with pseudocapsule formation as they are unresponsive to percutaneous drainage and thus prone to recurrence.23 28 29
Learning points.
Morel-Lavallee seroma (MLS) or post-traumatic pseudocyst is a soft tissue seroma developing due to closed degloving injury by a shearing force that causes separation of subcutaneous fatty layer from the deeper muscular fascia resulting in collection of fluid in the created space.
More frequently described in orthopaedic literature, it occurs more commonly over gluteal and trochanteric regions, knee and flanks with occurrence over back, thorax being a rare entity.
MLS should be distinguished from lymphocele, which can also occur following closed trauma.
Management entails multiple treatment options and since response to treatment is slow and swelling subsides gradually and patient counselling forms integral part of treatment.
Footnotes
Contributors: AS and SM contributed in preparing the first manuscript draft. AA and AAS approved the final draft. All authors contributed to the diagnosis and management of the patient.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morel-Lavallee VA. Decollements traumatiques de la peau et des couches sous-jacentes. Arch Gen Med 1863;1:20–38, 172–200, 300-32 [in French]. [Google Scholar]
- 2.Hak DJ, Olson SA, Matta JM. Diagnosis and management of closed internal degloving injuries associated with pelvic and acetabular fractures: the Morel-Lavallée lesion. J Trauma 1997;42:1046–51. 10.1097/00005373-199706000-00010 [DOI] [PubMed] [Google Scholar]
- 3.Parra JA, Fernandez MA, Encinas B et al. Morel-Lavallée effusions in the thigh. Skeletal Radiol 1997;26:239–41. 10.1007/s002560050228 [DOI] [PubMed] [Google Scholar]
- 4.Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee twenty-seven cases in The National football league. Am J Sports Med 2007;35:1162–7. 10.1177/0363546507299448 [DOI] [PubMed] [Google Scholar]
- 5.Kottmeier SA, Wilson SC, Born CT et al. Surgical management of soft tissue lesions associated with pelvic ring injury. Clin Orthop Relat Res 1996;329:46–53. 10.1097/00003086-199608000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Hudson DA, Knottenbelt JD, Krige JE. Closed degloving injuries: results following conservative surgery. Plast Reconstr Surg 1992;89:853–5. 10.1097/00006534-199205000-00013 [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee K, Perrin SM, Hughes PM. Morel-Lavallee lesion in an adolescent with ultrasound and MRI correlation. Skeletal Radiol 2007;36(Suppl 1):S43–5. 10.1007/s00256-006-0122-4 [DOI] [PubMed] [Google Scholar]
- 8.Neal C, Jacobson JA, Brandon C et al. Sonography of Morel-Lavallee lesions. J Ultrasound Med 2008;27:1077–81. [DOI] [PubMed] [Google Scholar]
- 9.Mellado JM, Péerez del Palomar L, Díaz L et al. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol 2004;182:1289–94. 10.2214/ajr.182.5.1821289 [DOI] [PubMed] [Google Scholar]
- 10.Shen C, Peng JP, Chen XD. Efficacy of treatment in peri-pelvic Morel-Lavallee lesion: a systematic review of the literature. Arch Orthop Trauma Surg 2013;133:635–40. 10.1007/s00402-013-1703-z [DOI] [PubMed] [Google Scholar]
- 11.van Gennip S, van Bokhoven SC, van den Eede E. Pain at the knee: the Morel-Lavallée lesion, a case series. Clin J Sport Med 2012;22:163–6. 10.1097/JSM.0b013e318246ee33 [DOI] [PubMed] [Google Scholar]
- 12.Moriarty JM, Borrero CG, Kavanagh EC. A rare cause of calf swelling: the Morel-Lavallee lesion. Ir J Med Sci 2011;180:265–8. 10.1007/s11845-009-0386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anakwenze OA, Trivedi V, Goodman AM et al. Concealed degloving injury (the Morel-Lavallee lesion) in childhood sports: a case report. J Bone Joint Surg Am 2011;93:e148 10.2106/JBJS.K.00219 [DOI] [PubMed] [Google Scholar]
- 14.Bansal A, Bhatia N, Singh A et al. Doxycycline sclerodesis as a treatment option for persistent Morel-Lavallée lesions. Injury 2013;44:66–9. 10.1016/j.injury.2011.11.024 [DOI] [PubMed] [Google Scholar]
- 15.Carlson DA, Simmons J, Sando W et al. Morel-Lavalée lesions treated with debridement and meticulous dead space closure: surgical technique. J Orthop Trauma 2007;21:140–4. 10.1097/BOT.0b013e31802f19ca [DOI] [PubMed] [Google Scholar]
- 16.Ciaschini M, Sundaram M. Radiologic case study. Prepatellar Morel-Lavallée lesion. Orthopedics 2008;31:719–21. [PubMed] [Google Scholar]
- 17.Efrimescu CI, McAndrew J, Bitzidis A. Acute lumbar Morel-Lavallee haematoma in a 14-year-old boy. Emerg Med J 2012;29:433 10.1136/emermed-2011-200660 [DOI] [PubMed] [Google Scholar]
- 18.Harma A, Inan M, Ertem E [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc 2004;38:270–3. [PubMed] [Google Scholar]
- 19.Luria S, Applbaum Y, Weil Y et al. Talc sclerodhesis of persistent Morel-Lavallée lesions (posttraumatic pseudocysts): case report of 4 patients. J Orthop Trauma 2006;20:435–8. 10.1097/00005131-200607000-00013 [DOI] [PubMed] [Google Scholar]
- 20.Moran DE, Napier NA, Kavanagh EC. Lumbar Morel-Lavallée effusion. Spine J 2012;12:1165–6. 10.1016/j.spinee.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 21.Penaud A, Quignon R, Danin A et al. Alcohol sclerodhesis: an innovative treatment for chronic Morel-Lavallée lesions. J Plast Reconstr Aesthet Surg 2011;64:e262–4. 10.1016/j.bjps.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Sawkar AA, Swischuk LE, Jadhav SP. Morel-Lavallee seroma: a review of two cases in the lumbar region in the adolescent. Emerg Radiol 2011;18:495–8. 10.1007/s10140-011-0975-2 [DOI] [PubMed] [Google Scholar]
- 23.Scaranelo AM, Davanço RA. Pseudocyst formation after abdominal liposuction-extravasations of Morel-Lavallée on MR images. Br J Plast Surg 2005;58:849–51. 10.1016/j.bjps.2004.12.016 [DOI] [PubMed] [Google Scholar]
- 24.Steiner CL, Trentz O, Labler L. Management of Morel-Lavallee lesion associated with pelvic and/or acetabular fractures. European J Trauma Emerg Surg 2008;34:554–60. 10.1007/s00068-007-7056-y [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Morgan SJ, Smith WR et al. Postoperative surgical site infection following acetabular fracture fixation. Injury 2010;41:396–9. 10.1016/j.injury.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Tran W, Foran J, Wang M et al. Postsurgical bleeding following treatment of a chronic Morel-Lavallée lesion. Orthopedics 2008;31:814 10.3928/01477447-20080801-34 [DOI] [PubMed] [Google Scholar]
- 27.Tseng S, Tornetta P III. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am 2006;88:92–6. 10.2106/JBJS.E.00021 [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz A, Yener O. Giant post-traumatic cyst after motorcycle injury: a case report with review of the pathogenesis. Prague Med Rep 2013;114:123–7. 10.14712/23362936.2014.30 [DOI] [PubMed] [Google Scholar]
- 29.Zecha PJ, Missotten FE. Pseudocyst formation after abdominoplasty—extravasations of Morel-Lavallée. Br J Plast Surg 1999;52:500–2. 10.1054/bjps.1999.3154 [DOI] [PubMed] [Google Scholar]