Abstract
Purpose of review
Discuss the current status and obstacles that need to be overcome in the future to provide patient-centered care with left ventricular assist device (LVAD) therapy.
Recent findings
LVADs offer both longer survival and improvements in quality of life for carefully selected patients with inotrope-dependent heart failure (HF). Yet, this technology does not come without significant risk of adverse effects and burdens. Recent observational data comparing LVAD to medical therapy in ambulatory, non-inotrope dependent patients with advanced HF suggest that survival may be similar and changes in quality of life may depend on baseline status. As both LVAD technology and medical therapy continue to evolve, there are many unanswered questions regarding the benefits, risks, and burdens of LVAD therapies in less severe HF populations.
Summary
Further research is needed to ensure the optimal delivery of LVAD therapy including improved patient selection, implantation timing, device type, and decision support.
Keywords: Heart failure, LVAD, mechanical circulatory support, decision-making
Introduction
Left ventricular assist device (LVAD) therapy is an increasingly utilized treatment for select patients with end-stage heart failure (HF). While LVAD therapy has the potential to improve survival and quality of life, patients, and their families must weigh the trade-offs related to the numerous potential complications and burdens related to this therapy. The Institute of Medicine defines patient-centered care as “providing care that is respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions.”1 Providing patient-centered care specifically in the realm of LVAD therapy requires that we 1) understand the short and long-term risks and benefits of LVAD therapy among broad populations of patients, 2) understand how to optimally deliver LVAD therapy including appropriate patient selection, implantation timing, choice of device type and settings, and concomitant medical therapy, and 3) individualize the risk/benefit profile of LVAD therapy to individual patients who are considering this treatment and can effectively communicate the complexities of these individualized risks and benefits to patients and their caregivers. In this review, we highlight the current state of knowledge in each of these domains and comment on the key unanswered questions that are needed to allow the advanced HF community to provide truly patient-centered LVAD therapy.
Risks and Benefits of LVAD Therapy—Current Knowledge and Knowledge Gaps
Next generation LVADs are engineered with non-pulsatile, rotary designs that allow for smaller profiles with improved durability. Currently approved continuous-flow (CF) LVADs proved superior to pulsatile LVADs in terms of survival, durability, and rate of adverse events in both BTT and DT populations.2–4 However, the clinical trials that established the superiority of CF LVADS were limited to select patients with a limited follow-up time of approximately 2 years.4–8
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry has provided longer-term, real-world outcomes data. Since the approval of the first CF LVAD in April 2008, overall actuarial survival rates for CF LVADs have been 80% at 1 year, 70% at 2 years, 59% at 3 years, and 47% at 4 years.9,10 Recent data from INTERMACS suggest that the DT population has actuarial survival rates of 76% at 1 year and 57% at 3 years,10 but longer-term outcome data are needed. Current generation devices also improve functional status and quality of life metrics.7*
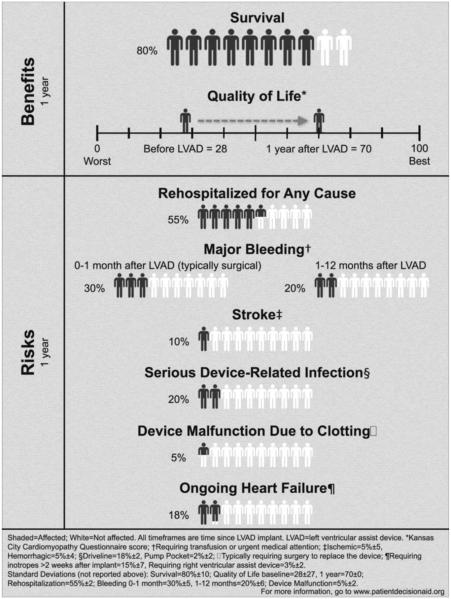
However, individual patients must weigh these potential benefits against the potential for numerous adverse events and lifestyle changes. In a recent systematic review, adverse event rates in the year following DT LVAD implantation were 55% for rehospitalization, 30% for major bleeding, 10% for disabling stroke, 20% for device-related infection, 5% for device malfunction, and 18% for persistent HF requiring inotrope therapy or additional mechanical circulatory support. This cumulative 1 year risk/benefit profile is presented in a simplified pictogram to aid patients as they are facing the decision about DT LVAD (Figure 1).7*
Figure 1.
One-Year Benefits and Risks of LVAD Therapy
Reproduced with permission from McIlvennan et al. Circ Heart Fail. 2014;7:1003-1013
A major limitation in our current knowledge base regarding the complex trade-offs of LVAD therapy is that the long-term risks and benefits are rarely stratified by indication or other important patient factors. BTT and DT patients have been combined in studies with longer follow-up periods while the survival outcomes are likely different among these types of patients.10,11 In addition, many single center reports have small numbers of patients with incomplete baseline characteristics, inconsistent definitions of adverse events, and variable follow-up periods which make it impossible to pool data to get a more accurate estimate of rates of complications. In order to provide tailored estimates of risks and benefits, larger studies with longer follow-up periods, clearly defined baseline patient characteristics, and standardized definitions of adverse events are needed. For the DT population in particular—who are faced with life with a LVAD without the bailout of transplantation—we currently may be underestimating their individualized risks compared to benefits as most literature relies on data largely obtained from healthier BTT populations.12
The Who, What, When, Why, and How of Optimal Delivery of LVAD Therapy—More Questions Than Answers
Who and When—Patient Selection
The optimal delivery of LVAD therapy—including appropriate patient selection, implantation timing, device type and settings, and concomitant medical therapy—is largely unknown, but certainly has important implications for individual patients.
In the realm of patient selection, the INTERMACS patient profiles serve as a reference for categorizing patients. Lower (worse) INTERMACS profiles are associated with small increases in mortality after LVAD implantation, and large increases in mortality without LVAD. Specifically, for patients categorized as INTERMACS profile 1 (“crash and burn” with critical organ hypoperfusion), outcomes with medical therapy are abysmal but risk with chronic durable LVAD therapy is also relatively high, such that most centers have shifted towards temporary forms of MCS to first assess whether these patients can resolve multi-organ failure and recover to a lower INTERMACS profile prior to durable LVAD implantation.5,13,14 For patients categorized as INTERMACS profiles 2-3 (inotrope dependent) multiple studies have clearly demonstrated superior outcomes with chronic durable LVAD vs. medical therapy15–18, and patients falling into these profiles currently account for the largest proportion of patients currently receiving LVAD therapy10.
By contrast, our current state of knowledge is considerably more limited for the large group of HF patients that fall into INTERMACS profiles 4-7 (ambulatory, non-inotrope dependent patients with advanced HF). The recently published Risk Assessment and Comparative Effectiveness of LVAD and Medical Management in Ambulatory HF Patients (ROADMAP) study was a non-randomized, prospective, observational study of patients in INTERMACS profiles 4-7 that illustrates the current clinical dilemma regarding the appropriate timing of LVAD therapy in this cohort of patients. Based on a composite primary endpoint of survival on original therapy with improvement in 6-minute walk distance of >75 meters at 12 months, the authors concluded that early LVAD implantation was superior to ongoing medical therapy. The early LVAD group also showed greater improvements in quality of life and depression scores. However, there were no differences in survival based on intention-to-treat analysis (mortality was 20% versus 21% at 12 months) and a substantially higher number of adverse events and hospitalizations occurred in the LVAD therapy arm.19** Furthermore, the improvements in quality of life, walk distance, and depression were greater in the LVAD group at least in part because patients selecting early LVAD implantation started with lower scores for all of these measures and thus had more room for improvement. Overall, ROADMAP suggests that movement of current generation LVAD devices upstream in the HF disease process creates a highly preference sensitive medical decision, where survival appears to be more equivalent between early versus deferred LVAD therapy and patients must seriously consider whether their current quality of life is diminished enough to take on the risks of major surgery and possible device complications. Additional observational data defining the outcomes of ambulatory non-inotrope dependent patients that fall into INTERMACS profiles 4-7 is currently being collected in the ongoing Medical Arm of Mechanically Assisted Circulatory Support (MEDAMACS) registry and the Registry Evaluation of Vital Information for VADs in Ambulatory Life (REVIVAL) study. Ultimately randomized, controlled data assessing a wide range of patient-centered outcomes is needed in the INTERMACS 4-7 population.
What, Why, and How—LVAD Type, Device Settings, and Role for Concomitant Medical Therapy
In order to improve durability and minimize complications, technological advances in LVAD pump design resulted in CF LVADs that have now completely replaced older pulsatile-flow LVADs. However, there may be unintended consequences of alterations in the flow patterns and differences between the types of LVAD related mechanical unloading that warrant further investigation.
Compared to first-generation pulsatile pumps, CF LVADS provide a lesser degree of left ventricular unloading as assessed by echocardiography, invasive hemodynamics, as well as circulating natriuretic peptide levels.20. Compared to second-generation axial flow CF LVAD designs (HeartMate II), third-generation centrifugal CF LVADs (HeartWare HVAD and HeartMate III) are more sensitive to pump-head pressure (afterload minus preload) and thus will see lower flow rates and less left ventricular unloading if systemic vascular resistance and volume status are not optimized.
CF LVADs, which rotate at thousands of rounds per minute, are associated with alterations in hematologic properties that may contribute to the adverse events of hemolysis, bleeding, and thrombosis.21 In addition, the long-term effects of non-pulsatile blood flow on end-organ and vascular function are unknown, but may contribute to patient outcomes while on chronic LVAD support. For example, CF LVADs have been found to alter cerebral auto-regulation of blood flow22, increase aortic vascular stiffness via alterations in vessel collagen and elastin content23, and increase sympathetic nervous system activity24. All of these findings in theory could contribute to the pathophysiology of CF LVAD related complications such as gastrointestinal bleeding, hypertension, and stroke. In addition, the effects of modulating the degree of pulsatility by adjusting pump settings or restoring intermittent pulsatility in LVAD pump technology warrants further investigation. The HeartMate III device is programmed to ramp up the rotor speed by 2000 rotations per minute (rpm) then down by 4000 rpm then back up 2000 rpm to the set speed over 0.3 seconds every 2 seconds, creating an artificial pulse pressure 30 times per minute. Whether partial restoration of hemodynamic pulsatility will decrease CF LVAD complications and improve patient outcomes will be assessed in the Multi-center Study of MagLev Technology in Patients Undergoing MCS Therapy With HeartMate 3 (MOMENTUM 3), which plans to randomize 514 patients to the newer device versus 514 patients to Heart Mate II, and complete data collection in November 2018.25
Finally, little is known about the role of concomitant medical therapy in LVAD patients. For example, it is unknown if LVAD patients should receive neurohormonal blockade to preserve right ventricular function, beta-blockers to limit arrhythmias, and renin-angiotensin-aldosterone system blockade to reduce vascular stiffness. Additional systematic studies looking at different devices, settings, and concomitant medical therapy are necessary to refine the optimal delivery of LVAD therapy.
Individualizing Risks and Benefits of LVAD Therapy
A major challenge to HF clinicians and patients contemplating LVAD therapy is trying to apply the risks and benefits from clinical trials and registries to an individual patient’s unique characteristics and condition. Many of the large registries that report long-term outcomes have combined BTT and DT patients, making it difficult to apply this data to patients with extremes of age, varying comorbidity, and disparate social support.11,26,27 Similarly, current HF risk models predicting survival for patients on medical therapy, such as the Seattle HF Model and HF Survival Score, have limitations and should be interpreted cautiously in the patient considering advanced HF therapies.13,14,28 Furthermore, the majority of LVAD clinical trial and HF risk score data is derived from study population’s made up of mostly Caucasian men with a significant under-representation from minorities and women.6,11,17 This may explain in part why the Seattle HF Model underestimates risks in African-Americans.29 Larger clinical studies and registries that include and characterize a wide spectrum of patients including older patients with multi-morbidity, women, and patients with racial and ethnic diversity are needed to provide better point estimates of individual risk for all patients considering LVAD therapy. This can be inherently challenging in the face of rapidly evolving device technology, where long-term outcomes from large numbers of patients are outdated by the time such data can be accrued.
Finally, HF clinicians must take a serious look at whether LVAD therapy is being considered equitably. Women and racial/ethnic minorities have a high prevalence of HF30, yet receive a disparagingly low percentage of LVADs.31,32 This has been hypothesized to be secondary to several issues including differing etiologies of HF, reduced referrals for advanced therapies, poor access to care, patient choice, provider bias, and poor candidacy for advanced therapies such as body size, comorbidities, insurance, and social support.28,32–35 Providers and policy makers should monitor practice patterns to ensure that equitable access and evaluation for advanced therapies is provided.
Communication of Individualized Risks and Benefits of LVAD Therapy
Advanced HF patients who are considering LVAD therapy are forced to confront a complex technology that has the potential to improve survival and quality of life but also comes with risks for numerous complications and burdens. Determination of the outcomes that matter most to patients may help direct individualized therapy since survival may not be the most important outcome for all patients.28 Patients place differing value on quality of life (symptoms, physical function, mental, emotional, and social facets of life), avoidance of major surgery, and burdens of therapy (lifestyle changes, lost opportunities, dependence on an electric machine, direct medical costs, indirect costs, and caregiver burden).28,36,37 As LVAD therapy is increasingly considered for INTERMACS 4-7 patients, where early implantation of an LVAD does not appear to significantly change survival, sorting through the complex trade-offs of therapy becomes even more important for all patients. HF clinicians therefore must be able to 1) effectively inform patients and their families of the expected benefits, risks, and burdens of LVAD therapy compared to other management options (often including palliative care), 2) recognize the wide uncertainty for an individual patient, 3) solicit patient values, goals, and preferences, and then 4) facilitate shared decision-making.
Family caregivers are particularly involved in LVAD therapy.14,28 The 2013 ISHLT Guidelines for MCS recommend that a caregiver and his/her burden be assessed during the selection process.14 Patient caregivers post LVAD often experience anxiety, guilt, depression, and increased sense of intimacy with the patient.38–40 It is the responsibility of clinicians to share the full spectrum of potential outcomes with the patient and caregiver(s).
Unfortunately, the most commonly utilized materials to inform patients about the risks and benefits of LVAD therapy have significant shortcomings.7*,41 Educational materials developed by industry tend to focus on the benefits of therapy while minimizing or omitting risks.41 Patient advocates currently supported with an LVAD have a tendency to be biased towards the benefits of LVAD therapy since patients with negative outcomes such as death or stroke are not available to communicate with patients considering LVAD therapy.41
Decision support tools that utilize pictographs and other novel formats to convey complex statistics can help clinicians provide patients with less biased risks/benefit profiles, clearly compare treatment options, and facilitate values clarification, all of which help lead to a well-informed decision (Figure 2).7*,28,42 One such multimedia LVAD decision support tool that follows the International Patient Decision Aid Standards is available for use for free at https://patientdecisionaid.org.43 Its ability to enhance patient and caregiver knowledge, improve decision quality, and integrate into routine clinical care is currently being studied in the multi-center Trial of a Decision Support Intervention for Patients and Caregivers Offered Destination Therapy Heart Assist Device (DECIDE-LVAD).44
Figure 2.
Development of LVAD decision aid materials that can help clinicians facilitate shared decision making between clinicians, patients, and their family caregivers.
Reproduced with permission from Thompson et al. JACC Heart Fail. 2015 Dec;3(12):965–76
Conclusions
Improvements in pump technology have resulted in better outcomes for advanced HF patients requiring LVAD therapy; however the potential for serious adverse events remains significant—necessitating a renewed focus on providing patient-centered care with this therapy. In order to achieve this goal, we must better understand the long-term risks and benefits of LVAD therapy in a broad population of patients including older patients who may not be candidates for transplant, women, minorities, and those with comorbidities. Furthermore, better information is needed regarding the optimal patient, timing, and type of LVAD on patient-centered outcomes such as survival, quality of life, hospitalizations, and adverse events. Finally, we must develop effective methods of tailoring and communicating the outcomes with LVAD therapy to individual patients and their families in a non-biased way that allows for informed and shared decision-making.
Supplementary Material
Key Points.
Left ventricular assist device (LVAD) therapy has the potential to improve survival and quality of life for patients with advanced heart failure (HF), but comes with a risk of numerous potential complications.
There is a clear survival benefit of continuous flow (CF) LVAD therapy compared to optimal medical therapy among patients with inotrope-dependent advanced HF.
There are unanswered questions regarding the role of LVAD therapy compared to medical therapy in ambulatory, non-inotrope dependent patients with advanced HF and this is a topic under active clinical investigation.
Further research is needed to ensure the optimal delivery of LVAD therapy including appropriate patient selection, timing of implant, type of device and settings (CF vs. intermittently pulsatile), and the role of concomitant medical therapy.
Decision support aids may help facilitate informed shared decision-making in LVAD therapy
Acknowledgements
None
Funding/Support: Dr. Breathett is supported by a T32 training grant (5T32 HL116276-02) from the National Institute of Health. Dr. Allen is supported by NIH (K23 HL105896) and PCORI (CDR-1310-06998) grants. Dr. Ambardekar is supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program.
Conflict of Interest Disclosures: Dr. Breathett received support to attend an LVAD Conference for Fellows sponsored by Thoratec. Dr. Allen serves as a consultant for J&J, Novartis, and St. Jude.
References and Recommended Reading*
- 1.Crossing the Quality Chasm: A New Health System for the 21st Century. Institute of Medicine. http://iom.nationalacademies.org/Reports/2001/Crossing-the-Quality-Chasm-A-New-Health-System-for-the-21st-Century.aspx (accessed 17 November 2015)
- 2.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an Intrapericardial, Continuous-Flow, Centrifugal Pump in Patients Awaiting Heart Transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 5.Stewart GC, Stevenson LW, Miller LW. Keeping Left Ventricular Assist Device Acceleration on Track. Circulation. 2011;123:1559–1568. doi: 10.1161/CIRCULATIONAHA.110.982512. [DOI] [PubMed] [Google Scholar]
- 6.Xie A, Phan K, Yan TD. Durability of continuous-flow left ventricular assist devices: a systematic review. Ann Cardiothorac Surg. 2014;3:547–556. doi: 10.3978/j.issn.2225-319X.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.McIlvennan CK, Magid KH, Ambardekar AV, et al. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003–1013. doi: 10.1161/CIRCHEARTFAILURE.114.001391. This study is a systematic review of the risks and benefits of LVAD therapy, and it provides a summary pictogram that can be used for instructing patients and caregivers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampropulos JF, Kim N, Wang Y, et al. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open Heart. 2014;1:e000109. doi: 10.1136/openhrt-2014-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 10**.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. doi: 10.1016/j.healun.2015.10.003. http://www.sciencedirect.com/science/article/pii/S1053249815014503 (accessed 18 November 2015). This study provides the latest INTERMACS registry outcomes data. Mortality data for Destination Therapy patients are included and can be compared to Bridge-To-Transplant patients. [DOI] [PubMed]
- 11.Lietz K, Long JW, Kfoury AG, et al. Outcomes of Left Ventricular Assist Device Implantation as Destination Therapy in the Post-REMATCH Era Implications for Patient Selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 12.Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Rogers JG, Butler J, Lansman SL, et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 16.Hershberger RE, Nauman D, Walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–187. doi: 10.1054/jcaf.2003.24. [DOI] [PubMed] [Google Scholar]
- 17.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 18.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2011;30:402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 19**.Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol. 2015;66:1747–1761. doi: 10.1016/j.jacc.2015.07.075. This study provides the latest outcomes data on LVAD usage in non-inotrope dependent ambulatory heart failure patients. This study helps to instruct the patient and provider about risks and benefits of LVAD implantation versus medical management in patients with INTERMACS profiles 4-7. [DOI] [PubMed] [Google Scholar]
- 20.Kato TS, Chokshi A, Singh P, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ Heart Fail. 2011;4:546–553. doi: 10.1161/CIRCHEARTFAILURE.111.962142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarzia V, Buratto E, Bortolussi G, et al. Hemorrhage and thrombosis with different LVAD technologies: a matter of flow? Ann Cardiothorac Surg. 2014;3:582–584. doi: 10.3978/j.issn.2225-319X.2014.08.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornwell WK, Tarumi T, Aengevaeren VL, et al. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2014;33:1295–1303. doi: 10.1016/j.healun.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Ambardekar AV, Hunter KS, Babu AN, et al. Changes in Aortic Wall Structure, Composition, and Stiffness With Continuous-Flow Left Ventricular Assist Devices: A Pilot Study. Circ Heart Fail. 2015;8:944–952. doi: 10.1161/CIRCHEARTFAILURE.114.001955. [DOI] [PubMed] [Google Scholar]
- 24.Cornwell WK, Tarumi T, Stickford A, et al. Restoration of Pulsatile Flow Reduces Sympathetic Nerve Activity Among Individuals With Continuous-Flow Left Ventricular Assist Devices. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.017647. CIRCULATIONAHA.115.017647. [DOI] [PubMed] [Google Scholar]
- 25.MOMENTUM 3 IDE Clinical Study Protocol (HM3™) https://clinicaltrials.gov/ct2/show/NCT02224755 (accessed 30 November 2015)
- 26.Dunlay SM, Park SJ, Joyce LD, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2014;33:359–365. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvao M, Saeed O, Immekus J, et al. An international survey to assess referral thresholds for destination therapy in non-inotrope-dependent patients: results of the CONSENSUS-DT study. J Card Fail. 2014;20:492–497. doi: 10.1016/j.cardfail.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, et al. Utility of the Seattle Heart Failure Model in Patients With Advanced Heart Failure. J Am Coll Cardiol. 2009;53:334–342. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2015 Update A Report From the American Heart Association. Circulation. 2014 doi: 10.1161/CIR.0000000000000152. CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal A, Gupta A, Pappas PS, et al. Racial differences in patients with left ventricular assist devices. ASAIO J Am Soc Artif Intern Organs 1992. 2012;58:499–502. doi: 10.1097/MAT.0b013e318268ea80. [DOI] [PubMed] [Google Scholar]
- 32.Joyce DL, Conte JV, Russell SD, et al. Disparities in access to left ventricular assist device therapy. J Surg Res. 2009;152:111–117. doi: 10.1016/j.jss.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 33.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 34.Pinney SP. Understanding and Eliminating Racial Disparities in TransplantationStill a Ways to Go∗. J Am Coll Cardiol. 2013;62:2316–2317. doi: 10.1016/j.jacc.2013.07.070. [DOI] [PubMed] [Google Scholar]
- 35.Aaronson KD, Schwartz JS, Goin JE, et al. Sex Differences in Patient Acceptance of Cardiac Transplant Candidacy. Circulation. 1995;91:2753–2761. doi: 10.1161/01.cir.91.11.2753. [DOI] [PubMed] [Google Scholar]
- 36.McIlvennan CK, Jones J, Allen LA, et al. Decision-making for destination therapy left ventricular assist devices: implications for caregivers. Circ Cardiovasc Qual Outcomes. 2015;8:172–178. doi: 10.1161/CIRCOUTCOMES.114.001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIlvennan CK, Allen LA, Nowels C, et al. Decision making for destination therapy left ventricular assist devices: ‘there was no choice’ versus ‘I thought about it an awful lot’. Circ Cardiovasc Qual Outcomes. 2014;7:374–380. doi: 10.1161/CIRCOUTCOMES.113.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick JN, Kellom K, Hull SC, et al. Caregivers and Left Ventricular Assist Devices as a Destination, Not a Journey. J Card Fail. 2015 doi: 10.1016/j.cardfail.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Baker K, Flattery M, Salyer J, et al. Caregiving for patients requiring left ventricular assistance device support. Heart Lung J Crit Care. 2010;39:196–200. doi: 10.1016/j.hrtlng.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Bekelman DB, Nowels CT, Retrum JH, et al. Giving voice to patients’ and family caregivers’ needs in chronic heart failure: implications for palliative care programs. J Palliat Med. 2011;14:1317–1324. doi: 10.1089/jpm.2011.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iacovetto MC, Matlock DD, McIlvennan CK, et al. Educational resources for patients considering a left ventricular assist device: a cross-sectional review of internet, print, and multimedia materials. Circ Cardiovasc Qual Outcomes. 2014;7:905–911. doi: 10.1161/CIRCOUTCOMES.114.000892. [DOI] [PubMed] [Google Scholar]
- 42.Thompson J, Matlock D, McIlvennan C, et al. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Heart Fail. 2015;3:965–976. doi: 10.1016/j.jchf.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A decision aid for Left Ventricular Assist Device (LVAD) for Destination Therapy: A device for patients with advanced heart failure. https://decisionaid.ohri.ca/AZsumm.php?ID=1799 (accessed 30 November 2015)
- 44.PCORI-1310-06998 Trial of a Decision Support Intervention for Patients and Caregivers Offered Destination Therapy Heart Assist Device (DECIDE-LVAD) https://clinicaltrials.gov/ct2/show/NCT02344576 (accessed 30 November 2015)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.