Summary
Cellular zinc homeostasis ensures that the intracellular concentration of this element is kept within limits that enable its participation in critical physiological processes without exerting toxic effects. We report here the identification and characterization of the first mediator of zinc homeostasis in Leishmania infantum, LiZIP3, a member of the ZIP family of divalent metal-ion transporters. The zinc transporter activity of LiZIP3 was first disclosed by its capacity to rescue the growth of Saccharomyces cerevisiae strains deficient in zinc acquisition. Subsequent expression of LiZIP3 in Xenopus laevis oocytes was shown to stimulate the uptake of a broad range of metal ions, among which Zn2+ was the preferred LiZIP3 substrate (K0.5 ≈ 0.1 μM). Evidence that LiZIP3 functions as a zinc importer in L. infantum came from the observations that the protein locates to the cell membrane and that its overexpression leads to augmented zinc internalization. Importantly, expression and cell-surface location of LiZIP3 are lost when parasites face high zinc bioavailability. LiZIP3 decline in response to zinc is regulated at the mRNA level in a process involving (a) short-lived protein(s). Collectively, our data reveal that LiZIP3 enables L. infantum to acquire zinc in a highly regulated manner, hence contributing to zinc homeostasis.
Keywords: Leishmania, trypanosomatid, ZIP, zinc-transport, zinc-homeostasis
Introduction
Zinc is a fast and flexible ligand exchanger without inherent redox properties. These characteristics make zinc a versatile micronutrient that plays fundamental roles in a multitude of physiological processes (reviewed in Stefanidou et al., 2006, and in Chasapis et al., 2012). The biological protagonism of zinc is evident when one considers that this metal serves as a structural and catalytic cofactor for about 10% of any proteome and, as more recently discovered, as an intra- or extracellular cell messenger (reviewed in Maret, 2013). Consistent with its numerous functions, zinc deficiency leads to severe defects and, ultimately, to cell death (reviewed in Chasapis et al., 2012). However, accumulation of zinc above certain thresholds is also hazardous to cells (reviewed in Plum et al., 2010; Wang & Fierke, 2013). To guarantee that intracellular zinc levels are kept within tolerable limits, organisms are endowed with a sophisticated system of transporters and chelating molecules (e.g. metallothionein) that respond to zinc in a coordinated way, adjusting uptake, storage and/or elimination according to the concentration of the metal in the environment and their own individual demands.
Several families of transporters have been shown capable of transporting zinc across the plasma membrane. One of the most important is the ZRT/IRT-like Protein (ZIP) family of divalent metal transporters which often transport, apart from zinc, other metal ions such as iron, cadmium and manganese with either broad or restricted substrate specificity (reviewed in Guerinot, 2000, and in Gaither & Eide, 2001a). The precise mode of function of ZIP proteins has yet to the elucidated. The family encompasses 4 subfamilies, I, II, LIV-1 and gufA, classified on the basis of sequence similarity (reviewed in Gaither & Eide, 2001a), which, in addition to being present at the cell surface, can locate to the membranes of organelles. Nevertheless, it is consensually accepted that ZIP proteins transport metal ions into the cytosol (reviewed in Eide, 2004). Analysis of how ZIP transporters contribute to finely tune the level of zinc that permeates cells has indicated that some of these molecules are positively or negatively modulated by the low and high bioavailability of zinc, respectively, through transcriptional and/or post-transcriptional mechanisms (reviewed in Guerinot, 2000, and in Gaither & Eide, 2001a).
Protozoan parasites of the genus Leishmania are agents of leishmaniasis, a group of vector-borne diseases that afflicts approximately 12 million people worldwide and results in severe disfigurement, disability and even death. These parasites cycle between two main stages, the promastigote in the insect vector and the amastigote in mammalian hosts. As in other organisms, zinc is an essential nutrient for parasite replication and infectivity. Illustrating this, several essential Leishmania proteins are known or predicted to bind zinc, perhaps the most prominent example being the major surface protease (MSP or GP63), a zinc metalloprotease implicated in several functions along parasite development, and a recognized virulence factor (Olivier et al., 2012). Despite the biological importance of zinc to Leishmania and of its potential toxic effects (Sharquie et al., 1997), no information exists regarding the processes underlying zinc homeostasis in these parasites. That Leishmania must adapt to fluctuations in zinc bioavailability appears, however, evident as they often thrive in people with deficits in this metal (Pasa et al., 2003; Van Weyenbergh et al., 2004; Bern et al., 2007; Amini et al., 2009; Mishra et al., 2010).
This study is focused on zinc acquisition in Leishmania. In order to advance our understanding of the mechanism employed by these parasites to regulate zinc uptake, we searched the genome of Leishmania infantum for ZIP homologues and identified a candidate gene for zinc internalization in this parasite, LiZIP3. We report here that LiZIP3 is a zinc transporter, the first characterized in Leishmania. Furthermore, we show that LiZIP3 enables L. infantum to acquire zinc in a tightly regulated way.
Results
The L. infantum ZIP3 gene encodes a putative zinc transporter
Analysis of the L. infantum genomic database (TriTrypDB, http://www.tritrypdb.org/tritrypdb/) identified three putative ZIP members. One of these, LiZIP1, encoded by a pair of identical genes (LinJ.31.3180 and LinJ.31.3190), is the orthologue of the Leishmania amazonensis ferrous-ion transporter LIT1 described by Huynh et al. (2006). The second, LiZIP2, specified by a single-copy gene (LinJ.33.3350), is a non-characterized protein glossed in the database as a putative cation transporter. The third putative ZIP member is encoded by another pair of identical genes (LinJ.28.2050 and LinJ.28.2060) and annotated as a zinc transporter-like protein. The work reported in this manuscript refers to the last of these molecules, herein designated as LiZIP3.
The predicted LiZIP3 protein is composed of 462 amino acids and has an expected molecular mass of 48.364 kDa and isoelectric point of 6.67. We generated a topology model of this molecule (Fig. 1) using TopPred software (http://mobyle.pasteur.fr/cgibin/portal.py?#forms::toppred). Specific features of its primary structure led us to consider LiZIP3 as both a ZIP member and a candidate for zinc transport, namely (i) the presence of a conserved ZIP-family consensus sequence [LIVFA] [GAS] [LIVMD] [LIVSCG] [LIVFAS] H [SAN] [LIVFA] [LIVFMAT] [LIVDE] G [LIVF] [SAN] [LIFVF] [GS] (reviewed in Eng et al., 1998), except that, in positions 4 and 15, a threonine (T) and an alanine (A) substitute for [LIVSCG] and [GS] respectively. These deviations from the consensus sequence do not exclude LiZIP3 from the ZIP family of transporters according to the PFAM database (Punta et al., 2012); and (ii) like most ZIP members, LiZIP3 is predicted to possess eight transmembrane domains (TMDs), amino (N)- and short carboxyl (C)-termini exposed to the extracellular milieu or to the lumen of organelles, and a non-conserved domain between TMDs III and IV facing the cytosol (Fig. 1). The latter region contains a HQHG sequence akin to the (HX)n motif present in most ZIPs and postulated to serve as a metal coordinating site (Eide et al., 1996; Zhao & Eide, 1996a,b; Milon et al., 2006; Nishida et al., 2008; Jacques et al., 2010). Another noteworthy characteristic of the LiZIP3 sequence, shared with some other ZIP members, is the presence of a long N-terminus, comprising one quarter of the protein and evidencing several histidine-rich clusters. LiZIP3 contains those amino acid residues previously identified by mutational analysis to be critical for zinc transport activity (Rogers et al., 2000; Jacques et al., 2010; Taudte & Grass, 2010) (Fig. 1). The L. infantum protein also contains amino acid residues previously found to be important for ZIP proteins to transport other metal ions namely iron, manganese and cadmium (Rogers et al., 2000; Jacques et al., 2010) (Fig. 1). In conclusion, sequence analysis indicates that LiZIP3 is a putative zinc transporter in L. infantum.
Fig. 1.
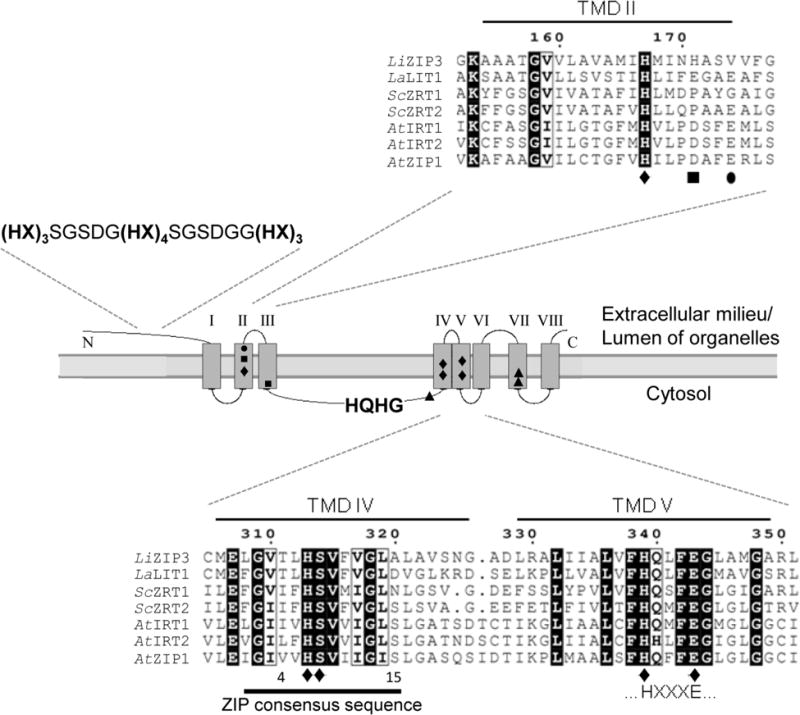
LiZIP3 is predicted to encode a member of the ZIP family. Alignment of selected residues of the predicted amino acid sequence of LiZIP3 with experimentally characterized ZIP members from L. amazonensis (La), S. cerevisiae (Sc) and A. thaliana (At) using MultAlin interface (Corpet, 1988). Also depicted is the topology of LiZIP3 predicted with TopPred 0.01 (Claros & von Heijne, 1994). Highlighted are the LiZIP3 transmembrane domains (TMDs) and the residues shown to be important for transport of zinc (●), of iron, manganese, zinc and cadmium (◆), and of iron and manganese (■) in AtIRT1, and of iron (▲) in LaLIT1. Histidine-rich regions, the ZIP consensus sequence and the HXXXE motif, characteristic of ZIP members, are also represented. Accession numbers: LiZIP3, LinJ.28.2050; ScZRT1, CAA96975.1; ScZRT2, CAA97701.1; AtIRT1, NP_567590.3; AtIRT2, NP_001031670.1; AtZIP1, NP_187881.1; LaLIT1 sequence was obtained by modification of the L. major orthologue LmjF.31.3060 according to Huynh et al. (2006).
LiZIP3 exhibits zinc transporter activity
As a starting point to analyse the function of LiZIP3, we examined the time course of its expression during L. infantum promastigote growth by using western blot analysis. To that end, we produced an antibody against a 14-amino acid peptide located in the variable loop between putative TMDs III and IV of LiZIP3. The antibody recognized a protein band of 45 kDa, close to the predicted size of the LiZIP3 nascent peptide (Fig. 2A). This band was undetectable at 0 and 24 h of culture but became more intense from 48 h onwards. This increase in LiZIP3 expression was accompanied by the progressive depletion of zinc from the culture medium, as determined by monitoring zinc content in the parasite supernatants at each time-point by using atomic absorption spectrometry. Together, these observations suggest that LiZIP3 is expressed in L. infantum in conditions of lower zinc concentrations and support a role of this protein in zinc acquisition.
Fig. 2. LiZIP3 transports zinc.
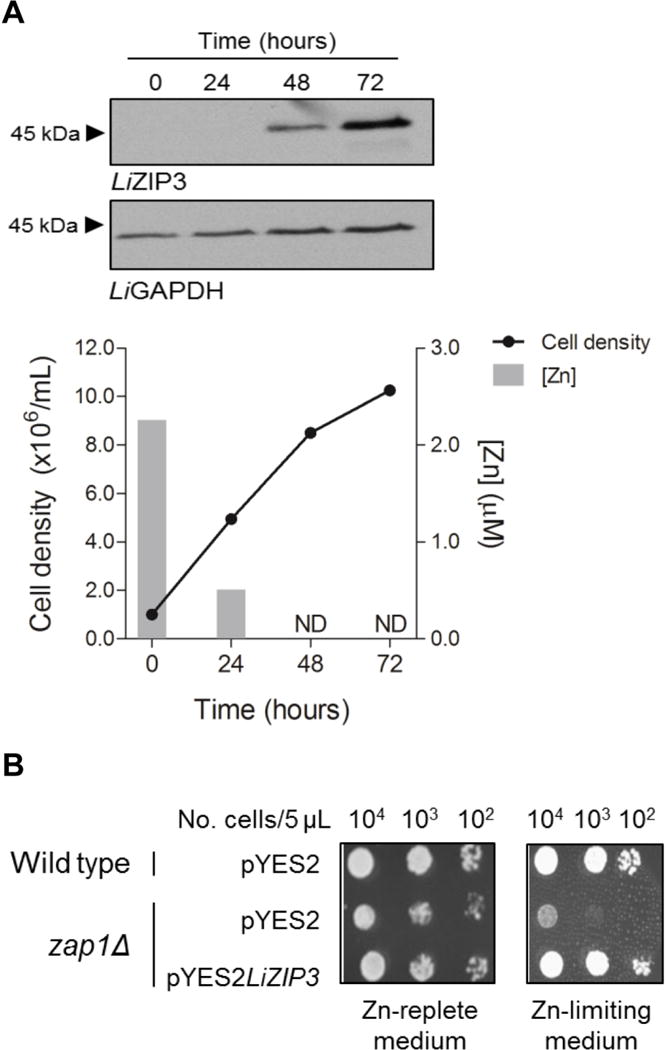
A. LiZIP3 is expressed in L. infantum under low zinc concentrations. Aliquots from a L. infantum culture set at 106 mL−1 were harvested daily and counted to determine cell density. Pellets were subjected to western blotting using an antibody raised against a LiZIP3 14-amino acid peptide. A band of approximately 45 kDa corresponding to LiZIP3 was detected at 48 and 72 h. In parallel, culture supernatants were analysed by atomic absorption spectrometry for their content in zinc. ND, not detected (detection limit = 0.38 μM).
B. LiZIP3 rescues the deficient zinc acquisition phenotype of the zap1Δ yeast strain. The growth of zap1Δ bearing pYES3LiZIP3 was compared with that of the same cells and of wild type carrying the empty vector (pYES2) in zinc-replete (SD-glucose) and zinc-limiting media (SD-glucose, 1 mM EDTA, 0.9 mM ZnCl2).
We indirectly tested the zinc-transport activity of LiZIP3 by using heterologous complementation in S. cerevisiae. The zap1Δ (ZHY6) strain, which is defective in high- and low-affinity zinc acquisition (Zhao & Eide, 1997) and is therefore unable to grow in zinc-limiting conditions, was transfected with an expression vector carrying the LiZIP3 gene (pYES2LiZIP3). The ability of LiZIP3-complemented yeast to grow in low-zinc medium was next compared with that of the wild type and of the mutant strain transfected with the empty pYES2 vector (Fig. 2B). Both the wild type and the zap1Δ strains were able to grow in SD-glucose, a zinc-rich medium; however, when cultured in zinc-limited medium [SD-glucose, 1 mM EDTA (a divalent metal chelator), 0.9 mM ZnCl2], the zap1Δ strain proliferated only if transfected with the LiZIP3-containing plasmid. In other words, LiZIP3 is required for zinc acquisition by this yeast strain. Similar results were observed for the zrt1zrt2 (ZHY3) strain (Fig. S1), lacking the high- and low-affinity zinc transporters ScZRT1 and ScZRT2 (Zhao & Eide, 1996b). Therefore, functional complementation in yeast indicates that LiZIP3 is an active zinc transporter.
Properties of transport by LiZIP3
To more directly establish the properties of LiZIP3-mediated transport we expressed LiZIP3 in RNA-injected Xenopus laevis oocytes. In oocytes expressing LiZIP3 we observed immunoreactivity at or near the cell perimeter that was not present in control oocytes (Fig. 3A). We also observed in all oocytes some intracellular immunoreactivity that was not specific to LiZIP3 expression.
Fig. 3. Properties of LiZIP3-mediated metal transport in X. laevis oocytes.
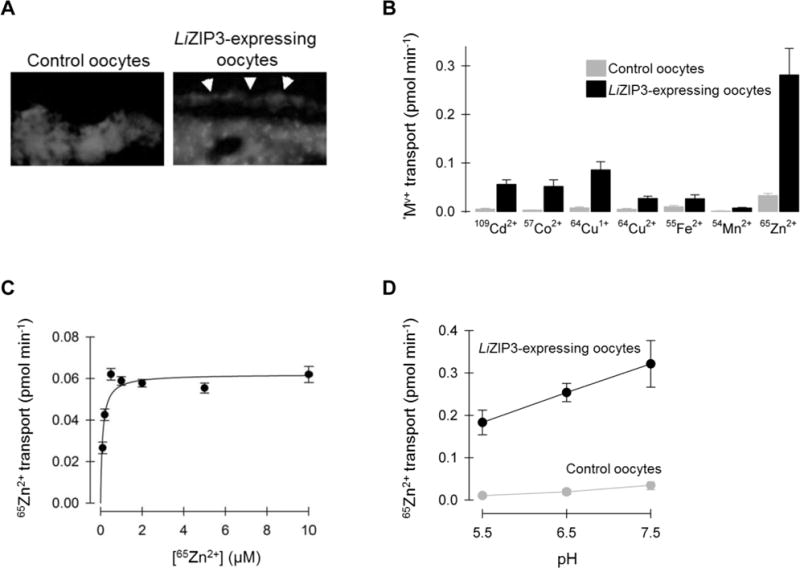
A. LiZIP3 RNA-injected X. laevis oocytes express LiZIP3 at the cell membrane. Immunofluorescence of sections from control and LiZIP3-expressing oocytes probed with the anti-LiZIP3 antibody. Arrowheads point to immunostaining in the approximate position of the cell membrane.
B. Metal-ion substrate profile of LiZIP3. Uptake of 5 μM radiotracer metal—ion (*Mv+, where *represents the radioactive isotope and v the valence) was measured in control oocytes (grey) and oocytes expressing LiZIP3 (black) at pH 7.5, over 10 min at 23°C. Media containing 64Cu1+ or 64Cu2+ were supplemented with 1 mM L-histidine and media with 64Cu+ or 55Fe2+ were supplemented with 1 mM L-ascorbic acid. Data are mean ± SD of 14–19 oocytes per group. Two-way ANOVA revealed an interaction (P < 0.001); LiZIP3 differed from control for all metals (P ≤ 0.003), except for 54Mn2+ (P = 0.30); within LiZIP3-expressing oocytes, all metals differed from one another (P ≤ 0.004) except 109Cd2+ cf. 57Co2+ (P = 0.70) and 64Cu2+ cf. 55Fe2+ (P = 0.93).
C. Saturation kinetics of LiZIP3—mediated 65Zn2+ transport at pH 7.5. The uptake of 0.1—10 μM 65Zn2+ in control oocytes and oocytes expressing LiZIP3 was measured in standard medium at pH 7.5, over 30 min at 23°C. The LiZIP3-mediated component was calculated as the difference between means for control and LiZIP3-expressing oocytes and the error (SEM) was propagated by using the root of the sum of squares of SEM (control) and SEM (LiZIP3). Data were fit by a Michaelis–Menten function (Equation 1) from which the following estimates were obtained: VSmax = 0.062 ± 0.01 pmol min−1; K S0.5= 0.10 ± 0.03 μM; r2 = 0.84; P = 0.004.
D. Uptake of 2 μM 65Zn2+ as a function of extracellular pH in control oocytes and in oocytes expressing LiZIP3 measured over 10 min. Data are mean ± SD (n = 9–16). Two-way ANOVA revealed an interaction (P < 0.001); within LiZIP3-expressing oocytes, each pH differs from one another (P < 0.001).
Since several ZIP family members exhibit broad metal reactivity with metal ions, we examined the substrate profile of LiZIP3 in X. laevis oocytes at pH 7.5 (Fig. 3B). Expression of LiZIP3 stimulated the uptake of a broad range of transition metal ions compared with control oocytes. The largest absolute increase was, by far, that for 65Zn2+, whereas modest increases were detected for 109Cd2+, 57Co2+, 64Cu1+, 64Cu2+, and 55Fe2+, but not for 54Mn2+. These results confirm that LiZIP3 is a metal-ion transporter with an apparent preference for zinc ions.
We examined the saturation kinetics of LiZIP3-mediated zinc transport over the concentration range 0.1–10 μM at pH 7.5 (Fig. 3C). LiZIP3-mediated zinc transport data (calculated as the difference between the uptake in LiZIP3-expressing and that in control oocytes, and by propagating the error) were fit by Equation 1 (Experimental Procedures), revealing that zinc transport by LiZIP3 is saturable with modestly high affinity for zinc judged from the zinc concentration at which transport was half-maximal of 0.1 μM. By measuring 65Zn2+ over the pH range 5.5 to 7.5, we observed maximal activity at pH 7.5 (Fig. 3D); however, substantial activity remained at pH 6.5 and pH 5.5 whereas mammalian ZIP transporters are more strongly inhibited at lower pH (Pinilla-Tenas et al., 2011; Wang et al., 2012).
LiZIP3 is a zinc uptake transporter in L. infantum
Having ascertained that LiZIP3 possesses zinc-transport activity in heterologous systems, we considered its potential role in the uptake of zinc in L. infantum. We first examined the subcellular location of the protein in the parasite by immunofluorescence using the anti-LiZIP3 antibody (Fig. 4A). We found LiZIP3 to be mostly present at the cell surface, a location consistent with a role in zinc internalization. Importantly, cell-surface localization of LiZIP3 was observed only when parasites were cultured in the presence of 1 mM EDTA. Supplementation of this culture with 50 μM zinc for 24 h led to the disappearance of the protein from the parasite cell membrane.
Fig. 4. LiZIP3 is a cell surface zinc transporter in L. infantum.
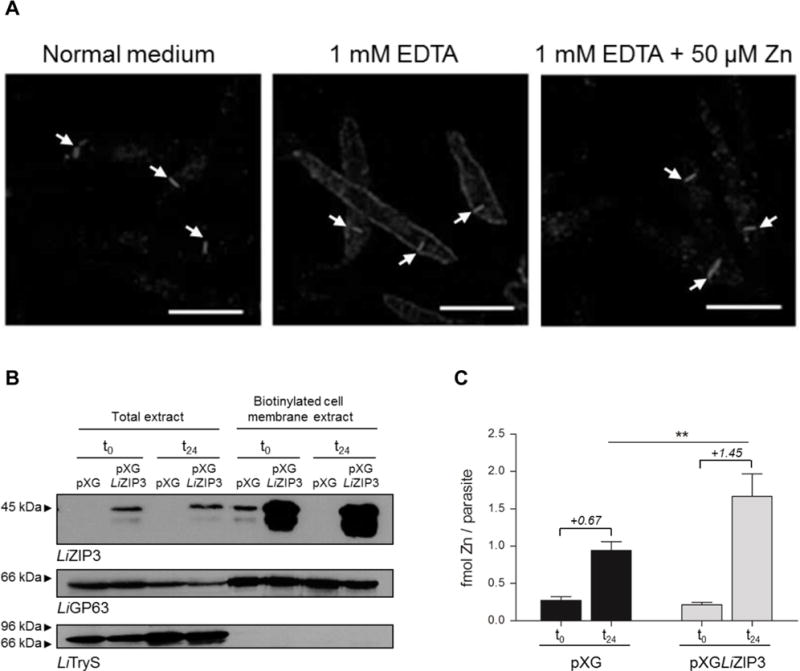
A. Zinc restriction stimulates the expression of LiZIP3 at the cell membrane of L. infantum. Parasites were grown at an initial density of 106 mL−1 in normal medium or in the presence of 1 mM EDTA. At 48 h of growth, 50 μM zinc were added to an aliquot of the EDTA-treated culture and, 24 h later, LiZIP3 localization was assessed by immunofluorescence using the anti-LiZIP3 antibody. The position of the kinetoplast in each parasite, observed by simultaneously staining samples with DAPI, is indicated by arrows. Scale bar: 5 μm. B and C. Overexpression of LiZIP3 at the cell surface results in an increased uptake of zinc.
B. Control (pXG) and LiZIP3-overexpressing (pXGLIZIP3) parasites with 72 h growth were exposed to a zinc stimulus by dilution to 106 mL−1 in fresh medium and cultured for an additional 24 h. The presence of LiZIP3 at the cell membrane, before (t0) and 24 h after (t24) medium replacement, was analysed by western blot analysis of cell membrane biotinylated proteins (the faster migrating band observed at approximately 38 kDa refers to a protein of unknown identity that is often recognized by the anti-LiZIP3 antibody). Upon stripping, LiGP63 and trypanothione synthetase (LiTryS) (Sousa et al., 2014) were used as controls for cell membrane and cytosolic proteins, respectively.
C. The zinc content of pXG and LiZIP3-overexpressing parasites was measured by atomic absorption spectrometry at t0 and t24 (mean ± SD, n = 3; asterisks indicate significant differences between pXG and LiZIP3-overexpressing parasites **P < 0.01, Student’s t test). Numbers in italic refer to the mean of the differences between zinc content at t0 and t24 for each parasite line.
As a second approach to verify the involvement of LiZIP3 in zinc acquisition in L. infantum, we measured the amount of zinc internalized during 24 h by parasites overexpressing LiZIP3 and by controls. The former were obtained by transfection with the pXGLiZIP3 expression vector and confirmed to express increased levels of LiZIP3 at the parasite surface by western blotting of biotinylated cell-membrane proteins (Fig. 4B). For this analysis we used parasites at 72 h of growth, i.e., at a point of the growth curve where zinc concentration in the medium is already below detection limit (Fig. 2A). Consistent with being in zinc limiting medium, both parasite lines contained similar zinc levels, as measured by atomic absorption spectrometry (Fig. 4C). Conversely, 24 h after a zinc stimulus (provided by diluting parasites in fresh medium), LiZIP3-overexpressing cells presented higher zinc content than controls indicating that they internalized more zinc (1.45 vs 0.67 fmol/parasite). This experiment provided direct evidence that LiZIP3 functions in zinc uptake in Leishmania.
Collectively, data reported here indicate that LiZIP3 functions as a zinc importer in L. infantum. Furthermore, they suggest that expression of this protein is modulated by the extracellular zinc levels.
Expression of LiZIP3 in L. infantum responds specifically to zinc
To more precisely test the effect of zinc on the expression of LiZIP3 we examined how zinc deprivation affects LiZIP3 in parasites grown in media containing varying concentrations of EDTA, by using western blotting. EDTA caused a dose-dependent up-regulation of LiZIP3 expression (Fig. 5A) that was not the result of a nonspecific effect of the chelator since the control protein LiGAPDH was unchanged. When EDTA-containing cultures were supplemented with zinc, the level of LiZIP3 decreased (Fig. 5B). A similar effect, i.e., induction of LiZIP3 that was reversed upon introduction of zinc, was observed when parasites were grown in the presence of the zinc chelator TPEN (Fig. 5C). These observations strengthen the conclusion that the expression of this protein is regulated according to the concentration of zinc in the medium, and furthermore suggest that parasites are able to sense very small concentrations of zinc. In fact, based on the high affinity of EDTA for zinc, one can assume that the concentration of free zinc in media containing 0.4 mM EDTA plus 50 μM zinc is very low, most likely within the sub-picomolar range. Nonetheless, these seemingly low concentrations of zinc are sensed by Leishmania parasites, which react by down-regulating LiZIP3 expression.
Fig. 5. LiZIP3 expression in Leishmania is modulated by the concentration of zinc in the culture medium.
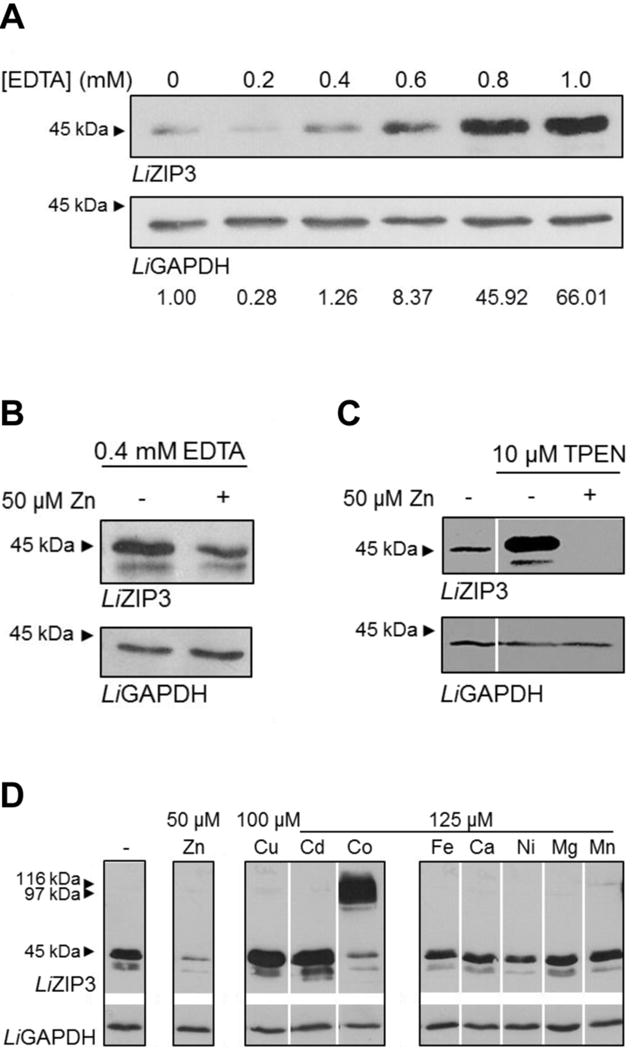
A. Dose-dependent effect of EDTA on LiZIP3 levels. The effect of the divalent metal chelator on the expression levels of LiZIP3 was addressed by adding increasing concentrations of EDTA to the culture medium for 48 h. Analysis was performed by western blotting. Values below the blots refer to the densitometry analysis (the intensity of the LiZIP3 band in the presence of each EDTA concentration was compared to that obtained in the absence of the chelator after loading correction with LiGAPDH.
B and C. The presence of zinc in the culture media reduces LiZIP3 expression. The ability of zinc to decrease the LiZIP3 levels in the presence of chelating agents was assessed (B) by supplementing a culture of parasites grown for 24 h in 0.4 mM EDTA with 50 μM zinc or (C) by adding 10 μM TPEN or 10 μM TPEN plus 50 μM zinc to a culture of parasites previously grown for 48 h in normal medium. Twenty four hours upon medium supplementation, parasites were harvested and processed for western blotting.
D. Effect of different divalent metals on LiZIP3 expression. L. infantum promastigotes were inoculated in normal medium at 106 mL−1 and, at 48 h of growth, several divalent metals were added to the cultures for an additional 24 h. The effect of metal supplementation on the level of LiZIP3 expression was analysed by western blotting. Expression of LiGAPDH in the same blots was used as loading control in all cases.
Since LiZIP3 can transport cadmium, cobalt, iron and copper (Fig. 3A) in addition to zinc, we investigated whether LiZIP3 expression is responsive also to a range of metals. For this analysis, promastigote cultures were supplemented with 100 μM copper or 125 μM cadmium, cobalt, iron, calcium, nickel, magnesium or manganese, or 50 μM zinc and incubated for 24 h after which changes in the protein level were assessed by western blotting. Whereas 50 μM zinc (or even 1 μM, Fig. S2) strongly down-regulated LiZIP3 levels, no other metal did so, despite their presence at concentrations of 100 or 125 μM (Fig. 5D). The slight reduction in the intensity of the 45 kDa band extracted from cultures treated with cobalt was likely due to protein aggregation during sample processing as deduced from the observation of high molecular weight bands. Interestingly, the presence of copper and cadmium led to an increase in the level of LiZIP3. As these two ions were previously reported to inhibit metal transport by some ZIP members (Eide et al., 1996; Grotz et al., 1998; Korshunova et al., 1999; Moreau et al., 2002; Ramesh et al., 2003; He et al., 2009; Taudte & Grass, 2010; Stephens et al., 2011), we considered that their competition with zinc for the transporter may explain the up-regulation of LiZIP3. We found that copper I and II were each capable of inhibiting zinc transport in oocytes expressing LiZIP3 (Fig. S3A and B); however, the degree of inhibition by copper was modest so other mechanisms may additionally account for the up-regulation of LiZIP3 expression in promastigote cultures.
In short, our results demonstrate that the L. infantum ZIP3 protein is a zinc-transporter tightly regulated by zinc but not by other metals. Together with the observation that zinc chelators promote LiZIP3 cell surface expression whereas zinc supplementation induces its loss, our findings also suggest that LiZIP3 is a key component of a cellular homeostatic system that allows the parasite to cope with fluctuations in zinc bioavailability. To gain insight into the mechanisms underlying the zinc-induced modulation of LiZIP3 we investigated the molecular basis for the decrease of this transporter in L. infantum during zinc surplus.
LiZIP3 decrease in response to zinc is regulated at the mRNA level
Rapid endocytosis of ZIP transporters in response to an increase in zinc levels appears to be a conserved mechanism by which cells prevent zinc overload (Gitan et al., 1998; Kim et al., 2004; Mao et al., 2007; Wang et al., 2004). Often, this process is accompanied by the subsequent degradation of the protein (Gitan et al., 1998; Mao et al., 2007) as well as the arrest of ZIP synthesis (Zhao & Eide, 1996a; Weaver et al., 2007). To test whether zinc induces the fast removal of surface-associated LiZIP3, we performed cell-membrane biotinylation assays of parasites following metal supplementation. We observed decreased surface-associated LiZIP3 after 12 h, but not 6 h, supplementation with 50 μM zinc (Fig. 6A, compare lanes 1, 2, and 3). Such regulation is clearly much slower than the rapid endocytosis (within a few minutes) reported for analogous transporters in response to zinc overload (Gitan et al., 1998; Kim et al., 2004; Mao et al., 2007; Wang et al., 2004). Removal of LiZIP3 from the membrane was not observed in control parasites that had not been supplemented with zinc (Fig. 6A, lanes 4 and 5). Moreover, the internalization of LiZIP3 recorded at 12 h was not accompanied by a decrease in the total amount of this protein (Fig. 6B, lanes 2 and 3), indicating that protein degradation also is unlikely to act as a mechanism by which LiZIP3 may be rapidly modulated. Instead, the early response of parasites to zinc appears to involve the arrest of LiZIP3 synthesis. This conclusion is supported by the observation that whereas untreated cells accumulated the transporter over 12 h culture (Fig. 6B, lanes 1, 4 and 5), zinc-supplemented parasites did not do so (Fig 6B, lanes 1, 2 and 3). That parasites rapidly react to zinc by blocking LiZIP3 synthesis is corroborated by the fact that LiZIP3 mRNA is down-regulated within 1 h of zinc addition (Fig. 6C). Such decrease of mRNA in response to zinc is specific for LiZIP3 as it was not observed for the two other L. infantum ZIP members, LiZIP1 and LiZIP2 (Fig. S5). Notably, this mechanism is triggered by concentrations as low as 1 μM, strengthening the importance of LiZIP3 mRNA down-regulation for homeostasis of zinc.
Fig. 6. Reduction of the LiZIP3 mRNA is an early event on the process of zinc-mediated LiZIP3 down-regulation.
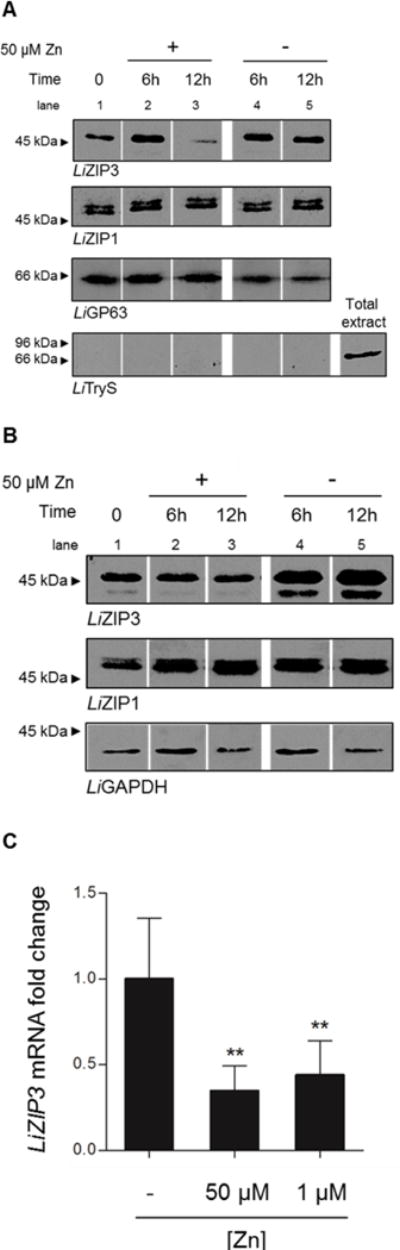
A and B. Zinc-induced decay of surface-associated LiZIP3 does not result from rapid endocytosis of the transporter but from protein synthesis arrest. A culture of promastigotes grown for 48 h in normal medium was supplemented with 50 μM zinc for different time-points. Before and 6 and 12 h after the addition of the metal, aliquots were pelleted for (A) cell membrane biotinylation and (B) total protein extraction. LiZIP3 levels were analysed by western blotting. The specificity of the zinc effect on cell membrane and total LiZIP3 levels was controlled, upon stripping, with antibodies recognizing other cell surface proteins, LiGP63 and LiZIP1, and soluble LiGAPDH. Exclusivity of biotinylation of cell surface proteins is shown with LiTryS. (Uncropped blots are in Fig. S4A and B).
C. Zinc supplementation causes a decrease in LiZIP3 mRNA level. Fifty or 1 μM zinc were added to cultures of promastigotes grown for 48 h in normal medium. Changes in LiZIP3 mRNA caused by metal supplementation were measured by qRT-PCR 1 h upon introduction of the metal in the culture (mean ± SD, n = 5; asterisks indicate significant differences to control, **P < 0.01, One-way ANOVA).
A labile protein mediates LiZIP3 down-regulation in response to zinc
Owing to the fact that transcription in Leishmania spp. is polycistronic, these parasites are unable to control gene expression at the transcription level (reviewed in Requena, 2011). Accordingly, one explanation for the observed decay of LiZIP3 mRNA upon zinc supplementation is that this metal alters the stability of the transcript. To test this hypothesis, we inhibited transcription by using actinomycin D and measured the levels of LiZIP3 mRNA in the absence or presence of 50 μM zinc by real-time reverse transcription-PCR (qRT-PCR) at varying time-points. We found a significant reduction in LiZIP3 mRNA levels in parasites supplemented with zinc for 30 and 60 min relatively to non-treated controls (Fig. 7A), an observation that supports the conclusion that this metal induces the destabilization of the transcript. Next, we measured the effect of zinc on LiZIP3 mRNA decay in parasites co-expressing LiZIP3 from its chromosome context and from an episome carrying the LiZIP3 gene devoid of its own untranslated regions (UTRs). We found that, while the mRNA derived from endogenous LiZIP3 showed the expected increased degradation rate when zinc was available, that from the episome was not affected by zinc (Fig. S6). These data indicate that the LiZIP3 UTRs are required for the mRNA destabilization induced by zinc and implicate the presence of elements in those regions responding to zinc concentration.
Fig. 7. Expression of LiZIP3 is regulated by zinc at the mRNA stability level through a process involving a short-lived protein.
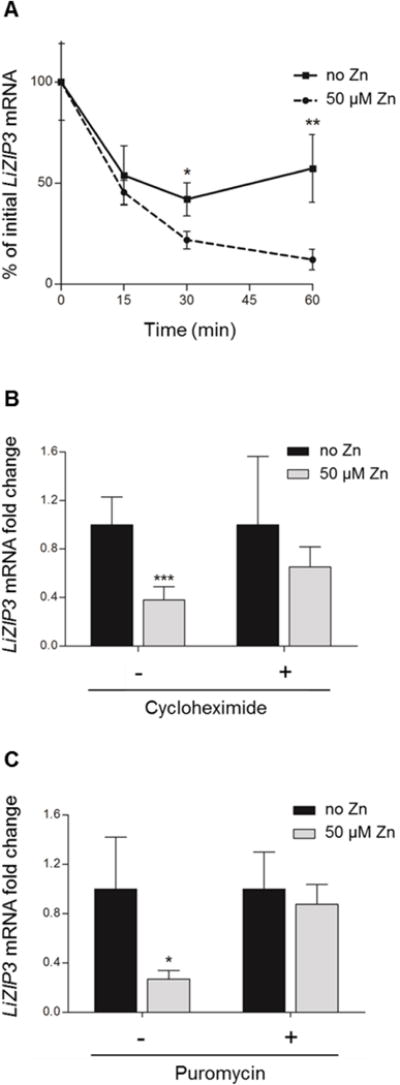
A. Effect of zinc on the degradation of LiZIP3 mRNA. Messenger RNA synthesis was blocked with 10 μg mL−1 actinomycin D and the degradation of LiZIP3 mRNA in the absence and in the presence of 50 μM zinc was accompanied by qRT-PCR (mean ± SD, n = 3–4; asterisks indicate significant differences in each time-point, *P < 0.05, **P < 0.01, Student’s t test).
B and C. Down-regulation of LiZIP3 mRNA requires the activity of a labile protein. Promastigotes grown for 48 h in normal medium were incubated with 50 μg mL−1 cycloheximide (B) or 200 μg mL−1 puromycin (C) during 2 h prior to addition of 50 μM zinc for 1 h. Fold changes in LiZIP3 mRNA were measured by qRT-PCR (mean ± SD, n = 5 for cycloheximide, n = 3 for puromycin; asterisks indicate significant differences between cultures not supplemented and supplemented with zinc in each condition tested, i.e. with or without cycloheximide or puromycin, *P < 0.05, ***P < 0.001, Student’s t test).
Short-lived proteins are often involved in regulation of mRNA stability in trypanosomatids (Wilson et al., 1993; Argaman et al., 1994; Graham & Barry, 1996; Quijada et al., 1997; Webb et al., 2005). To determine whether such molecules operate in the decay of LiZIP3 mRNA by zinc, we incubated parasites at the late-logarithmic phase of growth in the presence of cycloheximide or puromycin for 2 h to block protein synthesis and allow any short-lived protein to decay. We then added 50 μM zinc and assessed mRNA levels 1 h later by using qRT-PCR. The results of this analysis revealed that, unlike controls, parasites cultured in the presence of the inhibitors were unable to down-regulate the LiZIP3 transcript in response to zinc (Fig. 7B and C). These data suggest that zinc induces the expression of a labile protein which, directly or indirectly, causes destabilization of the LiZIP3 mRNA.
Discussion
The maintenance of an adequate intracellular zinc pool is crucial for normal cell physiology. One of the mechanisms through which this is achieved is by controlling the quantity of metal that is translocated across the cell membrane. This manuscript reports a novel member of the ZRT/IRT-like Protein (ZIP) family, LiZIP3, and provides evidence that it enables L. infantum to acquire zinc in a highly regulated way.
Support for a role of LiZIP3 in zinc internalization in L. infantum came from several observations. First, this protein was established to be a member of a family of transporters that contribute to elevate the cytosolic zinc content, the ZIP family, with conserved amino acids previously found to be important for the functionality of these molecules. These include several residues which are likely part of zinc binding sites associated to or within the channel through which substrates pass (Rogers et al., 2000; reviewed in Gaither & Eide, 2001a), and a (HX)2 in the cytosolic variable loop between TMDs III and IV, reminiscent of a (HX)n cluster which was also proposed to bind zinc. It is possible that, during transport, this histidine-rich motif becomes spatially close to histidines in TMDs IV and V and is, thus, an integral part of the mechanism for zinc translocation across membranes (Milon et al., 2006), either because it facilitates transport (Milon et al., 2006; Jacques et al., 2010) or because it imparts metal selectivity (Nishida et al., 2008). Alternatively, the (HX)n might bind cytoplasmic zinc functioning instead as a zinc sensor (Mao et al., 2007). A second argument strengthening the proposal that LiZIP3 enables zinc incorporation in parasites came from analysis in S. cerevisiae mutant strains and in X. laevis oocytes which experimentally confirmed that LiZIP3 displays zinc transport activity. Finally, the observation that LiZIP3 is present at the parasite cell membrane and that LiZIP3-overexpressing cells incorporate more zinc than wild type L. infantum provided solid evidence that this molecule is implicated in the internalization of zinc into the parasite. Importantly, we found that LiZIP3 is tightly modulated by the external concentration of this metal, its expression and cell surface location being either induced or repressed when bioavailability of zinc was low or high, respectively. Other metals did not affect LiZIP3 expression or localization indicating that these effects are zinc specific. These data suggest that LiZIP3 is important for the parasite to adjust the level of zinc entering the cell at a particular timing. Remarkably, Leishmania appear to be able to sense extremely low concentrations of zinc (within the sub-picomolar range), responding to these by regulating LiZIP3 expression.
Import of metals, as of other nutrients, into organisms is frequently carried out by more than one transporter displaying different affinities. Low-affinity transporters, which are constitutively expressed or are regulated only to a low extent, enable adequate influx of nutrients when these are plentiful but cannot provide sufficient substrate in case of scarcity. In these conditions, finely regulated high-affinity transporters take over, allowing organisms to maintain their vital activities (Eide, 2012). Our data indicating that zinc specifically modulates expression of LiZIP3 and that such regulation occurs in the face of small changes in zinc bioavailability strongly suggest that this protein functions as a high-affinity zinc transporter in L. infantum. This is in line with the estimated K0.5 of 0.1 μM for zinc transport in X. laevis oocytes expressing LiZIP3, similar to that of the high-affinity zinc transporters ScZRT1 (Zhao & Eide, 1996a), and ZIP5 and ZIP6 of Medicago truncatula (Stephens et al., 2011).
ZIP proteins can transport zinc and/or other metal ions. The substrate profile obtained in X. laevis oocytes indicates that LiZIP3 can transport Cu1+, Cu2+, Cd2+, Co2+ and Fe2+ in addition to Zn2+. Although we lack a complete inhibitory profile, the observation that 10-fold excess Cu1+ (the metal ion that, after zinc, is most efficiently internalized in X. laevis) and Cu2+ only modestly inhibited zinc uptake by LiZIP3 and the larger fluxes observed for zinc than for other metals, suggest that LiZIP3 is a zinc-preferring transporter. Nevertheless, it is possible that, under zinc-limited conditions in which the protein is maximally expressed at the cell membrane, LiZIP3 may supply L. infantum with cations other than zinc.
As argued above, LiZIP3 is one factor that allows L. infantum to withstand changes in zinc bioavailability. We addressed the zinc-response of this protein by investigating the mechanism behind LiZIP3 down-regulation upon zinc supplementation. A conserved mechanism operating in such situation involves rapid decrease of surface exposed transporter by induction of its endocytosis, simultaneously to synthesis arrest (Zhao & Eide, 1996a; Gitan et al., 1998; Mao et al., 2007; Weaver et al., 2007). Interestingly, we did not find evidence for accelerated LiZIP3 endocytosis, only for arrested protein synthesis. Protein internalization occurred only several hours after zinc addition raising the possibility that this is a late regulatory mechanism. In the absence of rapid protein removal from the cell membrane, the question arises of how Leishmania cope for over 6 h with excess zinc in their environment. One hypothesis is that LiZIP3 is promptly inactivated by post-translational modifications (PTMs). Although, up to now, no PTMs directly modulating ZIP activity have been reported, LiZIP3 holds a long histidine-rich extracellular N-terminal extension for which a role in modulation of zinc uptake has been previously proposed (Eide, 2005). The observed tolerance of L. infantum to the presence of surface LiZIP3 for several hours after zinc supplementation can also be explained by increased sequestration of the metal into specific subcellular proteins or sites. In this regard, acidocalcisomes arise as a putative storage organelle as they have been shown to contain zinc (Miranda et al., 2004; Lefurgey et al., 2005). Intake of zinc into these structures is presumably carried out by an acidocalcisome membrane protein previously described in Trypanosoma cruzi (Ferella et al., 2008) and with orthologues in Leishmania. Finally, we cannot rule out the possibility that a rapidly activated zinc cell exporter contributes to counteract excessive internalization of zinc in the parasite cytoplasm.
The present study reveals that a major mechanism by which zinc negatively modulates LiZIP3 expression occurs at the level of mRNA stability. This process may involve a short-lived regulatory protein which interacts directly or indirectly with zinc-sensing element(s) within the gene’s UTRs. A likely scenario is that the regulatory protein possesses a zinc-finger motif requiring zinc to become stable and exert its function. Future work should address the identity of this labile protein as well as of the zinc-responsive motif(s) within the LiZIP3 UTR(s) as this may be central to elucidate how zinc homeostatic responses are controlled in Leishmnaia. In other organisms the binding of specific transcription factors to promoter specific elements provides an important point to coordinate the zinc homeostatic response. In Leishmania and other trypanosomatids, which are virtually devoid of transcription regulation, no such mechanism can take place. Nonetheless, recent studies have shown that genes co-expressed specifically in a definite time-point share unique sequences in the untranslated regions of their mRNAs (Haile & Papadopoulou, 2007). It is, thus, reasonable to assume that the zinc-sensing element(s) within the LiZIP3 UTRs may be common to other genes sharing the same response to zinc and that their expression is controlled by the same protein factor.
In conclusion, by identifying and characterizing L. infantum ZIP3, this report uncovers one of the major players that allow Leishmania to obtain critical zinc without facing the dangers of zinc overload. This report also provides the first insight into the biology of zinc in a trypanosomatid organism.
Experimental Procedures
Reagents
Unless otherwise indicated, restriction enzymes were purchased from Fermentas and reagents were obtained from Sigma–Aldrich Corp. or Research Products International Corp.
Plasmid construction
To generate the constructs used in this work, the LiZIP3 ORF was excised by restriction from the p423ADHLiZIP3 plasmid (Supporting Experimental Procedures) and cloned into appropriate plasmids, as detailed next. In the case of the pYES2LiZIP3 construct, LiZIP3 was cloned into the HindIII and XhoI restriction sites of the pYES2 plasmid (Invitrogen). To generate pOX(+)LiZIP3, LiZIP3 was obtained by restriction with SpeI, Klenow end-filled, then XhoI and cloned into the EcoRV (Roche) and XhoI sites of pOX(+) (Takanaga et al., 2005). Finally, to assemble pXGLiZIP3, the LiZIP3 ORF was isolated by double digestion with HindIII and XhoI, Klenow end-filled and cloned into the SmaI site of pXG (Ha et al., 1996).
Functional expression in S. cerevisiae
The yeast strains used in this work were the following: wild type (DY1457, MATα ade6 can1 his3 leu2 trp1 ura3) and zap1Δ (ZHY6, MATα ade6 can1 his3 leu2 trp1 ura3 zap1Δ::TRP1) (Zhao & Eide, 1997). Yeast strains were grown in Synthetic Defined (SD)-glucose medium [0.67% (w/v) yeast nitrogen base, 2% (w/v) glucose] supplemented with the amino acidic auxotrophic requirements. Zinc-limiting medium was obtained by addition of 1 mM ethylenediaminetetraacetic acid (EDTA).
Expression of LiZIP3 in X. laevis oocytes
We performed laparotomy and ovariectomy on adult female X. laevis frogs (Nasco, Fort Atkinson, WI, USA) under 3-aminoethylbenzoate methanesulfonate anesthesia (0.1% in 1:1 water/ice, by immersion), following a protocol approved by the University of Cincinnati Institutional Animal Care and Use Committee. Ovarian tissue was isolated and treated with collagenase A (Roche) and oocytes were isolated and stored at 17°C in modified Barths’ medium as described (Mitchell et al., 2014). The construct pOX(+)LiZIP3 was linearized with SwaI and used to synthesize RNA in vitro using the mMESSAGE mMACHINE/SP6 RNA polymerase transcription kit (Applied Biosystems/Ambion) according to the manufacturers’ protocols. Defolliculate stage V–VI oocytes were injected with 50 ng of cRNA and incubated for 6–7 days before being used in functional assays and for immunofluorescence. Noninjected oocytes were used as controls.
Radiotracer uptake assays in X. laevis oocytes
We used the radiotracer metals 109Cd (added as CdCl2) at final specific activity of 28 MBq mg−1, 65Zn (added as ZnCl2) at 0.1–2.1 GBq mg−1 (both obtained from the National Laboratory, Oak Ridge, TN, USA), 57Co (added as CoCl2) at 2.94 GBq mg−1, 55Fe (added as FeCl3) at 230 MBq mg−1, and 54Mn (added as MnCl2) at 24 MBq mg−1 (purchased from Perkin–Elmer Life Science Products), and 64Cu (added as CuCl2) at 2.0 GBq mg−1 (Washington University–St Louis, MO, USA). To measure radiotracer metal-ion uptake, oocytes were incubated at room temperature (23°C) in a standard transport medium for 30 min as described (Wang et al., 2012). Media containing iron or copper were supplemented with 100 μM or 1 mM L-ascorbic acid or 100 μM L-histidine, when indicated in the figure legends. Radiotracer metal-ion uptake was measured over 10 or 30 min, i.e., well within the timecourse of linear uptake (> 2 h) of zinc, copper or iron in oocytes expressing mammalian ZIP orthologs (Pinilla-Tenas et al., 2011; Antala & Dempski, 2012) exhibiting higher fluxes than did LiZIP3. Uptake was terminated by rapidly washing the oocytes three times in ice-cold pH-7.5 transport medium. Oocytes were then solubilized using 5% (w/v) sodium dodecylsulfate (SDS) and radiotracer content assayed by liquid-scintillation counting using Scintisafe-30% liquid scintillation cocktail (Fisher Scientific). Saturation kinetics of zinc uptake were obtained by measuring the internalization of 65Zn in the range 0.1–10 μM in control and LiZIP3-expressing oocytes. Data were fit by a Michaelis–Menten function (Equation 1) (Illing et al., 2012), for which VS is the velocity (uptake) of substrate S (65Zn2+), the derived maximum velocity, S the concentration of substrate S, and the substrate concentration at which velocity was half-maximal.
| (Equation 1) |
Leishmania infantum cultures
Leishmania infantum promastigotes (strain MHOM/MA/67/ITMAP-263) were cultured at 25°C in RPMI 1640 GlutaMAX™-I medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBSi), 50 U mL−1 penicillin, 50 μg mL−1 streptomycin (all from Gibco) and 20 mM HEPES pH 7.4. For each assay parasites were first pre-passaged, i.e., they were subjected to 4–5 daily passages in culture medium at 106 mL−1, and seeded at 106 mL−1 at the beginning of the experiments.
Production of pXG and of pXGLiZIP3 parasites
Promastigotes in the logarithmic phase of growth were electroporated as described elsewhere (Beverley & Clayton, 1993) with 3–15 μg of plasmid DNA (pXG or pXGLiZIP3). Parasites were allowed to recover for 24 h before being selected on agar plates containing 15 μg mL−1 G418 (Sigma). Isolated clones were then cultured in selective liquid medium. The genotype of the transfectants was analysed by Southern blotting of genomic DNAs extracted by the proteinase K method (Blaxter et al., 1988).
Quantification of zinc in L. infantum parasites
To analyse the zinc content of pXG- and pXGLiZIP3-transfected L. infantum lines, parasites grown in normal medium for 72 h were seeded at 106 mL−1 in fresh medium for 24 h. Aliquots of approximately 108 parasites, harvested immediately before and 24 h after medium replacement, were washed twice in ice-cold PBS (0.1 M sodium phosphate buffer pH 7.2, 0.15 M NaCl) and pellets dried at 37°C, dissolved in 65% (v/v) nitric acid for 2 h at 65°C and analysed by atomic absorption spectrometry (flame atomization) using the Atomic Absorption Spectrometer PU 9200X (Philips).
Protein extracts and western blotting
Parasites were harvested at 3000 ×g for 10 min at 4°C and washed twice in ice-cold PBS. The pellet was suspended at 6.25×108 mL−1 in 4% (w/v) SDS in 50 mM Tris-HCl pH 7.4 containing a cocktail of protease inhibitors. The suspension was subjected to ultrasounds and stored at −70°C until further use. Before loading into SDS PAGE gels, extracts were treated with 4 M urea and 1% (v/v) β-mercaptoethanol, without boiling or heating. Western blotting was performed according to standard protocols. Control proteins were analysed upon membrane stripping and re-hybridization with the appropriate antibodies.
Cell membrane biotinylation
Parasites were harvested, washed twice in ice-cold PBS containing 1 mg mL−1 glucose (PBS-glucose) and suspended at approximately 1.5×108 mL−1 in 500 μL of 1 mg mL−1 EZ-Link™ Sulpho-NHS-SS-Biotin (Thermo Scientific) (prepared in ice-cold PBS-glucose). Upon 1 h incubation on ice with constant shaking, parasites were washed in PBS-glucose and cell pellets stored at −70°C. For further processing of samples, these were lysed in 20 μL 4% (w/v) SDS in 50 mM Tris-HCl pH 7.4, containing a cocktail of protease inhibitors, and diluted by adding 80 μL PBS-glucose. Cell lysates were then sonicated, diluted in 700 μL PBS-glucose and further incubated with 30 μL of a 1:10 dilution of High Capacity Neutravidin® Agarose Resin (Thermo Scientific), for 1 h at room temperature with shaking. Next, beads were pelleted by centrifugation at 3000 ×g for 2 min and washed twice in 1 mL PBS. Biotinylated proteins were eluted from the beads by incubation for 2 h at room temperature in Laemli sample buffer containing 50 mM 1,4-dithiothreitol (DTT) and 4 M urea. Eluted proteins were subjected to western blotting, without heating or boiling.
Antibodies
Polyclonal antibodies against LiZIP3 were produced by the GenScript Corp.’s custom antibody production services. A 14-amino acid peptide (LKDMYGGAEDGQGG) within the variable region of LiZIP3 was synthesized with an additional C-terminal cysteine and conjugated to the carrier protein keyhole limpet hemocyanin (KLH). After immunization of rabbits, antibodies were affinity-purified and used in western blotting and immunofluorescence. Sera against the glycosomal glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the major surface glycoprotease (GP63) were a gift from Dr. P. Michels and Dr. F. Opperdoes and Dr. M. Wilson, respectively.
Effect of metal concentration on LiZIP3 expression
To analyse protein expression throughout the days of promastigote culture, parasites were seeded at 106 mL−1 in normal culture medium. Cells were counted every 24 h by using a Neubauer chamber, pelleted and subjected to protein extraction. Zinc concentration of the corresponding supernatants was determined by atomic absorption spectrometry, as above. To examine the effect of metal supplementation on LiZIP3 expression, parasites were grown for 48 h in normal medium prior to the addition of metals in the following concentrations: 50 μM ZnCl2, 100 μM CuSO4·5H2O and 125 μM Fe-citrate, MnCl2·4H2O, MgCl2·6H2O, CaCl2, CdCl2·2.5H2O, NiSO4·6H2O and CoCl2·6H2O. Parasites were pelleted after 24 h and subjected to protein extraction. Finally, the impact of metal depletion on LiZIP3 expression was analysed by growing parasites in the presence of 0–1 mM EDTA or of 10 μM N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN). All solutions, except for TPEN, were aqueous. TPEN was prepared in 50% (v/v) dimethyl sulfoxide (DMSO).
Indirect immunofluorescence assay
To localize LiZIP3 in L. infantum, parasites were harvested, washed twice in ice-cold PBS, fixed in 4% paraformaldehyde (w/v) and spotted onto polylysine-treated slides. Cells were permeabilized with 0.1% (v/v) Triton X-100 and, after blocking with 1% (w/v) BSA, sequentially incubated with anti-LiZIP3 and Alexa Fluor® 488-conjugated (Invitrogen) antibodies. DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). Slides were mounted in 70% (v/v) glycerol in PBS. We performed immunolocalization of LiZIP3 in oocytes by using oocytes embedded and freshly frozen in Tissue-Tek O.C.T. (optimum cutting temperature) compound (Sakura Finetek U.S.A., Inc.). Five micrometer sections were cut by using a cryostat (Leica 3050S), collected onto polylysine-treated slides and dried overnight at room temperature. Fixation was performed with pre-cooled acetone (−20°C) for 10 min, after which the acetone was poured off and allowed to evaporate from the tissue. Oocyte sections were blocked with 1% (w/v) BSA and incubated with anti-LiZIP3 antibody followed by Alexa Fluor® 488-conjugated secondary antibody. Slides were mounted and visualized as described for parasites. Images were acquired with the Axiocam MR ver.3.0 camera (Carl Zeiss) coupled to the AxioImager Z1 microscope (Carl Zeiss) and with the laser scanning confocal microscope Leica TCS SP5 II (Leica Microsystems).
mRNA analysis
mRNA was measured by Real-Time Reverse Transcription-PCR (qRT-PCR). Promastigotes were seeded at 106 mL−1 and grown for 48 h prior to any treatment. To study the effect of zinc in the LiZIP3 mRNA, parasites were incubated for 1 h in the presence of 1 or 50 μM ZnCl2. The effect of zinc on the degradation of LiZIP3 transcript was determined in cells cultured in the presence of 10 μg mL−1 of actinomycin D and 50 μM ZnCl2 for 15, 30 and 60 min. The existence of a labile protein was evaluated on parasites treated with 50 μg mL−1 cycloheximide or 200 μg mL−1 puromycin for 2 h and subsequently incubated for 1 h with 50 μM ZnCl2. Following the specific treatments, parasites were harvested, washed once in ice-cold PBS and stored at −70°C until being processed for RNA extraction. Total RNA of parasites was extracted using the RNeasy Plus Mini Kit (QIAGEN). Synthesis of cDNA was achieved from 1 μg of total RNA using Superscript® VILO™ cDNA synthesis kit (Invitrogen). Real-Time PCR was performed using iQ™ SYBR® GREEN Supermix in an iCycler iQ5 from Bio-Rad according to the cycling conditions: an initial step at 95°C for 3.5 min, followed by 40 cycles at 95°C for 30 sec, 59°C for 45 sec and 72°C for 30 sec. The melt curve was obtained by decreasing the temperature by 0.5°C in each of the 10-sec 81 steps, starting on 55°C. Primers were designed to amplify sequences of approximately 200 bp (Table S1). The efficiency of each pair was tested and verified to be similar. Results were normalized by the L. infantum cytosolic GAPDH (LinJ.36.2480) as endogenous control. Relative expression levels (REL) were calculated according to Equation 2:
| (Equation 2) |
Statistical analysis
We used Student’s t test or one-way analysis of variance (ANOVA) in the analysis of the results obtained with parasites, followed by pairwise multiple comparisons using the Bonferroni post-hoc test. Radiotracer uptake data in oocytes were presented as mean and standard deviation (SD) for n independent observations and analysed using two-way ANOVA followed, where appropriate, by pairwise multiple comparisons using the Holm–Šidák test (for which P is the adjusted probability). Data were fit by Equation 1 by using the least-squares method of nonlinear regression followed by F-tests of the significance of the fit to the model; SE is the standard error of the estimate, and P refers to the significance of the fit.
Supplementary Material
Acknowledgments
We thank Sarah R. Anthony, from the University of Cincinnati, Ohio and Joana Passos from Instituto de Biologia Molecular e Celular, Porto for their help in the studies with X. laevis oocytes and Marisa Almeida, from the University of Porto, for zinc measurement assays.
Funding
This work was supported by FEDER funds through the Operational Competitiveness Programme - COMPETE, and by National funds through FCT - Fundação para a Ciência e Tecnologia (FCT), under the project FCOMP-01-0124-FEDER-009506 (PTDC/CVT/100090/2008, to AMT), and by Projects “NORTE-07-0124-FEDER-000002-Host-Pathogen Interactions” co-funded by Programa Operacional Regional do Norte (ON.2 - O Novo Norte), under the Quadro de Referência Estratégico Nacional (QREN), through FEDER and by FCT (to AMT), PHS grant R01 DK080047 (to BM) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the University of Cincinnati (to BM), and PHS grant R01 GM56285 (to DE) from the National Institute of General Medical Sciences (NIGMS). SC was partly financed by FCT fellowship SFRH/BD/28712/2006; HC is a recipient of FCT fellowship SFRH/BPD/80836/2011. AT and SC are members of COST Action TD1304 “The Network for the Biology of Zinc (Zinc-Net)”.
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- Amini M, Nahrevanian H, Khatami S, Farahmand M, Mirkhani F, Javadian S. Biochemical association between essential trace elements and susceptibility to Leishmania major in BALB/c and C57BL/6 mice. Braz J Infect Dis. 2009;13:83–85. doi: 10.1590/s1413-86702009000200002. [DOI] [PubMed] [Google Scholar]
- Antala S, Dempski RE. The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry. 2012;51:963–973. doi: 10.1021/bi201553p. [DOI] [PubMed] [Google Scholar]
- Argaman M, Aly R, Shapira M. Expression of heat shock protein 83 in Leishmania is regulated post-transcriptionally. Mol Biochem Parasitol. 1994;64:95–110. doi: 10.1016/0166-6851(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bern C, Haque R, Chowdhury R, Ali M, Kurkjian KM, Vaz L, Amann J, et al. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg. 2007;76:909–914. [PubMed] [Google Scholar]
- Beverley SM, Clayton CE. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol. 1993;21:333–348. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- Blaxter ML, Miles MA, Kelly JM. Specific serodiagnosis of visceral leishmaniasis using a Leishmania donovani antigen identified by expression cloning. Mol Biochem Parasitol. 1988;30:259–270. doi: 10.1016/0166-6851(88)90095-3. [DOI] [PubMed] [Google Scholar]
- Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86:521–534. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Colman A. Translation of eukaryotic messenger RNA in Xenopus oocytes. In: Hames BD, Higgins SJ, editors. Transcription and Translation: A Practical Approach. Oxford: IRL Press; 1984. pp. 271–302. [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- Eide DJ. The ZIP family of zinc transporters. In: Iuchi S, Kuldell N, editors. Zinc finger proteins: from atomic contact to cellular function. Springer; US: 2005. pp. 261–264. [Google Scholar]
- Eide DJ. An “inordinate fondness for transporters” explained? Sci Signal. 2012;5:pe5. doi: 10.1126/scisignal.2002837. [DOI] [PubMed] [Google Scholar]
- Eng BH, Guerinot ML, Eide D, Saier MH., Jr Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, Docampo R, Andersson B. Proteomics in Trypanosoma cruzi - localization of novel proteins to various organelles. Proteomics. 2008;8:2735–2749. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither LA, Eide D. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals. 2001a;14:251–270. doi: 10.1023/a:1012988914300. [DOI] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem. 2001b;276:22258–22264. doi: 10.1074/jbc.M101772200. [DOI] [PubMed] [Google Scholar]
- Gitan RS, Luo H, Rodgers J, Broderius M, Eide D. Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J Biol Chem. 1998;273:28617–28624. doi: 10.1074/jbc.273.44.28617. [DOI] [PubMed] [Google Scholar]
- Graham SV, Barry JD. Polysomal, procyclin mRNAs accumulate in bloodstream forms of monomorphic and pleomorphic trypanosomes treated with protein synthesis inhibitors. Mol Biochem Parasitol. 1996;80:179–191. doi: 10.1016/0166-6851(96)02674-6. [DOI] [PubMed] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci U S A. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. The ZIP family of metal transporters. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- Haile S, Papadopoulou B. Developmental regulation of gene expression in trypanosomatid parasitic protozoa. Curr Opin Microbiol. 2007;10:569–577. doi: 10.1016/j.mib.2007.10.001. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- He L, Wang B, Hay EB, Nebert DW. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharmacol. 2009;238:250–257. doi: 10.1016/j.taap.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med. 2006;203:2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem. 2012;287:30485–30496. doi: 10.1074/jbc.M112.364208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques I, Andrews NW, Huynh C. Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Mol Biochem Parasitol. 2010;170:28–36. doi: 10.1016/j.molbiopara.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–4530. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- Lefurgey A, Gannon M, Blum J, Ingram P. Leishmania donovani amastigotes mobilize organic and inorganic osmolytes during regulatory volume decrease. J Eukaryot Microbiol. 2005;52:277–289. doi: 10.1111/j.1550-7408.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews WR, Wang F, Eide DJ, Van Doren M. Drosophila fear of intimacy encodes a Zrt/IRT-like protein (ZIP) family zinc transporter functionally related to mammalian ZIP proteins. J Biol Chem. 2005;280:787–795. doi: 10.1074/jbc.M411308200. [DOI] [PubMed] [Google Scholar]
- Milon B, Wu Q, Zou J, Costello LC, Franklin RB. Histidine residues in the region between transmembrane domains III and IV of hZip1 are required for zinc transport across the plasma membrane in PC-3 cells. Biochim Biophys Acta. 2006;1758:1696–1701. doi: 10.1016/j.bbamem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Miranda K, Docampo R, Grillo O, de Souza W. Acidocalcisomes of trypanosomatids have species-specific elemental composition. Protist. 2004;155:395–405. doi: 10.1078/1434461042650361. [DOI] [PubMed] [Google Scholar]
- Mishra J, Carpenter S, Singh S. Low serum zinc levels in an endemic area of visceral leishmaniasis in Bihar, India. Indian J Med Res. 2010;131:793–798. [PubMed] [Google Scholar]
- Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am J Physiol Cell Physiol. 2014;306:C450–C459. doi: 10.1152/ajpcell.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, et al. GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem. 2002;277:4738–4746. doi: 10.1074/jbc.M106754200. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Müller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Nishida S, Mizuno T, Obata H. Involvement of histidine-rich domain of ZIP family transporter TjZNT1 in metal ion specificity. Plant Physiol Biochem. 2008;46:601–606. doi: 10.1016/j.plaphy.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Olivier M, Atayde VD, Isnard A, Hassani K, Shio MT. Leishmania virulence factors: focus on the metalloprotease GP63. Microbes Infect. 2012;14:1377–1389. doi: 10.1016/j.micinf.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Pasa S, Kargin F, Bildik A, Seyrek K, Ozbel Y, Ozensoy S. Serum and hair levels of zinc and other elements in dogs with visceral leishmaniasis. Biol Trace Elem Res. 2003;94:141–147. doi: 10.1385/BTER:94:2:141. [DOI] [PubMed] [Google Scholar]
- Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, et al. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am J Physiol Cell Physiol. 2011;301:C862–871. doi: 10.1152/ajpcell.00479.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada L, Soto M, Alonso C, Requena JM. Analysis of post-transcriptional regulation operating on transcription products of the tandemly linked Leishmania infantum hsp70 genes. J Biol Chem. 1997;272:4493–4499. doi: 10.1074/jbc.272.7.4493. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy R, Swihart KG, McCoy JJ, Wilson ME, Donelson JE. Intergenic regions between tandem gp63 genes influence the differential expression of gp63 RNAs in Leishmania chagasi promastigotes. J Biol Chem. 1995;270:12133–12139. doi: 10.1074/jbc.270.20.12133. [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 2003;133:126–134. doi: 10.1104/pp.103.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena JM. Lights and shadows on gene organization and regulation of gene expression in Leishmania. Front Biosci. 2011;17:2069–2085. doi: 10.2741/3840. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci U S A. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharquie KE, Najim RA, Farjou IB. A comparative controlled trial of intralesionally-administered zinc sulphate, hypertonic sodium chloride and pentavalent antimony compound against acute cutaneous leishmaniasis. Clin Exp Dermatol. 1997;22:169–173. [PubMed] [Google Scholar]
- Sousa AF, Gomes-Alves AG, Benítez D, Comini MA, Flohé L, Jaeger T, Passos J, et al. Genetic and chemical analyses reveal that trypanothione synthetase but not glutathionylspermidine synthetase is essential for Leishmania infantum. Free Radic Biol Med. 2014;73:229–238. doi: 10.1016/j.freeradbiomed.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- Stephens BW, Cook DR, Grusak MA. Characterization of zinc transport by divalent metal transporters of the ZIP family from the model legume Medicago truncatula. Biometals. 2011;24:51–58. doi: 10.1007/s10534-010-9373-6. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem. 2005;280:8974–8984. doi: 10.1074/jbc.M413027200. [DOI] [PubMed] [Google Scholar]
- Taudte N, Grass G. Point mutations change specificity and kinetics of metal uptake by ZupT from Escherichia coli. Biometals. 2010;23:643–656. doi: 10.1007/s10534-010-9319-z. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Van Weyenbergh J, Santana G, D’Oliveira A, Jr, Santos AF, Jr, Costa CH, Carvalho EM, Barral A, et al. Zinc/copper imbalance reflects immune dysfunction in human leishmaniasis: an ex vivo and in vitro study. BMC Infect Dis. 2004;4:50. doi: 10.1186/1471-2334-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem. 2012;287:34032–34043. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Fierke CA. The BaeSR regulon is involved in defense against zinc toxicity in E. coli. Metallomics. 2013;5:372–383. doi: 10.1039/c3mt20217h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Dufner-Beattie J, Kim BE, Petris MJ, Andrews G, Eide DJ. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J Biol Chem. 2004;279:24631–24639. doi: 10.1074/jbc.M400680200. [DOI] [PubMed] [Google Scholar]
- Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol Chem. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb H, Burns R, Ellis L, Kimblin N, Carrington M. Developmentally regulated instability of the GPI-PLC mRNA is dependent on a short-lived protein factor. Nucleic Acids Res. 2005;33:1503–1512. doi: 10.1093/nar/gki298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Paetz KE, Ramamoorthy R, Donelson JE. The effect of ongoing protein synthesis on the steady state levels of Gp63 RNAs in Leishmania chagasi. J Biol Chem. 1993;268:15731–15736. [PubMed] [Google Scholar]
- Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci U S A. 1996a;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem. 1996b;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- Zhao H, Eide DJ. Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:5044–5052. doi: 10.1128/mcb.17.9.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


