Abstract
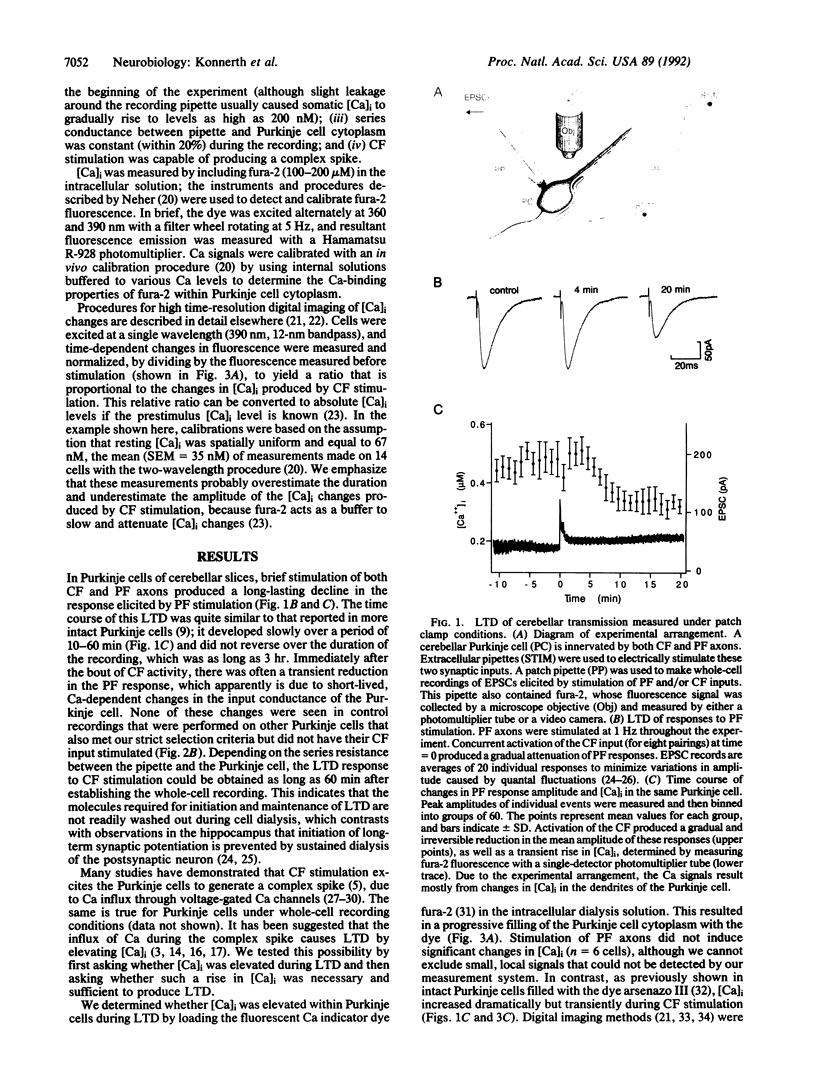
We have performed experiments designed to test the hypothesis that long-term depression (LTD) of excitatory synaptic transmission in the cerebellar cortex is caused by a rise in postsynaptic Ca concentration. These experiments combined measurements of synaptic efficacy, performed with the thin slice patch clamp technique, with fura-2 measurements of intracellular Ca concentration ([Ca]i) in single cerebellar Purkinje cells. Simultaneous activation of the climbing fiber and parallel fibers innervating single Purkinje cells caused a LTD of transmission of the parallel fiber-Purkinje cell excitatory synapse. This LTD was associated with large and transient rises in [Ca]i in the Purkinje cell and apparently was due to Ca entry through voltage-gated Ca channels in the Purkinje cell dendrites. The rise in [Ca]i produced by climbing fiber activity was necessary for LTD, because addition of the Ca chelator bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetate (BAPTA) to the interior of the Purkinje cell blocked LTD. Further, elevation of [Ca]i, produced by depolarizing pulses delivered in conjunction with parallel fiber activation, induced a depression of synaptic activity that closely resembled LTD in both time course and magnitude. Thus, a rise in [Ca]i appears to be sufficient to initiate LTD. From these results, we conclude that LTD of the parallel fiber-Purkinje cell synapse is initiated by a brief, climbing fiber-mediated rise in postsynaptic [Ca]i and that LTD is maintained by other, longer-lived processes that are triggered by the rise in postsynaptic [Ca]i.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Blackstone C. D., Supattapone S., Snyder S. H. Inositolphospholipid-linked glutamate receptors mediate cerebellar parallel-fiber-Purkinje-cell synaptic transmission. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4316–4320. doi: 10.1073/pnas.86.11.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6179–6183. doi: 10.1073/pnas.83.16.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Jaillard D. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. J Physiol. 1991 Jan;432:123–141. doi: 10.1113/jphysiol.1991.sp018380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. Neuroreport. 1990 Oct;1(2):133–136. doi: 10.1097/00001756-199010000-00013. [DOI] [PubMed] [Google Scholar]
- Crepel F., Krupa M. Activation of protein kinase C induces a long-term depression of glutamate sensitivity of cerebellar Purkinje cells. An in vitro study. Brain Res. 1988 Aug 23;458(2):397–401. doi: 10.1016/0006-8993(88)90486-6. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985 Sep 9;342(2):357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Ekerot C. F., Oscarsson O. Prolonged depolarization elicited in Purkinje cell dendrites by climbing fibre impulses in the cat. J Physiol. 1981 Sep;318:207–221. doi: 10.1113/jphysiol.1981.sp013859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirano T. Effects of postsynaptic depolarization in the induction of synaptic depression between a granule cell and a Purkinje cell in rat cerebellar culture. Neurosci Lett. 1990 Nov 13;119(2):145–147. doi: 10.1016/0304-3940(90)90819-u. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Synaptic control of excitability in turtle cerebellar Purkinje cells. J Physiol. 1989 Feb;409:157–170. doi: 10.1113/jphysiol.1989.sp017490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Karachot L. Messengers mediating long-term desensitization in cerebellar Purkinje cells. Neuroreport. 1990 Oct;1(2):129–132. doi: 10.1097/00001756-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D., Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. J Neurophysiol. 1990 Sep;64(3):948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kano M., Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987 Jan 15;325(6101):276–279. doi: 10.1038/325276a0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Knöpfel T., Audinat E., Gähwiler B. H. Climbing Fibre Responses in Olivo-cerebellar Slice Cultures. I. Microelectrode Recordings from Purkinje Cells. Eur J Neurosci. 1990;2(8):726–732. doi: 10.1111/j.1460-9568.1990.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Llano I., Armstrong C. M. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Connor J. A. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991 Dec 13;254(5038):1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Dickinson M. H., Smeyne M., Connor J. A. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991 Jul;7(1):81–89. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Llano I., Dreessen J., Kano M., Konnerth A. Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron. 1991 Oct;7(4):577–583. doi: 10.1016/0896-6273(91)90370-f. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y., Sugimori M. Isolated mammalian brain in vitro: new technique for analysis of electrical activity of neuronal circuit function. Fed Proc. 1981 Jun;40(8):2240–2245. [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Davis S., Butcher S. P. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc Lond B Biol Sci. 1990 Aug 29;329(1253):187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Calcium is an intracellular mediator of the climbing fiber in induction of cerebellar long-term depression. Proc Natl Acad Sci U S A. 1990 May;87(9):3383–3385. doi: 10.1073/pnas.87.9.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol. 1987 Dec;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Thompson R. F. The neurobiology of learning and memory. Science. 1986 Aug 29;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]





