SUMMARY
Background:
Tuberculosis is a serious health problem in Brazil so that the knowledge on the aspects of cutaneous tuberculosis is medically important.
Objective:
To assess the characteristics of patients with cutaneous tuberculosis treated at the Cassiano Antonio Moraes University Hospital, located in the city of Vitória, State of Espírito Santo, Brazil.
Methods:
This is a retrospective, descriptive, observational and cross-sectional study using the medical records of 29 patients with cutaneous tuberculosis treated at the Dermatology and Pulmonology services of the hospital from 1986 to 2011. The inclusion criterion was the confirmation of cutaneous tuberculosis taking into account clinical, epidemiological, immunological, and bacteriological findings, as well as the response to specific treatment.
Results:
Of the 29 studied patients; 18 (62%) were women with average age of 37 years; the predominant clinical condition was erythema induratum of Bazin in 12 (41.4%) cases; and the cutaneous lesions were in the lower limbs in 19 (65.8%) patients. Extra-cutaneous involvement occurred in eight (27.6%) cases. The tuberculin tests were positive in 15 (79%) individuals and the assessment of the infectious agent was negative in most of the investigated cases.
Conclusion:
The study found a low frequency (0.44%) of cutaneous tuberculosis in an endemic area of tuberculosis. There was a predominance of infection in women aged thirty to forty years. Erythema induratum was the most common clinical condition, affecting mainly the lower limbs, in contrast to other Brazilian studies that found scrofuloderma as the most common manifestation, predominating in the cervical region of male children and adolescents.
KEYWORDS: Tuberculosis, Cutaneous tuberculosis, Mycobacterium tuberculosis, Epidemiology, Tuberculid
INTRODUCTION
Tuberculosis occurs worldwide and in 2014 there were 9.6 million cases and 1.4 million deaths 1 . In Brazil, the incidence of tuberculosis was 36.7 cases/year per 100,000 inhabitants and the mortality rate was 2.4 cases/year per 100,000 inhabitants 2 . The State of Espírito Santo had similar national annual incidence (35.2) and its capital city, Vitória, had 45.3 cases/year per 100,000 inhabitants 2 . Cutaneous tuberculosis represented 1 to 2% of the tuberculosis cases and 0.1 to 1% of all cutaneous disorders 3 , 4 .
Cutaneous tuberculosis is an infectious disease mainly caused by M. tuberculosis, having exogenous, endogenous, or autoinoculation pathways of skin penetration 5 , 6 . The clinical types depend on the route of infection, virulence of the bacillus, and the immune status of the host, in particular, the cellular immunity7,8 . The classification systems of cutaneous tuberculosis are based on the propagation mechanism proposed by Tappeiner and Wolff, dividing the infection into endogenous and exogenous, and the bacterial load as described by Ridley and Jopling for leprosy, subdividing the cases into multibacillary and paucibacillary 4 , 5 , 6 , 7 . The clinical forms of most interest in Brazil are:
a) Scrofuloderma: is the most common in Brazil among children and adolescents and is associated with active pulmonary tuberculosis 3 , 8 - 10 . This type manifests as fistulizing erythematous brown nodules that discharge caseous and purulent material, resulting in retracted scars 6 . It occurs in the submandibular, cervical, supraclavicular, axillary, and inguinal regions 8 . The histopathological analysis shows necrosis, abscesses, tuberculoid granulomas, and the presence of gram-positive bacilli 10 , 11 .
b) Acute miliary tuberculosis: is a rare variant occurring in human immunodeficiency virus (HIV-positive) patients with CD4 counts below 100 cells/µL 5 . It occurs in disseminated tuberculosis with pulmonary or meningeal focuses 5 . Cutaneous lesions are multiple and they include pustules, macules, erythematous papules, etc., evolving to atrophic scars, mainly in the thorax 4 , 7 . The histopathological analysis exhibits necrosis foci and nonspecific inflammatory infiltrate in the dermis, without caseous granulomas, and bacilli around and within blood vessels 7 .
c) Tuberculid: results from cellular immunity to extra-cutaneous antigens of M. tuberculosis released periodically in the blood circulation 5 , 11 . It is subdivided into papulo-necrotic tuberculid, erythema induratum of Bazin and other less frequent types 6 , 7 , 11 .
c1) Papulo-necrotic tuberculids: usually occurs in children and adolescents 7 , 11 . The lesions are erythematous papules, symmetric, multiple, with a central necrotic umbilication, covered with crust and subsequently with varicelliform scars. Active lesions alongside the scars help in the diagnosis 6 , 7 , 11 and they are often observed in the extensor surfaces of the limbs 6 , 11 . The histopathology analysis shows necrosis of the epidermis and dermis, nonspecific inflammatory infiltrate or tuberculoid granulomas, granulomatous vascular lesions with thrombus formation, and absence of bacilli 6 , 11 .
c2) Erythema induratum of Bazin: is the most reported form of tuberculid in the literature. It is common in women with active pulmonary diseases 4 , 11 - 13 , resulting from the interaction of rare bacilli present in blood and from circulatory conditions of the patients 5 . From a clinical point of view, multiple, chronic, painful, recurrent, and ulcerated violaceous nodules appear in the posterior portion of the legs, which involute slowly, causing hyperpigmentation or atrophy4,6,11-13 . The histopathology analysis must find three out of four elements: lobular panniculitis; fat necrosis; vasculitis and granuloma; and poorly visualized acid-fast bacilli 4 , 12 , 13 .
d) Other forms of cutaneous tuberculosis: erythema nodosum, which has several etiologies, including M. tuberculosis and occurring more commonly in women in whom the lesions are erythematous, painful, non-ulcerated subcutaneous nodules that occur in the legs, with spontaneous resolution in eight weeks without scarring 6 . It is also characterized by septal panniculitis without vasculitis according to some histopathologic features 13 .
Diagnosis of cutaneous tuberculosis is based on clinical-epidemiological findings; purified protein derivative (PPD) results; suggestive cutaneous histopathology and bacilloscopy; culture and results of polymerase chain reaction (PCR) performed on cutaneous fragments; immunological test results; assessment of extra-cutaneous outbreaks; and, sometimes, therapeutic trials 3 , 5 , 6 , 11 .
The treatment of cutaneous tuberculosis consists of a six-month basic scheme comprising isoniazid, rifampin and pyrazinamide on a daily basis with ethambutol or streptomycin administered for eight weeks 14 . Desensitization with diluted plain tuberculin decreases hyperergic reactions and improves the clinical condition. It is used for all tuberculids or after chemotherapy treatment 15 .
In order to investigate the number of cases, clinical and epidemiological data, and the manifestations of cutaneous tuberculosis in a reference center, a study was conducted at the Dermatology and Tuberculosis services of the Cassiano Antonio Moraes University Hospital (HUCAM), in the city of Vitória, State of Espírito Santo, Brazil, from 1986 to 2011.
MATERIALS AND METHODS
This is a retrospective, descriptive, observational and cross-sectional study using data from the healthcare provided at the Dermatology and Pulmonology services of the HUCAM. The cases of cutaneous tuberculosis of the Dermatology Service were retrieved from the analysis of the non-electronic medical records of dermato-pathological weekly sessions held from November 1986 to July 2011. The other individuals were retrieved from the analysis of the written records of the Tuberculosis Program, Pulmonology Service. These cases were classified as other forms of tuberculosis, including cutaneous tuberculosis, and they were recorded from January 1999 to July 2011.
The medical records containing the clinical, epidemiological and demographic variables, laboratory tests and therapies were retrieved from non-electronic records of the HUCAM. All data from the Dermatology and Pulmonology services were collected from spreadsheets filed in both services and from medical records, in addition to the results of laboratory tests and the ones from the Pathology Service of HUCAM. The histopathological slides of the cutaneous lesions were reviewed by the pathologist and the researchers in the Pathology Service of the HUCAM and the abnormalities were subsequently recorded. The assessments of M. tuberculosis by culture analysis and by PCR after analysis of the skin biopsy were not routinely performed at the HUCAM at the time of this study.
The inclusion criteria used for the analysis of the records from the two services (Dermatology and Pulmonology) were related to the diagnosis of cutaneous tuberculosis, considering the following findings: (a) Clinical, epidemiological and histopathological abnormalities after hematoxylin-eosin staining and bacilloscopy after Ziehl-Neelsen staining; and (b) In other cases, further assessment was performed, such as: PPD; culture of skin with M. tuberculosis infection; assessment of tuberculosis in other organs using sputum bacilloscopy and culture; chest and bones X-rays; chest tomography; abnormal elements and urine sediment examination; urine culture; urography; fine-needle aspiration biopsy and lymph node biopsy; myelogram; soft tissue and abdominal ultrasound data; and therapeutic response to tuberculosis.
Variables were analyzed using the Statistical Package for the Social Sciences (SPSS) software, version 17.0, determining the absolute and relative frequencies for nominal, ordinals and dichotomous variables, as well as central index and dispersion measures for the dimensional variables. The research was approved by the Research Ethics Committee of the Health Sciences Center of the Federal University of Espírito Santo, Brazil.
RESULTS
A total of 5,875 patients attended the dermato-pathology sessions at the Dermatology Service with a confirmed diagnosis of cutaneous tuberculosis in 18 (0.3%) of them. Their medical records were retrieved and the data collected. Patients from the Tuberculosis Program of the Pulmonology Service treated from January 1999 to July 2011 totaled 2,510 patients, among whom 157 (6.2%) were diagnosed with other forms of tuberculosis (cutaneous, osteoarticular, laryngeal, pericardial, etc.). Regarding their medical records, 129 (82.2%) were retrieved and 28 were not located. From the total of 2,510 patients, 11 (0.44%) had clinical and pathological diagnosis of cutaneous tuberculosis.
This study enrolled 29 cases (0.35% of the 8,385). The median age was 35 years, the average age was 37 years, and the standard deviation was 15 years and nine months, ranging from a minimum of 10 to a maximum of 81 years. Eighteen (62%) patients were women, of which 20 (69%) were white, seven (24.1%) had mixed ethnicity (black and white), and two (6.9%) were black.
With respect to the occupation, nine (31.1%) patients were housewives, five (17.3%) were business women, four (13.8%) were farmers, three (10.3%) were health workers, two (6.9%) in each group of retirees were bricklayers and general services assistants, one (3.4%) was a student, and one (3.4%) was a teacher. Regarding the social habits, there were five (50%) cigarette smokers among ten analyzed medical records and three (33.3%) alcoholic patients out of nine medical records.
Two (14.3%) patients were HIV-positive according to 14 medical records containing this information. In addition, one hepatitis C-positive patient received immunosuppressant therapy due to a kidney transplantation; one patient had the Alport syndrome and was also submitted to a renal transplantation and immunosuppressant therapy; and another patient was diagnosed with Takayassu's arteritis. According to the 29 medical records, three (10.3%) patients had had contact with patients infected with M. tuberculosis. History of previous tuberculosis was found in three (10.3%) patients, one of them reported pulmonary tuberculosis, one bone tuberculosis, and one reported cutaneous tuberculosis. All of them did receive treatment.
The clinical forms of cutaneous tuberculosis were: 12 (41.4%) patients had erythema induratum of Bazin; six (20.8%) had scrofuloderma; three (10.3%) had erythema nodosum related to tuberculosis; three (10.3%) had non-classifiable forms; two (6.9%) had papulo-necrotic tuberculides; two (6.9%) had acute miliary tuberculosis; and one (3.4%) had erythema induratum of Bazin and papulo-necrotic tuberculides.
In 19 (65.8%) cases, cutaneous lesions were located in the lower limbs, three (10.3%) cases had disseminated lesions, three (10.3%) cases were located in the upper limbs, and there was one (3.4%) case with lesions in each of the following sites: neck, thorax, limbs, and genitalia, as shown in Figure 1. Extra-cutaneous tuberculosis was present in eight (27.6%) patients, three (37.5%) of them with involvement of lymph nodes, two (25%) with pulmonary tuberculosis, two (25%) with bone tuberculosis, and one (12.5%) with renal tuberculosis associated to enlarged renal lymph nodes, as shown in Table 1. A total of 19 medical records had information regarding PPD; in 12 cases (63.2%) they were strong reactors, four (21%) were non-reactors, and three (15.8%) were low reactors.
Fig. 1. Correlation of the clinical forms of cutaneous tuberculosis according to the location of the cutaneous lesions, in the patients of the study.
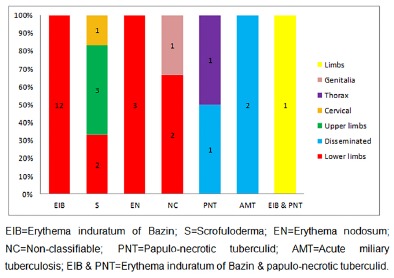
Table 1. Correlation of the clinical forms of cutaneous tuberculosis with the extracutaneous areas affected in the patients of the study.
| Extracutaneous areas | Erythema induratum of Bazin | Scrofuloderma | Erythema nodosum | Non- classifiable | Papulo-necrotic tuberculid | Acute miliary tuberculosis | Erythema induratum of Bazin and papulo-necrotic tuberculid | Total |
| Lymph nodes | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 3 |
| Pulmonary | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Bone | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| Renal and lymph nodes | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total | 0 | 2 | 0 | 2 | 2 | 1 | 1 | 8 |
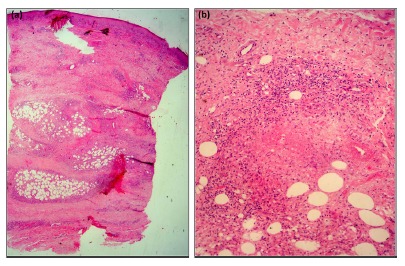
The histopathological analysis of 35 biopsies showed the presence of hypodermis containing chronic granulomatous inflammation and caseous necrosis in the lobular and/or septal region, with or without nodular vasculitis (Fig. 2) in 18 (51.4%) patients; chronic granulomatous inflammation and caseous necrosis in 14 (40%) patients; nonspecific reaction including necrosis and abscesses, but with no granulomas presenting central necrosis, in three (8.6%) patients.
Fig. 2. A patient's histopathological examination showing lobular hypodermitis and nodular vasculitis. (a) Image with lower magnification (H&E, x40). (b) Image with higher magnification (H&E,x400).
Bacilloscopy performed in cutaneous histopathological specimen was positive in one (2.9%) patient. M. tuberculosis culture was performed using samples of cutaneous lesion biopsies in nine patients, and two (22.2%) positive results were found.
A refinement of some variables assessed in this study can be observed in Table 2. Regarding treatment, 27 (93.1%) patients had undergone the basic scheme, and two (6.9%) patients had undergone monotherapy with isoniazid and tuberculin desensitizing therapy. The recurrence of the cutaneous lesions after treatment took place in three patients.
Table 2. Correlation of the clinical forms of cutaneous tuberculosis and some items assessed in the patients of the study.
| Assessed Items | Erythema induratum of Bazin | Scrofuloderma | Erythema nodosum | Non-classifiable | Papulo-necrotic tuberculid | Acute miliary tuberculosis | Erythema induratum of Bazin and papulo-necrotic tuberculid | Total |
| Number of patients (%) | 12 (41.4) | 6 (20.8) | 3 (10.3) | 3 (10.3) | 2 (6.9) | 2 (6.9) | 1 (3.4) | 29 (100) |
| Average age | 37 | 49 | 38 | 37 | 21 | 27.5 | 25 | 37 |
| Sex | ||||||||
| Male | 1 | 3 | 2 | 2 | 1 | 2 | 0 | 11 |
| Female | 11 | 3 | 1 | 1 | 1 | 0 | 1 | 18 |
| Immunosuppressed patients | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 4 |
| Extracutaneous tuberculosis | 0 | 2 | 0 | 2 | 2 | 1 | 1 | 8 |
| PPD (mm) | ||||||||
| < 5 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 4 |
| 5-9 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| > 9 | 3 | 3 | 3 | 0 | 2 | 0 | 1 | 12 |
| Not recorded | 8 | 1 | 0 | 0 | 0 | 1 | 0 | 10 |
| Positive bacilloscopy | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Culture | ||||||||
| Positive | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| Negative | 2 | 2 | 0 | 2 | 0 | 1 | 0 | 7 |
| Not recorded | 10 | 3 | 3 | 0 | 2 | 1 | 1 | 20 |
DISCUSSION
This study was the first clinical and epidemiological analysis of cutaneous tuberculosis in the State of Espírito Santo, Brazil. The scarce literature addressing this topic in Brazil contained a total of 105 cases found in the review of the literature regarding the national reference journal of dermatology (Brazilian Annals of Dermatology) from the beginning of the journal (1912) to the present day9,16-30 .
The frequency of cutaneous tuberculosis in the State of Espírito Santo between 2001 (beginning of the compulsory notification of cutaneous forms) and 2010 was 0.07% with respect to the total number of tuberculosis cases (data from the Espírito Santo State Department of Health), below the number of cases confirmed in the Pulmonology Service of the HUCAM in the same period totaling eight (0.42%) cases of cutaneous tuberculosis among 1,905 patients with tuberculosis, suggesting a possible lack of compulsory notification of cases in the State.
The percentage of cutaneous tuberculosis with respect to the clinical forms of tuberculosis was small, differing from the data of other authors, who found percentages between 2.4 and 4.8% 31 - 35 , as seen in Table 3.
Table 3. Studies on cutaneous tuberculosis assessed showing percentages among cutaneous disorders and clinical forms of tuberculosis with respect to the places surveyed.
| Authors (references) | % of cutaneous tuberculosis among cutaneous disorders | % of cutaneous tuberculosis among clinical forms of tuberculosis |
| Diógenes et al. 9 | 0.7 | Absent |
| Bopp et al. 21 | 0.12 | Absent |
| Fariña et al. 32 | 0.14 | 2.4 |
| Goyal et al. 33 | 0.7 | 3 |
| Kivanç-Altunay et al. 34 | Absent | 3.5 |
| Yates et al. 35 | Absent | 4.4 |
| García-Rodríguez et al. 36 | Absent | 4.8 |
| Kumar et al. 37 | 0.1 | Absent |
| Umapathy et al. 38 | 0.12 | Absent |
| Dwari et al. 39 | 0.12 | Absent |
| Hazarika et al. 40 | 0.25 | Absent |
| Terranova et al. 41 | 0.7 | Absent |
| Hamada et al. 42 | 0.034 | Absent |
| Ho et al. 43 | 0.04 | Absent |
| Chong et al. 44 | 0.066 | Absent |
| Zouhair et al. 45 | 2 | Absent |
Among skin diseases treated at the Dermatology Service of the HUCAM, cutaneous tuberculosis represented 0.3%, corroborating the data obtained by some authors 21 , 31 , 32 , 36 - 40 , and diverging from others 9 , 41 - 44 . The difference in percentages may be related to the characteristics of the studied places, namely socioeconomic and sanitary, health control system, vaccination coverage, individual habits, immunological status of the patients, and number of immigrants from endemic regions 3 - 7 , 14 , 45 . Differences may also be attributed to diagnostic difficulties requiring additional tests which are not always available.
The average age of most patients was similar to that found in studies conducted by Azulay & Serra 17 , Nascimento et al. 23 and Ranawaka et al. 46 However, the studies on cutaneous tuberculosis show a variation in the ages of the most affected. This difference can rely on whether or not neonates have received the Bacille Calmette-Guérin (BCG) vaccine as a prophylactic measure, thus generating a secondary immune response or postponing the onset of disease 40 .
Women were the most affected group in this study, corroborating data described in other Brazilian studies 9 , 22 - 26 , 28 , 29 , probably because the most observed clinical form was the erythema induratum of Bazin.
Cutaneous tuberculosis was more frequent among white individuals, corroborating data of other Brazilian studies 9 , 17 , 19 , 20 , 22 - 24 , 27 - 30 ; however, it is most often found among Asians and black Africans, possibly due to socioeconomic conditions rather than ethnic differences 47 .
Regarding occupation, housewives corresponded to almost a third of the cases, but the comparison of this datum was hindered by the lack of information on this topic in the literature. Thakur et al. 39 reported that 73.8% of patients in India worked in the production of tea, and Bhutto et al. 48 found that 90% of patients were farmers in Pakistan.
Among the cases enrolled according to their medical records, one-half of the patients were smokers and one-third of them were alcoholics. These are high percentages compared to 21.8% of smokers and 7.4% of alcoholic patients found by García-Rodríguez et al. 35 Smoking and drinking are habits that cause impairment of the immunological system 3 , 6 , 7 , 14 , favoring the emergence of tuberculosis.
The frequency of HIV-positive patients in the study was similar to that found by Fariña et al. (18.2%) in Spain 31 and Terranova et al. (22%), in Ethiopia 40 . It is worth noting that the percentage of HIV-positive individuals can be higher, because the serological test for the detection of the virus was not available at the beginning of the studied period (1986) and also taking into consideration that Brazil has a high prevalence of HIV-positive individuals.
The two immune deficient patients that were receiving immunosuppressive drugs after organ transplantation, as well as the two HIV-positive patients exhibited a more severe clinical form of the disease and a more difficult diagnosis. Therefore, research on immunosuppression in patients with cutaneous tuberculosis is essential, because cutaneous infection should be considered as a differential diagnosis in immunosuppressed patients with certain skin lesions.
Contact with tuberculosis patients had occurred in 10.3% of the patients, corroborating data obtained by Terranova et al. (18%) 40 . History of tuberculosis before the diagnosis of the cutaneous form can occur in some patients, as observed in the present study and the study conducted by Terranova et al. (18.3%) 40 . This fact could possibly result from a deficient disease control system that failed in tracking cases after the end of treatment.
The most common clinical form observed in the present study was the erythema induratum of Bazin, corroborating some Asian studies 41 - 43 . The explanation for this finding in the present study refers to the predominance of middle-aged women, with professional occupation that requires upright posture, since these factors are related to deficient circulatory conditions of the lower limbs, favoring the deposition of M. tuberculosis antigens, and stimulating immune reactions in the lower limbs in already sensitized (positive tuberculin test) individuals, as in the majority of the studied cases. In Brazil, Diógenes et al.9 diagnosed scrofuloderma as the most frequent type of cutaneous tuberculosis. The small number of patients with scrofuloderma found in this study, contrasting with data reported in the literature, can be explained by cultural changes, improved sanitation (consumption of pasteurized milk) and socioeconomic conditions, immunological differences due to host genetic factors, BCG vaccination coverage, and standardized treatment 8 , 40 . Another explanation for the national scenario would be the preference for publishing the rarest cases (tuberculosis gummas, erythema induratum of Bazin, lupus vulgaris) rather than the most common type (scrofuloderma), as seen in the reports of the Brazilian Annals of Dermatology 9 , 16 - 30 .
The most frequent site of the lesions was the lower limbs, being related to the most frequent clinical form found in this study, i.e., erythema induratum of Bazin, which requires deficient circulatory conditions of the legs for its emergence 5 .
One-third of the patients had cutaneous tuberculosis associated with extra-cutaneous tuberculosis, and lymph nodes were the most affected area, similar to data found by Bhutto et al. (22.2%) 48 . However, other studies found a higher frequency, such as those conducted by Terranova et al. (80.7%) 40 and Thakur et al. (52.4%) 39 . These results reflect the importance of conducting further research on other foci in the case of cutaneous tuberculosis, since they may be responsible for the persistence of cutaneous manifestations, transmission, and thus, the morbidity and mortality caused by this disease 45 .
In most assessed cases, positive PPD, negative bacilloscopy and culture exams matched the clinical form most frequently found, i.e., paucibacillary tuberculosis. Thakur et al. 39 submitted 42 patients to PPD tests, most of them being reactors, and also to cutaneous biopsies followed by histopathologic examination of the lesions, with no evidence of bacilli. These results are consistent with the predominant form, i.e., scrofuloderma.
Most of the patients submitted to the histopathological study of cutaneous lesions revealed the presence of chronic granulomatous, the most characteristic finding of this disease, corroborating data found in studies conducted in Asian countries and Spain 32 , 35 , 37 - 39 , 46 , 48 - 50 .
Most patients in this study had undergone basic treatment with good response, similar to data found by Ranawaka et al. 46 and Varshney et al. 32 . However, some patients had only undergone monotherapy with isoniazid or in association with the desensitizing therapy. Currently, these procedures are not recommended due to the possibility of drug resistance. In addition, this scheme is not adequate to treat the visceral forms sometimes associated to cutaneous involvement, even though these therapies had been used by the time the patients were treated, as observed in the studies conducted by Brown et al. 51 and Chong et al. 43 .
In conclusion, the present study found 0.44% of cutaneous forms among the clinical manifestations of tuberculosis and 0.3% among cutaneous diseases. Erythema induratum of Bazin was the most common skin manifestation, affecting the lower limbs and occurring mainly in middle-aged women. There was an extra-cutaneous involvement of tuberculosis in almost one-third of the individuals. According to the PPD tests, most patients were reactors, whereas the bacilloscopy was negative in nearly all of the histopathological tests.
Among the limitations in the present study, it can be observed that the exams to confirm the diagnosis were deficient in some respects, primarily the lack of data related to the bacillus culture and PCR tests performed on cutaneous fragments. On the other hand, data from patients treated in different years and examinations conducted by several professionals have limited the study. In addition, data collection was based on only one medical center, thus totaling a small sample, even though it was a reference institution for tuberculosis in the State of Espírito Santo.
The present study provides epidemiological data of cutaneous tuberculosis in the State of Espirito Santo, which has a high prevalence of tuberculosis in Brazil; identifies the most frequent clinical form of cutaneous tuberculosis, contrasting with the national literature data, and calls the attention of the healthcare professionals that they should improve the propaedeutics of a neglected disease that is prevalent worldwide.
ACKNOWLEDGMENTS
We are thankful to the patients and healthcare professionals of the Cassiano Antonio de Moraes University Hospital for their cooperation in the present research.
REFERENCES
- 1.World Health Organization . Global tuberculosis report. Geneva: WHO; 2015. [Google Scholar]
- 2.Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária . Série histórica da taxa de incidência de tuberculose. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 3.Almaguer-Chávez J, Ocampo-Candiani J, Rendón A. Panorama actual en el diagnóstico de la tuberculosis cutánea. Actas Dermosifiliogr. 2009;100:562–570. [PubMed] [Google Scholar]
- 4.Concha RM, Fich SF, Rabagliati BR, Pinto SC, Rubio LR, Navea DO. Tuberculosis cutánea reporte de dos casos y revisión de la literatura. Rev Chil Infectol. 2011;28:262–268. [PubMed] [Google Scholar]
- 5.Frankel A, Penrose C, Emer J. Cutaneous tuberculosis a pratical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19–27. [PMC free article] [PubMed] [Google Scholar]
- 6.Lai-Cheong JE, Perez A, Tang V, Martinez A, Hill V, Menagé HP. Cutaneous manifestations of tuberculosis. Clin Exp Dermatol. 2007;32:461–466. doi: 10.1111/j.1365-2230.2007.02352.x. [DOI] [PubMed] [Google Scholar]
- 7.Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis diagnosis and treatment. Am J Clin Dermatol. 2002;3:319–328. doi: 10.2165/00128071-200203050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Santos JB, Figueiredo AR, Ferraz CE, Oliveira MH, Silva PG, Medeiros VLS. Tuberculose cutânea aspectos epidemiológicos, etiopatogênicos e clínicos. Parte I. An Bras Dermatol. 2014;89:219–228. doi: 10.1590/abd1806-4841.20142334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diógenes MJN, Meireles TEF, Cabral SEX, Carvalho FF, Silva MAB, Almeida EP. Tuberculose cutânea avaliação retrospectiva (1981 a 1990) An Bras Dermatol. 1996;71:107–113. [Google Scholar]
- 10.Sethuraman G, Ramesh V, Ramam M, Sharma VK. Skin tuberculosis in children learning from India. Dermatol Clin. 2008;26:285–294. doi: 10.1016/j.det.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173–180. doi: 10.1016/j.clindermatol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Mascaró JM, Jr, Baselga E. Erythema induratum of Bazin. Dermatol Clin. 2008;26:439–445. doi: 10.1016/j.det.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis) diagnosis and management. Dermatol Ther. 2010;23:320–327. doi: 10.1111/j.1529-8019.2010.01332.x. [DOI] [PubMed] [Google Scholar]
- 14.Handog EB, Gabriel TG, Pineda RTV. Management of cutaneous tuberculosis. Dermatol Ther. 2008;21:154–161. doi: 10.1111/j.1529-8019.2008.00186.x. [DOI] [PubMed] [Google Scholar]
- 15.Pereira JCB. Análise comparativa entre tubercúlides e tuberculose extrapulmonar uma outra face do Mycobacterium tuberculosis. Rev Port Pneumol. 2008;14:391–407. [PubMed] [Google Scholar]
- 16.Braga RV. Tuberculose verrucosa atípica. An Bras Dermatol. 1951;26:29–33. [PubMed] [Google Scholar]
- 17.Azulay RD, Serra O. Caso de lúpus vulgar. An Bras Dermatol. 1954;29:29–32. [PubMed] [Google Scholar]
- 18.Azulay RD, Azulay JD. Primeiro caso de lúpus vulgar em paciente do nordeste brasileiro. An Bras Dermatol. 1955;30:195–202. [PubMed] [Google Scholar]
- 19.Guimarães NA. Lúpus vulgar na Bahia. An Bras Dermatol. 1955;30:243–254. [PubMed] [Google Scholar]
- 20.Zamith V de A. Lúpus vulgar angiomatoso. An Bras Dermatol. 1960;35:28–33. [Google Scholar]
- 21.Bopp C, Bernardi CDV, Müller P, Bakos L, Gervini RI, Kosminski B. Análise interpretativa das dermatoses mais frequentes em Porto Alegre, Rio Grande do Sul, Brasil. An Bras Dermatol. 1973;48:117–132. [Google Scholar]
- 22.Leão AL, Barros MJ, Rocha GL. Lupus vulgaris. An Bras Dermatol. 1981;56:45–47. [Google Scholar]
- 23.Nascimento do LV, Azulay RD, Neves RG, Rabello FE. Tuberculose cutânea indurativa de Bazin. An Bras Dermatol. 1983;58:147–152. [Google Scholar]
- 24.Wanke NCF. Eritema nodoso de etiologia obscura. An Bras Dermatol. 1984;59:97–99. [Google Scholar]
- 25.Ramos e Silva M, Marques A de S, Rocha GL. Tuberculose cutânea associada à tuberculose osteoarticular. An Bras Dermatol. 1986;61:245–250. [Google Scholar]
- 26.Fernandes NC, Fernandes L, Bonat EF. Eritema nodoso revisão de 72 casos. An Bras Dermatol. 1989;64:23–24. [Google Scholar]
- 27.Hinrichsen SL, Moura LV, Arraes LC, Reis L, Lamprea D, Gava R. Tuberculose cutânea e AIDS relato de caso. An Bras Dermatol. 1996;71:511–514. [Google Scholar]
- 28.Duquia RP, Almeida HL de, Junior, Duvelius ES, Wolter M. Caso para diagnóstico. An Bras Dermatol. 2006;81:490–492. [Google Scholar]
- 29.Martins EV, Junior, Marques BP, Reis ET, Neto, Lima BCMLS, Neumann YRB. Tuberculose cutânea disseminada com escrofuloderma associado à tuberculose de arco costal. An Bras Dermatol. 2007;82:343–347. [Google Scholar]
- 30.Ljubenovic MS, Ljubenovic DB, Binic II, Jankovic AS, Jancic SA. Cutaneous tuberculosis and squamous-cell carcinoma. An Bras Dermatol. 2011;86:541–544. doi: 10.1590/s0365-05962011000300017. [DOI] [PubMed] [Google Scholar]
- 31.Fariña MC, Gegundez MI, Piqué E, Esteban J, Matín L, Requena L. Cutaneous tuberculosis: a clinical, histopathologic and bacteriologic study. J Am Acad Dermatol. 1995;33:433–440. doi: 10.1016/0190-9622(95)91389-0. [DOI] [PubMed] [Google Scholar]
- 32.Varshney A, Goyal T. Incidence of various clinico-morphological variants of cutaneous tuberculosis and HIV concurrence a study from Indian subcontinent. Ann Saudi Med. 2011;31:134–139. doi: 10.4103/0256-4947.77495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kivanç-Altunay I, Baysal Z, Ekmekçi TR, Köslü A. Incidence of cutaneous tuberculosis in patients with organ tuberculosis. Int J Dermatol. 2003;42:197–200. doi: 10.1046/j.1365-4362.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- 34.Yates VM, Ormerod LP. Cutaneous tuberculosis in Blackburn district (U K.): a 15-year prospective series, 1981-95. Br J Dermatol. 1997;136:483–489. [PubMed] [Google Scholar]
- 35.García-Rodríguez JF, Monteagudo-Sánchez B, Mariño-Callejo A. Tuberculosis cutânea estudio decriptivo de 15 años. Enferm Infecc Microbiol Clin. 2008;26:205–211. doi: 10.1016/s0213-005x(08)72692-2. [DOI] [PubMed] [Google Scholar]
- 36.Kumar B, Rai R, Kaur I, Sahoo B, Muralidhar S, Radotra B. Childhood cutaneous tuberculosis: a study over 25 years from northern India. Int J Dermatol. 2001;40:26–32. doi: 10.1046/j.1365-4362.2001.01165.x. [DOI] [PubMed] [Google Scholar]
- 37.Umapathy KC, Begum R, Ravichandran G, Rahman F, Paramasivan CN, Ramanathan VD. Comprehensive findings on clinical, bacteriological, histophatological and therapeutic aspects of cutaneous tuberculosis. Trop Med Int Health. 2006;2:1521–1528. doi: 10.1111/j.1365-3156.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 38.Dwari B, Ghosh A, Paudel R, Kishore P. A clinicoepidemiological study of 50 cases of cutaneous tuberculosis in a tertiary care teaching hospital in Pokhara, Nepal. Indian J Dermatol. 2010;55:233–237. doi: 10.4103/0019-5154.70670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thakur B, Verma S, Hazarika D. A clinicopathological study of cutaneous tuberculosis at Dibrugarh district, Assam. Indian J Dermatol. 2012;57:63–65. doi: 10.4103/0019-5154.92685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terranova M, Padovese V, Fornari U, Morrone A. Clinical and epidemiological study of cutaneous tuberculosis in Northern Ethiopia. Dermatology. 2008;217:89–93. doi: 10.1159/000128284. [DOI] [PubMed] [Google Scholar]
- 41.Hamada M, Urabe K, Moroi Y, Miyazaki M, Furue M. Epidemiology of cutaneous tuberculosis in Japan a retrospective study from 1906 to 2002. Int J Dermatol. 2004;43:727–731. doi: 10.1111/j.1365-4632.2004.02238.x. [DOI] [PubMed] [Google Scholar]
- 42.Ho CK, Ho MH, Chong LY. Cutaneous tuberculosis in Hong Kong an update. Hong Kong Med J. 2006;12:272–277. [PubMed] [Google Scholar]
- 43.Chong LY, Lo KK. Cutaneous tuberculosis in Hong Kong a 10-year retrospective study. Int J Dermatol. 1995;34:26–29. doi: 10.1111/j.1365-4362.1995.tb04372.x. [DOI] [PubMed] [Google Scholar]
- 44.Zouhair K, Akhdari N, Nejjam F, Ouazzani T, Lakhdar H. Cutaneous tuberculosis in Marroco. Int J Infect Dis. 2007;11:209–212. doi: 10.1016/j.ijid.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Hay RJ. Cutaneous tuberculosis and the control of infection in resource-poor environments. Dermatology. 2008;217:94–96. doi: 10.1159/000128285. [DOI] [PubMed] [Google Scholar]
- 46.Ranawaka RR, Abeygunasekara PH, Perera E, Weerakoon HS. Clinico-histopathological correlation and the treatment response of 20 patients with cutaneous tuberculosis. Dermatol Online J. 2010;16:13–13. [PubMed] [Google Scholar]
- 47.World Health Organization . Global Tuberculosis Control. Geneva: WHO; 2010. [Google Scholar]
- 48.Bhutto AM, Solangi A, Khaskhely NM, Arakaki H, Nonaka S. Clinical and epidemiological observations of cutaneous tuberculosis in Larkana, Pakistan. Int J Dermatol. 2002;41:159–165. doi: 10.1046/j.1365-4362.2002.01440.x. [DOI] [PubMed] [Google Scholar]
- 49.Hsiao PF, Tzen CY, Chen HC, Su HY. Polymerase chain reaction based detection of Mycobacterium tuberculosis in tissues showing granulomatous inflammation without demonstrable acid-fast bacilli. Int J Dermatol. 2003;42:281–286. doi: 10.1046/j.1365-4362.2003.01461.x. [DOI] [PubMed] [Google Scholar]
- 50.Puri N. A clinical and histopathological profile of patients with cutaneous tuberculosis. Indian J Dermatol. 2011;56:550–552. doi: 10.4103/0019-5154.87153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown FS, Anderson RH, Burnett JW. Cutaneous tuberculosis. J Am Acad Dermatol. 1982;6:101–106. doi: 10.1016/s0190-9622(82)80205-3. [DOI] [PubMed] [Google Scholar]


