SUMMARY
Schistosomiasis remains one of the most prevalent neglected tropical diseases especially in Nigeria which has the greatest number of infected people worldwide. A cross-sectional study was conducted among 551 participants from Kano State, North Central Nigeria. Fecal samples were examined for the presence of Schistosoma mansoni eggs using the formalin-ether sedimentation method while the urine samples were examined using the filtration technique for the presence of S. haematobium eggs. Demographic, socioeconomic and environmental information was collected using a pre-validated questionnaire. The overall prevalence of schistosomiasis was 17.8%, with 8.9% and 8.3% infected with S. mansoni and S. haematobium, respectively and 0.5% presenting co-infection with both species. The multiple logistic regression analysis revealed that age < 18 years (OR = 2.13; 95% CI; 1.34- 3.41), presence of infected family members (OR = 3.98; 95% CI; 2.13-7.46), and history of infection (OR = 2.87; 95% CI; 1.87- 4.56) were the significant risk factors associated with schistosomiasis in these communities. In conclusion, this study revealed that schistosomiasis is still prevalent among Hausa communities in Nigeria. Mass drug administration, health education and community mobilization are imperative strategies to significantly reduce the prevalence and morbidity of schistosomiasis in these communities.
KEYWORDS: Schistosomiasis, Neglected tropical diseases, Prevalence, Risk factors, Nigeria
INTRODUCTION
Schistosomiasis, a parasitic infection caused by digenetic blood trematode worms of the family Schistosomatidae, is one of the most prevalent neglected tropical diseases (NTDs) and still considered as a major public health problem in about 77 developing countries in the tropics and subtropics 1 , 2 . It is estimated that over 240 million people are infected, with about 700 million people worldwide at risk of infection 3 . Over 90% of this infection occurs in sub-Saharan Africa with almost 300,000 deaths annually from schistosomiasis in Africa 4 , 5 .
Schistosomiasis prevalence and morbidity is highest among schoolchildren, adolescents and young adults 6 . Thus, the negative impacts on school performance and the debilitation caused by untreated infections demoralize both social and economic development in endemic areas 5 .
Urogenital schistosomiasis, caused by S. haematobium, is characterized by hematuria, dysuria, bladder wall pathology, hydronephrosis, and it can also lead to squamous cell carcinoma 5 , 7 . In adults, the infection can cause genital ulcers and other lesions 4 resulting in poor reproductive health, with sexual dysfunction and infertility 8 . On the other hand, intestinal schistosomiasis, caused by S. mansoni, presents with bloody diarrhoea and bowel ulceration, chronic infections progressing to hepatomegaly and/or associated with periportal liver fibrosis, portal hypertension, and hematemesis 4 , 5 . Although S. intercalatum can cause another form of intestinal schistosomiasis, its distribution is limited to West and Central Africa 9 .
Nigeria has the greatest number of cases of schistosomiasis worldwide 3 , with about 29 million infected people, among which 16 million are children, and about 101 million people are at risk of schistosomiasis 2 , 6 , 10 , 11 . In 1988, the Federal Ministry of Health (FMOH), in collaboration with the National Schistosomiasis Control Program (NSCP), deliberated on the possibility of bringing down the prevalence by 50% within 5 years in operational areas 12 . However, these efforts were hampered by the lack of baseline data on the distribution of the disease in a broad scale. According to the Nigeria master plan for NTDs 2013-2017, out of the 37 states of Nigeria, mapping and baseline surveys on schistosomiasis have been conducted in a total of 19 states, all located in southern and western parts of Nigeria, so that schistosomiasis has been completely mapped in only 9 of those states 13 . Apart from several reports on the prevalence of schistosomiasis 14 - 18 , there is a scarcity of research on the risk factors associated with this infection in the majority of the federation, particularly in Kano State. This makes intervention and control measures more difficult as such information is crucial to identify and implement effective control measures. Considering this context, the present study has aimed to investigate the prevalence and risk factors of schistosomiasis in Kano State, North Central Nigeria.
MATERIALS AND METHODS
Ethical statement
The present study was carried out according to the guidelines proposed by the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethics Committee of the University of Malaya Medical Centre, Malaysia. Permission was also obtained from Kano State's Ministry of Health, Kano State Hospitals Management Board, the local government authorities and the district heads of communities. When seeking the consent from the research participants in each village, the objectives and procedures of the study were clearly explained to them in the local language, Hausa. Participants were also informed that they could withdraw from the study without any consequences. Thus, written and signed or thumb-printed informed consents were obtained from all the adult participants and guardians/parents of the children before starting the survey, and these procedures were also approved by the ethics committees. All the infected individuals were treated with a single dose of 40 mg/kg body weight of praziquantel under the supervision of a researcher and a medical officer (Direct Observed Therapy) 19 .
Study area
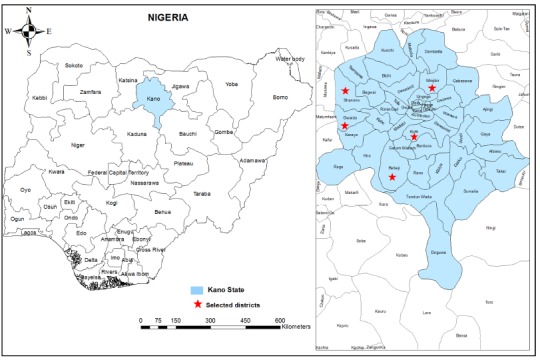
A cross-sectional community-based study was conducted between May and June 2013 among participants aged between one to 90 years old, in five rural areas of Kano State, North Central Nigeria. Five districts namely Kura, Bebeji, Gwarzo, Shanono and Minjibir were randomly selected from the available district list provided by the primary health care personnel and traditional rulers (Fig. 1). The total population of the selected districts ranges from 140,607 people in Shanono to 213,794 people in Minjibir, with a mean of 149,170 people 20 . Kano State (8.5o E and 11.5o N) is the most populous state of the Nigerian Federation with a total population of more than 11 million people and a total area of 20,131 km 2 comprising 1,754,200 hectares dedicated to agriculture and 75,000 hectares of forest vegetation and grazing land 20 . Kano State has been a commercial and agricultural state known for the production of groundnuts and cotton. It is also the second largest industrial center in Nigeria with textile, tanning, footwear, cosmetics, plastic and other industries. Hence, residents of Kano State are predominantly farmers and merchants, and the selected districts have a homogenous population with respect to socio-cultural and daily economic activities. The climate of the study area is the tropical dry-and-wet type which lasts from May to October, typical of the Western African savannah, while the dry season lasts from October to April. The annual mean rainfall is between 800 and 900 mm with a mean annual temperature of about 26 °C 21 .
Fig. 1. A geographic map showing Kano State and the districts involved in the study.
Study population
Before the beginning of the study, the objectives and plan were explained to the heads of the selected villages in order to get their cooperation and permission to conduct the survey. Then, the heads informed all the residents to gather at the school or clinic where they received explanation about the objectives of the survey and their participation. All the residents who agreed voluntarily to participate were included in this study (universal sampling). They received labeled containers and were instructed to bring their stool and urine samples the next day.
A total of 609 individuals had agreed voluntarily to participate in this study and received stool and urine containers. Of them, 551 (90.5%) individuals, aged between one and 90 years, had met the inclusion criteria (written signed consent, completed questionnaire and delivered stool and urine samples for examination). In these communities, children and male adolescents were seen bathing/swimming in the streams and ponds especially at midday. Although toilets were available in almost all of the houses, human and animal excreta were seen around the water bodies and in the farmlands.
Questionnaire survey
A pre-validated questionnaire was applied to the participants in order to collect demographic data (age, gender and family size), socio-economical background (educational level, occupation and household income), behavioural risks (personal hygiene such as hand washing, habit of wearing shoes outside the house and water contact activities), environmental sanitation and living conditions (types of water supply, latrine system, presence of domestic animals, water proximity) and health conditions (history of infection, haematuria). The participants were interviewed by two research assistants who received a specific training on how to apply the questionnaire.
Parasitology
Following the administration of the questionnaire, wide mouth 100 mL screw-capped containers pre-labelled with the participant's name and code were distributed to each participant for the collection of stool and urine samples. The samples were transported within 5 hours of collection in suitable cool boxes at temperatures between 4 and 6 °C for subsequent examination at the Aminu Kano Teaching Hospital, Kano, Nigeria.
All the stool samples were examined using direct smear, formalin-ether sedimentation, and Kato-Katz techniques for the presence of S. mansoni eggs 22 . To determine the worm burden, egg counts were performed and recorded as eggs per gram of faeces (EPG) for positive samples and the intensity of infections was then graded as heavy (≥ 400 EPG), moderate (100-399 EPG) or light (1-99 EPG) according to the criteria proposed by the WHO 19 . Likewise, urine samples were examined for haematuria using a dipstick test (Chuncheon, Korea) 23 , and then examined for the presence of S. haematobium eggs by a sedimentation method previously described by Cheesbrough 22 . Egg counts of S. haematobium were performed and recorded as eggs per 10 millilitres of urine (EP10 mL), and the intensity of infection was graded as heavy (> 50 EP10 mL) or light (1-50 EP10 mL) 19 . In addition, 20% of the samples were re-examined for the presence of Schistosoma eggs by another parasitologist to ensure the quality control.
Data analysis
Data were double-entered by two different researchers into spreadsheets of IBM SPSS Statistics, version 18.0 (IBM Corporation, NY, USA). Then, a third researcher crosschecked the two data sets for accuracy and created a single data set for data analysis. Demographic, socioeconomic, environmental and behavioral characteristics were treated as categorical variables and presented as frequencies and percentages. Egg counts were found not to have a normal distribution, however, there are biological reasons for using the arithmetic mean (± standard deviation (SD) rather than the median or geometric mean to express the egg counts 24 . Pearson's Chi square test was used to examine the associations of infection prevalence with the demographic, socioeconomic, environmental and behavioral factors. Moreover, a multivariable logistic regression analysis was used to identify the risk factors that were significantly associated with infection. For each statistically significant factor, an Odds Ratio (OR) and a 95% confidence interval (CI) were computed by the univariate and multivariate logistic regression analyses. The level of statistical significance was set as p < 0.05.
RESULTS
General characteristics of the respondents
The demographic and socioeconomic characteristics of the participants are shown in Table 1. Five hundred and fifty one individuals (61.7% males and 38.3% females) aged between one and 90 years, with a median age of 25 years (IQR 14-37 years) were enrolled in this study. Of these, 127 (23.0%) were from Kura, 119 (21.6%) reside in Bebeji, 97 (17.6%) in Gwarzo, 99(18.0%) in Shanono and 109 (19.8%) in Minjibir. Overall, 463 (84.0%) of the participants referred at least six years of formal education while only 270 (49.0%) were employed. Accordingly, those with an overall family monthly income of N 32,000 (equivalent to US$ 200) and above were 231 (41.9%). In all the communities, houses are made mostly of mud (78%) or concrete (27%). All the houses had toilets, but most (87.3%) were traditional pit toilets, and about two-thirds of the houses had access to a piped water supply. Moreover, about one-third (38.8%) of the participants claimed that they had a past history of schistosomiasis.
Table 1. Demographic and socioeconomic characteristics of the participants (n = 551).
| Variables | N | % |
| Gender | ||
| Males | 340 | 61.7 |
| Females | 211 | 38.3 |
| Age groups (years) | ||
| ≤ 10 | 56 | 10.2 |
| 11 - 20 | 190 | 34.5 |
| 21 - 30 | 104 | 18.9 |
| 31 - 40 | 89 | 16.1 |
| > 40 | 112 | 20.3 |
| Educational level | ||
| Educated (at least primary education) | 463 | 84.0 |
| Non educated (no formal education) | 88 | 16.0 |
| Household monthly income | ||
| ≥ NGN32000 | 231 | 41.9 |
| < NGN32000 (low) | 320 | 58.1 |
| Family size | ||
| <10 members | 267 | 48.5 |
| ≥ 10 members | 284 | 51.5 |
| Type of toilet in the household | ||
| Pour flush system | 70 | 12.7 |
| Pit (ground dug) | 481 | 87.3 |
| Drinking water | ||
| Safe (piped) | 356 | 64.6 |
| Unsafe | 195 | 35.4 |
| Have contact with a water body | 257 | 50.9 |
| Reasons for water contact | ||
| Swimming | 66 | 25.7 |
| Domestic purposes | 175 | 68.1 |
| Fishing | 13 | 5.1 |
| Waste disposal | 3 | 1.2 |
| Had history of infection | 214 | 38.8 |
| Experienced haematuria | 219 | 39.7 |
| Experienced blood in stool | 217 | 39.4 |
NGN, Nigerian Naira; (US$1 = NGN 165).
Prevalence and distribution of schistosomiasis
Overall, 17.8% (98/551) of the participants were found to be positive for schistosomiasis. Of them, 49 (8.9%) were infected with S. mansoni and 46 (8.3%) were infected with S. haematobium; three (0.5%) had co-infections of both Schistosoma species. Of the 49 S. haematobium-positive individuals, 15 (30.6%) were intensely infected with a mean ± standard deviation (SD) of 98 (19.2) EP 10 mL, while 34 (69.4%) cases were of light intensity with a mean of 32 ± 10.3 EP 10 mL. Likewise, eight (15.4%) and two (3.8%) S. mansoni cases were of moderate and heavy intensity with a mean of 128 ± 10.4 and 458 ± 6.7 EPG, respectively. Moreover, 42 (80.8%) S. mansoni cases were light infections with a mean of 65 ± 24.3 EPG.
Table 2 shows the distribution of schistosomiasis according to age, gender and location. The results showed that those aged 11 - 20 years had the highest prevalence (27.4%) while children aged ≤ 10 years had the lowest prevalence (10.7%) compared to other age groups (p = 0.001). Likewise, the infection rate was significantly higher among males than females (20.6% vs 13.3%; p = 0.029). Regarding the communities, a significant variable prevalence (p < 0.001) was observed, with Gwarzo having the highest (30.9%) prevalence, and the lowest was in found Bebeji (11.8%). According to the participants, the percentage of males who had history of infection and hematuria was significantly higher than the one in females (49.1% vs 22.3%; OR = 3.4; 95% CI; 2.2-5.0). Similarly, the percentage of males who experienced blood in stools was significantly higher than in females (42.4%, 34.6%; OR = 1.4; 95% CI; 1.0-2.0).
Table 2. Prevalence and distribution of schistosomiasis among the participants according to age, gender and location (n = 551).
| Prevalence | No. examined | No. positive | % |
| Overall schistosomiasis | 551 | 98 | 17.8 |
| S. mansoni | 551 | 49 | 8.9 |
| S. haematobium | 551 | 46 | 8.3 |
| Co-infection with both S. mansoni and S. haematobium | 551 | 3 | 0.5 |
| Gender | |||
| Male | 340 | 70 | 20.6 |
| Female | 211 | 28 | 13.3 |
| Age groups (years) | |||
| ≤ 10 | 56 | 6 | 10.7 |
| 11 - 20 | 190 | 52 | 27.4 |
| 21 - 30 | 104 | 15 | 14.4 |
| 31 - 40 | 89 | 11 | 12.4 |
| > 40 | 112 | 14 | 12.5 |
| Location | |||
| Kura | 127 | 20 | 15.7 |
| Bebeji | 119 | 14 | 11.8 |
| Gwarzo | 97 | 30 | 30.9 |
| Shanono | 99 | 21 | 21.2 |
| Minjibir | 109 | 13 | 11.9 |
Risk factors of schistosomiasis
Results of the univariate analysis for the association of schistosomiasis with demographic, socioeconomic, environmental and behavioral factors are presented in Table 3. Besides the significant association of schistosomiasis with age and gender, the results showed that the prevalence of schistosomiasis was significantly higher among those who were not working when compared to the working participants (21.7% vs 13.7%; p = 0.014). Moreover, the presence of other family members infected with schistosomiasis was significantly associated with higher rates of infection (p < 0.001). Likewise, the prevalence of schistosomiasis was significantly higher among those who had a history of infection compared to their counterparts (p < 0.001).
Table 3. Univariate analysis of factors associated with schistosomiasis among the participants (n = 551).
| Variables | Schistosomiasis | OR(95% CI) | P | |
| No. examined | % infected | |||
| Age group | ||||
| Children (< 18 years) | 198 | 23.2 | 1.76 (1.13, 2.73) | 0.012* |
| Adult (≥ 18 years) | 353 | 14.7 | 1 | |
| Gender | ||||
| Male | 340 | 20.6 | 1.70 (1.05, 2.73) | 0.029* |
| Female | 211 | 13.3 | 1 | |
| Educational levels | ||||
| Non educated | 88 | 17.0 | 0.83 (0.43, 1.60) | 0.573 |
| Primary education | 272 | 16.5 | 080 (0.50, 1.29) | 0.355 |
| Secondary/tertiary education | 191 | 19.9 | 1 | |
| Occupational status | ||||
| Not working | 281 | 21.7 | 1.75 (1.12, 2.74) | 0.014* |
| Working | 270 | 13.7 | 1 | |
| Household monthly income | ||||
| < NGN 32,000 (low) | 231 | 18.2 | 1.05 (0.67, 1.63) | 0.836 |
| ≥ NGN 32,000 | 320 | 17.5 | 1 | |
| Family size | ||||
| > 10 members (large) | 284 | 20.4 | 1.46 (0.94, 2.27) | 0.095 |
| ≤ 10 members | 267 | 15.0 | 1 | |
| Type of toilet in house | ||||
| Pit latrine | 481 | 17.3 | 0.77 (0.41, 1.42) | 0.394 |
| Pour flush toilet | 70 | 21.4 | 1 | |
| Source of drinking water | ||||
| Unsafe source (stream, rain, well,..etc) | 195 | 15.9 | 0.82 (0.51, 1.30) | 0.391 |
| Safe source (pipe) | 356 | 18.8 | 1 | |
| Source of household water | ||||
| Unsafe source (stream, rain, well,..etc) | 203 | 16.7 | 0.89 (0.57, 1.41) | 0.627 |
| Safe source (pipe) | 348 | 18.4 | 1 | |
| Water proximity | ||||
| Near (≤ 250 meters) | 380 | 16.6 | 0.77 (0.49, 1.22) | 0.269 |
| Far (> 250 meters) | 171 | 20.5 | 1 | |
| Water contact | ||||
| Yes | 257 | 18.7 | 1.04 (0.66, 1.63) | 0.877 |
| No | 248 | 18.1 | 1 | |
| Presence of domestic animals | ||||
| Yes | 228 | 18.0 | 1.02 (0.66, 1.59) | 0.919 |
| No | 323 | 17.6 | 1 | |
| Presence of infected family member | ||||
| Yes | 55 | 38.2 | 3.36 (1.85, 6.10) | < 0.001* |
| No | 496 | 15.5 | 1 | |
| Wearing shoes when go outside | ||||
| No | 142 | 19.0 | 1.12 (0.68, 1.83) | 0.657 |
| Yes | 409 | 17.4 | 1 | |
| History of schistosomiasis | ||||
| Yes | 214 | 26.6 | 2.62 (1.68, 4.09) | < 0.001* |
| No | 337 | 12.2 | 1 | |
NGN, Nigerian Naira; (US$1 = NGN 165). OR, Odds ratio. CI, Confidence interval. * Significant association (P < 0.05).
Five variables that showed significant associations (p < 0.05) with the prevalence of schistosomiasis were considered for the multiple logistic regression analysis (Table 4). Overall, three variables were retained as the significant risk factors of schistosomiasis among the examined participants. The results confirmed that participants aged below 18 years had higher odds for schistosomiasis when compared to the adult participants by 2.13 times (OR = 2.13; 95% CI; 1.34-3.41). Moreover, the presence of other family members infected with schistosomiasis increased the participants' odds for the infection by almost 4 times (OR = 3.98; 95% CI; 2.13-7.46). Similarly, participants who had history of schistosomiasis had 2.87 higher odds for the infection when compared to their counterparts (OR = 2.87; 95% CI; 1.81- 4.56).
Table 4. Multivariate analysis of factors associated with schistosomiasis among the participants (n = 551).
| Variables | Schistosomiasis | ||
| Adjusted OR | 95% CI | P | |
| Age (< 18 years) | 2.13 | 1.34, 3.41 | 0.002* |
| Gender (male) | 1.01 | 0.59, 1.85 | 0.876 |
| Family size (≥ 10 members) | 1.21 | 0.75, 1.94 | 0.433 |
| Occupational status (not working) | 1.38 | 0.79, 2.42 | 0.264 |
| Presence of infected family member | 3.98 | 2.13, 7.46 | < 0.001* |
| History of schistosomiasis | 2.87 | 1.81, 4.56 | < 0.001* |
OR, Odds ratio. CI, Confidence interval. * Significant key risk factors (P < 0.05).
DISCUSSION
Schistosomiasis remains a major public health problem in many developing countries particularly among rural populations in sub-Saharan Africa. Nigeria is considered as the most endemic country for schistosomiasis, with approximately 29 million infected people and 101 million people at risk of infection 2 , 10 . The present study revealed that the prevalence of schistosomiasis in the study area was 17.8% with no significant difference in the prevalence of urogenital (8.3%) and intestinal schistosomiasis (8.9%). This prevalence is in accordance with other rates reported by previous studies; 11.5% in Adamawa State 25 , 15.3% in Ebonyi State 17 , 17.4% in Oyo State 18 , and 18.7% in Plateau and Nasarawa States of Nigeria 16 . However, higher prevalence rates were reported earlier in the same state, Kano 14 , 15 , 26 . A previous study among 493 school children in the Minjibir local government area of Kano State found that 44.2% of the children were infected with S. haematobium 15 . Another study showed that 50.3% (352/700) of children, aged 5-17 years, were infected with S. mansoni 14 . Moreover, similarly high prevalence of urogenital schistosomiasis was reported among preschool children from Ogun and Benue States, Southern Nigeria 27 , 28 . The lower prevalence reported by the present study could be attributed to the integrated and cost-effective approaches implemented by the Federal Ministry of Health to eliminate multiple NTDs in Nigeria including lymphatic filariasis, onchocerciasis, schistosomiasis, human African trypanosomiasis and leprosy by the year 2020 3 .
On the other hand, lower prevalence rates were reported in other states of Nigeria. An overall schistosomiasis prevalence of 6% was reported in Yobe State, Northeastern Nigeria (10% S. haematobium and 2% S. mansoni) 14 . Similarly, another study from Ogun State reported that the prevalence of S. mansoni and S. haematobium infections was 2.3% and 0.6% respectively 29 . A recent study among 2,064 participants from Anambra State, Nigeria reported that 15.7% of them were infected with S. haematobium while none of the participants was found to be positive for S. mansoni 30 . Moreover, it was shown that schistosomiasis is focally distributed and prevalence rates vary in different communities and locations of Nigeria 28 , 31 , 32 . In this regard, the present study revealed a significantly variation of prevalence rates among the studied communities, with the Gwarzo area having the highest prevalence (30.9%) while the lowest prevalence was found in Bebeji (11.8%). The geographic distribution of each Schistosoma species is closely dependent on the presence of appropriate freshwater snails that serve as the obligatory molluscan hosts. Both genus Bulinus and Biomphalaria are found in Nigeria, with Bulinus having wider distribution and more species, such as B. globosus, B. truncates and B. senegalensis, compared to Biomphalaria 17 , 33 , 34 . Globally, high prevalence rates of urogenital and intestinal schistosomiasis have been reported in other countries in Africa (Tanzania, Ghana and Ethiopia) 10 , 35 , Asia (Philippines) 36 , and Latin America (Brazil) 37 .
Our findings showed that the prevalence of infection was significantly higher among male participants compared to females and this is consistent with previous reports in Nigeria 15 , 17 , 26 , 31 . Likewise, this finding is in agreement with previous studies from Brazil, Yemen, Zanzibar and South Darfur 38 - 41 . By contrast, a significantly higher prevalence of schistosomiasis was reported among females in comparison to males in Ghana 42 . In the present study, we found that males have a more intense exposure to the sources of infection compared to females. Our findings showed that 50.9% of the participants admitted to have contact with a water body, for domestic purposes (68.1%) and swimming (25.7%), and these were the most reported reasons. Moreover, the percentage of male participants who had contact with a water body (swimming) was significantly higher than their female peers (38.0% vs 5.3%; p < 0.001) 43 . This could be attributed to religious and cultural practices. For instance, in Islamic communities, females are not allowed to swim or bathe in the open water sources and also do not participate in fishing and irrigation activities 18 , 44 . Moreover, males were more likely to be knowledgeable of the existence of an open water source in their area compared to females 45 .
Similarly, we found that the prevalence of infection was significantly higher among participants aged below 18 years compared to those aged ≥ 18 years; the highest prevalence rate (27.4%) was reported among those aged 11-20 years, while children aged 10 years and below had the lowest prevalence (10.7%) compared to other age groups. A previous study among 167 preschool children from Ogun State, Southern Nigeria revealed that 58.1% of these children had urogenital schistosomiasis 28 . The control of schistosomiasis in Nigeria consists of a school-based mass drug administration, with an absence of any provision for preschool children. Hence, provision for their treatment should be considered in control programs. In accordance with our findings, previous studies have shown the age-dependent occurrence of schistosomiasis and indicated that the prevalence peak occurs during the adolescence and then decreases slowly 26 , 30 , 46 , 47 . The excessive mobility of adolescents in terms of swimming, bathing and playing in open water could explain the higher prevalence rate in this age group. Moreover, previous studies from Nigeria, Kenya and Malawi reported an increasing trend of infection among children aged 6-13 years with a decline from the age of 14 years 45 , 48 , 49 .
The present study investigated the potential risk factors associated with schistosomiasis among the studied participants and revealed that age < 18 years, presence of infected family members and having history of past Schistosoma infection were the key factors found to be associated with infection in these communities. These findings are in agreement with previous studies from Nigeria 27 , 32 , 50 and other countries 44 , 45 , 51 . Moreover, a previous study from Brazil found that individuals aged between 10-19 years had about seven time higher risks of infection than those aged between 0-10 years 38 . Our findings showed that individuals residing in these communities with the presence of other infected family members conferred a 4-fold higher risk of getting schistosomiasis. A recent study among children in Yemen suggested that infected family members served as a source of infection and the presence of an infected family member may contribute to the transmission of infection among other family members who may have similar water contact exposure and behavior 44 . This factor has also been identified as a significant predictor of intestinal polyparasitism among aboriginal children in rural Malaysia 52 . The occupational risk of schistosomiasis is well documented and considered as a proxy for the nature and intensity of water contact 30 , 53 . A significant association between schistosomiasis and employment status was reported in Brazil and China 54 - 56 . Unemployed individuals might have the responsibility to fetch water for domestic purposes and have more leisure time to go for swimming and other recreational activities; hence, they have more exposure to sources of infection compared to employed individuals. The present study showed that unemployed participants had a higher prevalence of schistosomiasis compared to employed participants, however, this association was not retained by the logistic regression model.
Our findings showed that participants who had a history of schistosomiasis were 2.87 times more likely to be infected compared with individuals that did not have history of schistosomiasis. This could be partially attributed to the clustering of communities with high infection rates around infested water sources, exposing the residents to a higher risk of re-infection 45 , 57 . Moreover, this finding may indicate the poor knowledge of these people on schistosomiasis transmission and prevention. Knowledge, attitude and practices (KAP) of these participants about schistosomiasis have been assessed and previously published 43 . However, the knowledge gained by own account might not be enough to protect these people from infection as the lack of access to safe drinking water and adequate sanitation are the driving forces behind the risk behavior of individual community members 58 . Likewise, chemotherapy for the treatment of schistosomiasis in highly endemic areas does not ensure protection against infection and has not had long-lasting success. Previous studies have reported rapid reinfection within a period of 6 to 8 months following chemotherapy, and the prevalence rate returning to its pre-treatment level, and this finding emphasizes the need for effective health education interventions 59 - 61 .
Hence, our findings suggest that improving socioeconomic status alone may not contribute to a significant reduction of schistosomiasis prevalence rate in these communities so that integrated control measures should be implemented. In this regard, community awareness and better understanding of the social, cultural and behavioral determinants are imperative for designing effective control strategies 62 . Moreover, participation of the target communities in the control activities is one of the essential strategies for the success and sustainability of disease control programs 44 , 63 . In low socioeconomic level communities, intervention through public awareness is often recommended as a first line of action to create the enabling environment for other strategies to thrive 64 . Stories of success in eliminating and reducing the transmission, prevalence and intensity of schistosomiasis have been documented in Africa (Egypt and Morocco) 65 , 66 , Asia (China and Japan) 67 , 68 , and Latin America (Dominican Republic and Puerto Rico) 2 , 67 . Moreover, a recent study suggested and discussed an agenda to enhance collaboration between China and Africa on schistosomiasis control in order to translate and apply the Chinese experience in African countries 69 .
We acknowledge some limitations of our methodology. This study had to rely on a single fecal sample collection and a single Kato-Katz smear instead of the ideal three consecutive samples and multiple Kato-Katz smears examination 70 . Thus, the prevalence rates of schistosomiasis as well as the co-infection with both species are likely to be underestimated due to the temporal variation in egg excretion over hours and days. Moreover, information on water contact activities was collected using only the questionnaire, while the frequency and duration of water contact were not investigated. Quantifying water contact activities is essential to assess the contribution of water contact behavior to schistosomiasis in endemic communities 71 .
In conclusion, the present study shows that schistosomiasis is still prevalent among Hausa communities in Kano State, Nigeria; 17.8% of the participants were found to be positive for schistosomiasis. Screening of other family members and treating the infected individuals should be adopted by the public health authorities to combat this infection in these communities. Besides mass drug administration, school and community-based health education regarding good personal hygiene and sanitary practices is imperative among these communities in order to significantly reduce the transmission and morbidity of schistosomiasis.
ACKNOWLEDGEMENTS
We gratefully acknowledge the Schistosomiasis National Control Project and Ministry of Health, Kano State, Nigeria for their generous cooperation during this study. We also thank the participants for their voluntary participation in this study. The work presented in this paper was funded by the University of Malaya Research Grants; RG510-13HTM and RG331-15AFR.
REFERENCES
- 1.Bruun B, Aagaard-Hansen J. The social context of schistosomiasis and its control. Geneva: WHO; 2008. [Google Scholar]
- 2.World Health Organization . Schistosomiasis: progress report 2001-2011 and strategic plan 2012-2020. Geneva: WHO; 2013. [Google Scholar]
- 3.Hotez PJ, Asojo OA, Adesina AM. Nigeria ''Ground Zero'' for the high prevalence neglected tropical diseases. PLoS Negl Trop Dis. 2012;6:54. doi: 10.1371/journal.pntd.0001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 5.Van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 6.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:54. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 8.Swai B, Poggensee G, Mtweve S, Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health a retrospective histopathological study from Tanzania. BMC Infect Dis. 2006;6:134–134. doi: 10.1186/1471-2334-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchuem Tchuenté LA, Southgate VR, Jourdane J, Webster BL, Vercruysse J. Schistosoma intercalatum an endangered species in Cameroon? Tren Parasitol. 2003;19:389–393. doi: 10.1016/s1471-4922(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 10.Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 12.Ekpo UF, Mafiana CF. Epidemiological studies of urinary schistosomiasis in Ogun State, Nigeria identification of high-risk communities. Niger J Parasitol. 2004;25:111–119. [Google Scholar]
- 13.Nigeria. Federal Ministry of Health . Nigeria master plan for neglected tropical disease (NTDs) 2013-2017. 2012. http://docplayer.net/2404435-Nigeria-master-plan-for-neglected-tropical-diseases-ntds-2013-2017.html [Google Scholar]
- 14.Bassey SE, Umar Z. A re-assessment of schistosomiasis infection in Garun-Babba, Kadawa and Kura in Kano State, Nigeria. Niger J Parasitol. 2004;25:107–109. [Google Scholar]
- 15.Duwa MR, Oyeyi TI, Bassey SE. Prevalence and intensity of urinary schistosomiasis among primary school pupils in Minjibir local government area of Kano State, Nigeria. Bayero J Pure Appl Sci. 2009;2:75–78. [Google Scholar]
- 16.Evans DS, King JD, Eigege A, Umaru J, Adamani W, Alphonsus K. Assessing the WHO 50% prevalence threshold in school-aged children as indication for treatment of urogenital schistosomiasis in adults in central Nigeria. Am J Trop Med Hyg. 2013;88:441–445. doi: 10.4269/ajtmh.12-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivoke N, Ivoke ON, Nwani CD, Ekeh FN, Asogwa CN, Atama CI. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop Biomed. 2014;31:77–88. [PubMed] [Google Scholar]
- 18.Okoli EI, Odaibo AB. Urinary schistosomiasis among schoolchildren in Ibadan, an urban community in south-western Nigeria. Trop Med Int Health. 1999;4:308–315. doi: 10.1046/j.1365-3156.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva: WHO; 2002. [PubMed] [Google Scholar]
- 20.Nigeria . National Bureau of Statistics. 2015. http://www.nigerianstat.gov.ng [Google Scholar]
- 21.Olofin EA, Nabegu AB, Dambazau AM. Wudil within Kano region: a geographical synthesis. Wudil: Kano University of Science and Technology, Department of Geography; 2008. [Google Scholar]
- 22.Cheesbrough M. District laboratory practice in tropical countries, part 1. 2. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 23.Kosinski KC, Bosompem KM, Stadecker MJ, Wagner AD, Plummer J, Durant JL. Diagnostic accuracy of urine filtration and dipstick tests for Schistosoma haematobium infection in a lightly infected population of Ghanaian schoolchildren. Acta Trop. 2011;118:123–127. doi: 10.1016/j.actatropica.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Montresor A. Arithmetic or geometric means of eggs per gram are not appropriate indicators to estimate the impact of control measures in helminth infections. Trans R Soc Trop Med Hyg. 2007;101:773–776. doi: 10.1016/j.trstmh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nale Y, Galadima M, Yakubu SE. Index of potential contamination for urinary schistosomiasis in Zaria, Nigeria. Niger J Parasitol. 2003;24:95–101. [Google Scholar]
- 26.Abdullahi MK, Bassey SE, Oyeyi TI. A comprehensive mapping of urinary schistosomiasis using geographic information systems (GIS) in Kano State, Nigeria. Bayero J Pure Appl Sci. 2009;2:41–46. [Google Scholar]
- 27.Amuta EU, Houmsou RS. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac J Trop Med. 2014;7:34–39. doi: 10.1016/S1995-7645(13)60188-1. [DOI] [PubMed] [Google Scholar]
- 28.Ekpo UF, Laja-Deile A, Oluwole AS, Sam-Wobo SO, Mafiana CF. Urinary schistosomiasis among preschool children in a rural community near Abeokuta, Nigeria. Parasit Vectors. 2010;3:58–58. doi: 10.1186/1756-3305-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agbolade OM, Agu NC, Adesanya OO, Odejayi AO, Adigun AA, Adesanlu EB. Intestinal helminthiases and schistosomiasis among school children in an urban center and some rural communities in southwest Nigeria. Korean J Parasitol. 2007;45:233–238. doi: 10.3347/kjp.2007.45.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ugochukwu DO, Onwuliri CO, Osuala FO, Dozie IN, Opara FN, Nwenyi UC. Endemicity of schistosomiasis in some parts of Anambra State, Nigeria. J Med Lab Diagn. 2013;4:54–61. [Google Scholar]
- 31.Bigwan EI, Tinja B, Damen JG. Prevalence of Schistosomiasis among secondary school boarding students in Potiskum Metropolis, Yobe State, Northeastern Nigeria. Bayero J Pure Appl Sci. 2012;5:155–158. [Google Scholar]
- 32.Ekpo UF, Mafiana CF, Adeofun CO, Solarin AR, Idowu AB. Geographical information system and predictive risk maps of urinary schistosomiasis on Ogun State, Nigeria. BMC Infect Dis. 2008;8:74–74. doi: 10.1186/1471-2334-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akinwale OP, Kane RA, Rollinson D, Stothard JR, Ajayi MB, Akande DO. Molecular approaches to the identification of Bulinus species in south-west Nigeria and observations on natural snail infections with schistosomes. J Helminthol. 2011;85:283–293. doi: 10.1017/S0022149X10000568. [DOI] [PubMed] [Google Scholar]
- 34.Ndifon GT, Ukoli FM. Ecology of freshwater snails in south-western Nigeria I: Distribution and habitat preferences. Hydrobiologia. 1989;171:231–253. [Google Scholar]
- 35.Abebe N, Erko B, Medhin G, Berhe N. Clinico-epidemiological study of schistosomiasis mansoni in Waja-Timuga, District of Alamata, northern Ethiopia. Parasit Vectors. 2014;7:158–158. doi: 10.1186/1756-3305-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olveda DU, Li Y, Olveda RM, Lam AK, McManus DP, Chau TN. Bilharzia in the Philippines past, present, and future. Int J Infect Dis. 2014;18:52–56. doi: 10.1016/j.ijid.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Martins-Melo FR, Pinheiro MC, Ramos AN, Jr, Alencar CH, Bezerra FS, Heukelbach J. Trends in schistosomiasis-related mortality in Brazil, 2000-2011. Int J Parasitol. 2014;44:1055–1062. doi: 10.1016/j.ijpara.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Enk MJ, Lima AC, Barros HS, Massara CL, Coelho PM, Schall VT. Factors related to transmission of and infection with Schistosoma mansoni in a village in the South-eastern Region of Brazil. Mem Inst Oswaldo Cruz. 2010;105:570–577. doi: 10.1590/s0074-02762010000400037. [DOI] [PubMed] [Google Scholar]
- 39.Azazy AA, Raja'a YA. Malaria and intestinal parasitosis among children presenting to the Paediatric Centre in Sana'a, Yemen. East Mediterr Health J. 2003;9:1048–1053. [PubMed] [Google Scholar]
- 40.Rudge JW, Stothard JR, Basáñez MG, Mgeni AF, Khamis IS, Khamis AN. Micro-epidemiology of urinary schistosomiasis in Zanzibar local risk factors associated with distribution of infections among schoolchildren and relevance for control. Acta Trop. 2008;105:45–54. doi: 10.1016/j.actatropica.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Deribe K, Eldaw A, Hadziabduli S, Kailie E, Omer MD, Mohammed AE. High prevalence of urinary schistosomiasis in two communities in South Darfur implication for interventions. Parasit Vectors. 2011;4:14–14. doi: 10.1186/1756-3305-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nkegbe E. Sex prevalence of schistosomiasis among school children in five communities in the lower river Volta basin of South Eastern Ghana. Afr J Biomed Res. 2010;13:87–88. [Google Scholar]
- 43.Dawaki S, Al-Mekhlafi HM, Ithoi I, Ibrahim J, Abdulsalam AM, Ahmed A. The menace of schistosomiasis in Nigeria knowledge, attitude, and practices regarding schistosomiasis among rural communities in Kano State. Plos One. 2015;10:54. doi: 10.1371/journal.pone.0143667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, Surin J. Prevalence and associated factors of schistosomiasis among children in Yemen implications for an effective control programme. PLoS Negl Trop Dis. 2013;7:54. doi: 10.1371/journal.pntd.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapito-Tembo AP, Mwapasa V, Meshnick SR, Samanyika Y, Banda D, Bowie C. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis. 2009;3:54. doi: 10.1371/journal.pntd.0000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekwunife CA, Ukaga CN, Okafor FC. Urinary schistosomiasis in Anambra State, Nigeria. Nigerian J Parasit. 2004;25:127–131. [Google Scholar]
- 47.Nmorsi OP, Egwanyenga OA, Ukwandu NC, Nwokolo NQ. Urinary schistosomiasis in a rural community in Edo State Nigeria eosinophiluria as a diagnostic marker. Afr J Biotechnol. 2005;4:183–186. [Google Scholar]
- 48.Nduka FO, Ajaero CM, Nwoke BE. Urinary schistosomiasis among school children in an endemic community in south-eastern Nigeria. Appl Parasitol. 1995;36:34–40. [PubMed] [Google Scholar]
- 49.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 50.Nmorsi OP, Kwandu UN, Ebiaguanye LM. Schistosoma haematobium and urinary tract pathogens co-infections in a rural community of Edo State, Nigeria. J Commun Dis. 2007;39:85–90. [PubMed] [Google Scholar]
- 51.El-Khoby T, Galal N, Fenwick A, Barakat R, El-Hawey A, Nooman Z. The epidemiology of schistosomiasis in Egypt summary findings in nine governorates. Am J Trop Med Hyg. 2000;62(Suppl 2):88–99. doi: 10.4269/ajtmh.2000.62.88. [DOI] [PubMed] [Google Scholar]
- 52.Al-Delaimy AK, Al-Mekhlafi HM, Nasr NA, Sady H, Atroosh WM, Nashiry M. Epidemiology of intestinal polyparasitism among Orang Asli schoolchildren in rural Malaysia. PLOS Negl Trop Dis. 2014;8:54. doi: 10.1371/journal.pntd.0003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y, Manderson L. Schistosomiasis and the social patterning of infection. Acta Trop. 1992;51:175–194. doi: 10.1016/0001-706x(92)90037-x. [DOI] [PubMed] [Google Scholar]
- 54.Coutinho EM, Abath FG, Barbosa CS, Domingues AL, Melo MC, Montenegro SM. Factors involved in Schistosoma mansoni infection, in rural areas of Northeast Brazil. Mem Inst Oswaldo Cruz. 1997;92:707–715. doi: 10.1590/s0074-02761997000500027. [DOI] [PubMed] [Google Scholar]
- 55.Spear RC, Seto E, Liang S, Birkner M, Hubbard A, Qiu D. Factors influencing the transmission of Schistosoma japonicum in the mountains of Sichuan Province of China. Am J Trop Med Hyg. 2004;70:48–56. [PubMed] [Google Scholar]
- 56.Yang J, Zhao Z, Li Y, Krewski D, Wen SW. A multi-level analysis of risk factors for Schistosoma japonicum infection in China. Int J Infect Dis. 2009;13:e407–e412. doi: 10.1016/j.ijid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Gomes EC, Leal-Neto OB, de Oliveira FJ, Jr, Campos JV, Souza-Santos R, Barbosa CS. Risk analysis for occurrences of schistosomiasis in the coastal area of Porto de Galinhas, Pernambuco, Brazil. BMC Infect Dis. 2014;14:101–101. doi: 10.1186/1471-2334-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis a review. Parasit Vectors. 2015;8:156–156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Clercq D, Hanne C, Vercruysse J. Selected chemotherapy and seasonally transmitted Schistosoma haematobium infections in the middle valley of the Senegal River Basin. Trans R Soc Trop Med Hyg. 2000;94:198–199. doi: 10.1016/s0035-9203(00)90276-1. [DOI] [PubMed] [Google Scholar]
- 60.Senghor B, Diaw OT, Doucoure S, Sylla SN, Seye M, Talla I. Efficacy of praziquantel against urinary schistosomiasis and reinfection in Senegalese school children where there is a single well-defined transmission period. Parasit Vectors. 2015;8:362–362. doi: 10.1186/s13071-015-0980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin monitoring treatment success and re-infection patterns. Acta Trop. 2013;128:292–302. doi: 10.1016/j.actatropica.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Nyantekyi LA, Legesse M, Belay M, Tadesse K, Manaye K. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiop J Health Dev. 2010;24:185–190. [Google Scholar]
- 63.Alemu A, Alemu A, Esmael N, Dessie Y, Hamdu K, Mathewos B. Knowledge, attitude and practices related to visceral leishmaniasis among residents in Addis Zemen town, South Gondar, Northwest Ethiopia. BMC Public Health. 2013;13:382–382. doi: 10.1186/1471-2458-13-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ekeh HE, Adeniyi JD. Health education strategies for tropical disease control in school children. J Trop Med Hyg. 1988;91:55–59. [PubMed] [Google Scholar]
- 65.Barakat RM. Epidemiology of schistosomiasis in Egypt travel through time: review. J Adv Res. 2013;4:425–432. doi: 10.1016/j.jare.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hotez PJ, Savioli L, Fenwick A. Neglected tropical diseases of the Middle East and North Africa review of their prevalence, distribution and opportunities of control. PLoS Negl Trop Dis. 2012;6:54. doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N. Schistosomiasis and neglected tropical diseases towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, Yu Q, Tchuenté LT, Bergquist R, Sacko M, Utzinger J. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 2016;16:376–383. doi: 10.1016/S1473-3099(15)00360-6. [DOI] [PubMed] [Google Scholar]
- 70.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. 2004;92:205–212. doi: 10.1016/j.actatropica.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Sow S, de Vlas SJ, Stelma F, Vereecken K, Gryseels B, Polman K. The contribution of water contact behavior to the high Schistosoma mansoni infection rates observed in the Senegal River Basin. BMC Infect Dis. 2011;11:198–198. doi: 10.1186/1471-2334-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]


