Abstract
Hybridizing different antimicrobial peptides (AMPs) is a particularly successful approach to obtain novel AMPs with increased antimicrobial activity but minimized cytotoxicity. The hybrid peptide cecropin A (1–8)-LL37 (17–30) (C-L) combining the hydrophobic N-terminal fragment of cecropin A (C) with the core antimicrobial fragment of LL37 (L) was designed and synthesized. C-L showed higher antibacterial activity against all indicator strains than C and L, and no hemolytic activity to sheep erythrocytes was observed. C-L kills bacterial cells and causes disruption of surface structure, as determined by scanning electron microscopy. Synergistic effects were observed in the combination of C-L with several antibiotics (chloramphenicol, thiamphenicol, or neomycin sulfate) against Escherichia coli and Staphylococcus aureus.
Keywords: hybrid peptide, cecropin A (1–8)-LL37 (17–30), antibacterial activity, hemolytic activity, synergistic interaction
1. Introduction
The increasing microbial resistance to conventional antibiotics is a universal public concern [1], which results in great efforts to develop new safe therapeutic agents [2,3]. Antimicrobial peptides (AMPs) are attracting more and more attention as a therapeutic alternative in the field of disease control and prevention. AMPs are naturally distributed short amphipathic cationic molecules with a unique mechanism of action and broad spectrum of antimicrobial activity [4,5,6]. Many AMPs, such as melittin, human cathelicidin cationic antimicrobial peptide LL37, indolicidin, and IsCT, show undesirable hemolytic activity toward eukaryotic cells while killing microbes, which largely compromised their development as pharmaceuticals [2,7,8]. Hybridizing different AMPs has been demonstrated to be an effective method to avoid these innate limitations [9,10]. Additionally, the combination of antibiotics with proteinaceous AMPs is a good option to enhance the antibacterial activity and eliminate the adverse effects [11].
Cecropin A (C), produced by Hyalophora cecropia, is a linear helical antibacterial peptide composed of 37 amino acid residues. It is divided into an N-terminal amphipathic α-helix domain and C-terminal hydrophobic α-helix domain. C shows broad bioactivities, such as antibacterial, anti-inflammation, and anticancer activities, but does not lyse eukaryotic cells [12,13,14,15]. The N-terminal amphipathic α-helix domain corresponding to the first 7–8 residues has been widely applied to design a large group of novel peptides, such as CA (1–8)-M (1–12) [10,16], CA (1–8)-M (1–18) [17], CA (1–8)-MA (4–12) [9], CA (1–7)-M (1–13) [10], and CA (1–7)-M (2–9) [18], by hybridizing with segments from melittin whose hemolytic concentration was extremely lower than its minimal inhibitory concentrations (MICs). Antimicrobial studies on these hybrid peptides showed that their lethal concentrations against a panel of bacteria ranged from 0.1 to 15 µM, while hemolytic concentrations were >300 µM [10,17,19,20].
The human cathelicidin cationic antimicrobial peptide LL-37 (L), which is a linear helical peptide containing 37 amino acid residues, is isolated from human leukocytes and epithelia. L plays a critical role in the process of antimicrobial infection, angiogenesis, wound healing, and apoptosis [7,21,22,23,24]. However, it shows undesirable hemolytic activity against eukaryotic cells by inducing membrane disruption at concentrations of 25–30 µM, which is slightly higher than its MIC value. This property greatly limits its clinical application [24]. Numerous antimicrobial studies on a series of L fragments showed that a well-defined helical structure in the region corresponding to residues 17–30 was responsible for its antimicrobial, anticancer, and antiviral activities [7,25,26].
Recently, many studies suggested that net positive charge and helical content are important factors to the antibacterial activity of AMPs [27,28]. Early studies from our laboratory also demonstrated the importance of helical structure to the antibacterial activity of AMPs [9]. In the present study, the hybrid peptide C (1–8)-L (17–30) (C-L) was designed by combining the N-terminal amphipathic α-helix fragment of C with the core antimicrobial fragment of L. This approach was on the basis of the hypothesis that the combination will elevate the antimicrobial property and minimize the hemolytic activity by forming more net positive charges and higher content of helical structure. Positive charge was believed to be closely related to the electrostatic bonding force between the AMPs and the negatively charged bacterial cell membrane [29,30]. Helical structure could promote deep insertion of AMPs into bacterial cell membrane [31,32]. In order to pave the way for its clinical application, various tests, including antibacterial activity, hemolytic activity, time-killing curve (TKC), and bacterial morphological changes, were carried out in vitro to evaluate the antibacterial efficacy of C-L.
2. Results
2.1. Primary Structural Parameters of Peptides
As shown in Table 1, Matrix-assisted laser desorption ionization (MALDI) mass spectrometry indicated the purity of final peptides products were all above 95%. The amino acid sequence and molecular weight were confirmed to be correct. The molecular modeling results showed the secondary structure of C-L differed from that of the parental peptides. In comparison with C and L, C-L had a higher net charge (+8) and contained a higher concentration of helical residues (90.9%) (Table 2). C and C-L had the same hydrophobic residue concentration (45%), and this was higher than that of L (35%). The predicted secondary structural type of each residue (Figure 1) showed that C-L had a higher propensity for helical structure than L and C, particularly in the analysis of SSpro [33], 20 amino acids of C-L took the form of a helical structure, accounting for 90.9% of the total amino acid residues, which was greater than both L (81.1%) and C (81.1%).
Table 1.
Amino acid sequence and molecular weight (MW) of C-L, L, and C determined by Matrix-assisted laser desorption ionization (MALDI) mass spectrometry.
| Peptide | Amino Acid Sequence | Theoretical MW | Measured MW |
|---|---|---|---|
| C-L | KWKLFKKIFKRIVQRIKDFLRN | 2905.70 | 2905.60 |
| L | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 4490.57 | 4490.60 |
| C | KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK | 4010.60 | 4010.82 |
Table 2.
Structure parameters of the peptides predicted by online software Jpred 4 and SSpro.
| Peptide | Structure | Net Charge | Helical Residue (%) | Hydrophobic Residue (%) |
|---|---|---|---|---|
| C-L | α-helical | +8 | 90.9 | 45 |
| L | α-helical | +6 | 81.1 | 35 |
| C | α-helical | +7 | 81.1 | 45 |
Figure 1.
The secondary structure of hybrid peptides predicted by Jpred 4 and SSpro. “H” in red represents α-helical structure and “–” represents non α-helical structure.
2.2. Antibacterial and Hemolytic Activity
The antibacterial and hemolytic activity (HC50) of the peptides were determined through micro-broth dilution method (Table 3). When compared to parental peptides, the antibacterial activity of C-L was greatly improved. Results showed that all the six indicator strains were more susceptible to C-L (MICs ranged 2–16 µg/mL) than C (MICs ranged 4.0–198 µg/mL) and L (MICs ranged 13–90 µg/mL) (p < 0.01). C-L treated group showed lower HC50 to sheep erythrocyte cells than the C-treated group and L-treated group (p < 0.01). C-L did not show cytotoxicity (HC50 was 221 µg/mL, HC50 > 128 µg/mL was determined as no cytotoxicity) while the HC50 of L and C was 32 and 169 µg/mL, respectively.
Table 3.
The minimal inhibitory concentrations (MICs) and hemolytic activity (HC50) of C-L, C, and L against six bacterial strains and sheep erythrocyte cell.
| Bacteria Strains | Antimicrobial Peptide | p-Value | |||
|---|---|---|---|---|---|
| C | L | C-L | |||
| MIC (µg/mL) | E. coli K88 | 64 ± 0.72 a | 61 ± 1.78 b | 8 ± 0.33 c | <0.01 |
| E. coli CVCC 245 | 4 ± 0.28 c | 84 ± 1.54 a | 7 ± 0.29 b | <0.01 | |
| S. aureus CVCC 26003 | 128 ± 7.57 a | 36 ± 1.55 b | 7 ± 0.28 c | <0.01 | |
| S. aureus ATCC 25923 | 198 ± 10.1 a | 58 ± 1.68 b | 2 ± 0.03 c | <0.01 | |
| L. mono. CVCC 1599 | 64 ± 2.99 a | 13 ± 0.43 b | 2 ± 0.06 c | <0.01 | |
| M. luteus CVCC 28001 | 154 ± 5.49 a | 90 ± 1.43 b | 16 ± 0.18 c | <0.01 | |
| HC50 (µg/mL) | Sheep erythrocyte cell | 169 ± 15.1 b | 32 ± 0.68 c | 221 ± 3.27 a | <0.01 |
MICs, minimal inhibitory concentrations; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli; L. mono., Listeria monocytogenes; M. luteus., Micrococcus luteus; a,b,c Means with different superscripts within the same row differ (p < 0.01).
2.3. Sterilization Speed and Efficacy
Both Escherichia coli (E. coli) CVCC 245 and Staphylococcus aureus (S. aureus) ATCC 25923 were susceptible to the peptides tested. Table 4 showed the sterilization rate of L, C, and C-L on S. aureus ATCC 25923 and E. coli CVCC 245. For S. aureus ATCC 25923, C-L caused 57% viability decrease at 1 h, while L and C caused about 10% and 46% decreases, respectively. The sterilization rate of C-L remained the highest in the following 4 h. For E. coli CVCC 245 the survival rate to C-L at 1, 2, and 3 h were 29%, 16%, and 5%, respectively. However, the strain survival rate was 44%, 35%, and 14% at 1, 2, and 3 h, respectively, when L was used as a bactericide, and 44%, 30%, and 15%, respectively, when C was used (Table 4). No matter S. aureus or E. coli, the C-L-treated group showed lower viability than the C-treated group and L-treated group at 1, 2, and 3 h (p < 0.05). According to the results shown in Table 4, C-L was found to be more effective than C or L in sterilization, especially in the first 3 h.
Table 4.
The sterilization rate of L, C, and C-L on S. aureus ATCC 25923 and E. coli CVCC 245.
| Bacteria Strains | Time (h) | Antimicrobial Peptides | p-Value | ||
|---|---|---|---|---|---|
| C | L | C-L | |||
| Viable S. aureus concentration (×108 CFU/mL) | 1 | 0.54 ± 0.007 b | 0.90 ± 0.020 a | 0.43 ± 0.017 c | <0.01 |
| 2 | 0.40 ± 0.035 b | 0.53 ± 0.026 a | 0.30 ± 0.012 c | <0.01 | |
| 3 | 0.21 ± 0.014 a | 0.23 ± 0.011 a | 0.18 ± 0.020 b | 0.014 | |
| 4 | 0.09 ± 0.005 | 0.09 ± 0.002 | 0.08 ± 0.003 | 0.079 | |
| 5 | 0.01 ± 0.0005 | 0.01 ± 0.0003 | 0.01 ± 0.0001 | 0.298 | |
| Viable E. coli concentration (×108 CFU/mL) | 1 | 0.44 ± 0.028 a | 0.44 ± 0.027 a | 0.29 ± 0.015 b | <0.01 |
| 2 | 0.30 ± 0.021 b | 0.35 ± 0.014 a | 0.16 ± 0.026 c | <0.01 | |
| 3 | 0.15 ± 0.014 a | 0.14 ± 0.020 a | 0.05 ± 0.003 b | <0.01 | |
| 4 | 0.06 ± 0.002 | 0.05 ± 0.002 | 0.05 ± 0.003 | 0.061 | |
| 5 | 0.01 ± 0.0002 | 0.01 ± 0.003 | 0.01 ± 0.0001 | 0.659 | |
a,b,c Means with different superscripts within the same row differ (p < 0.05).
2.4. Disruption of Bacterial Cell Surface Structure Integrity
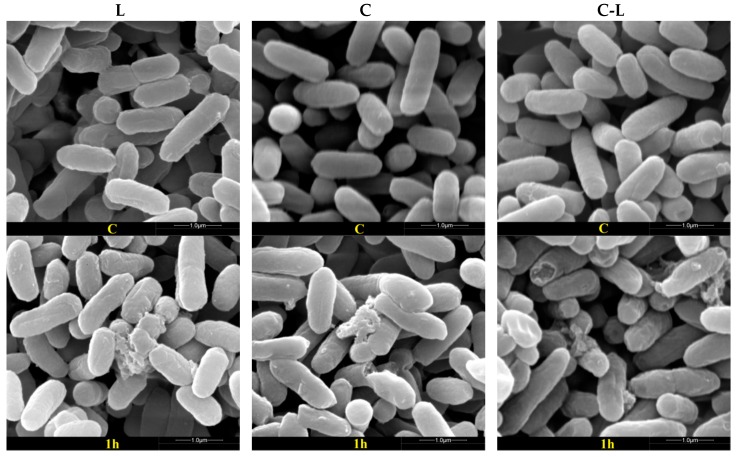
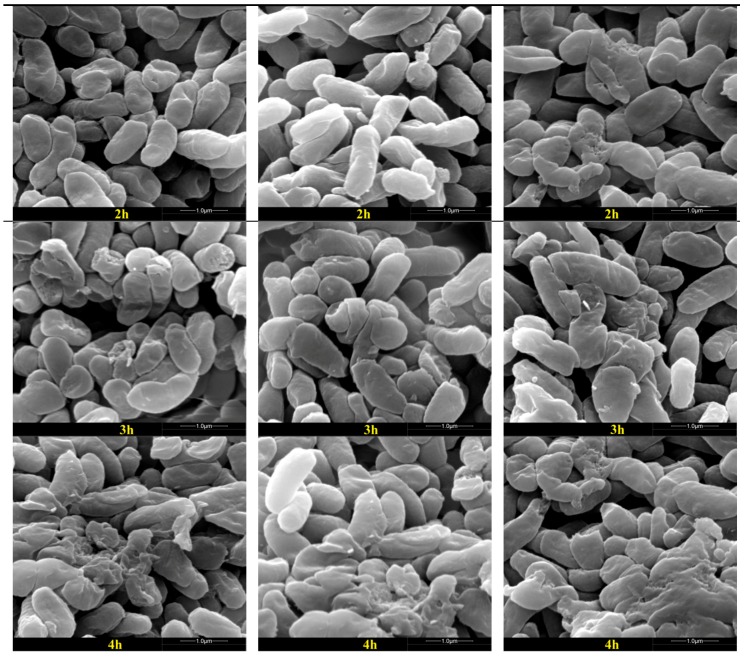
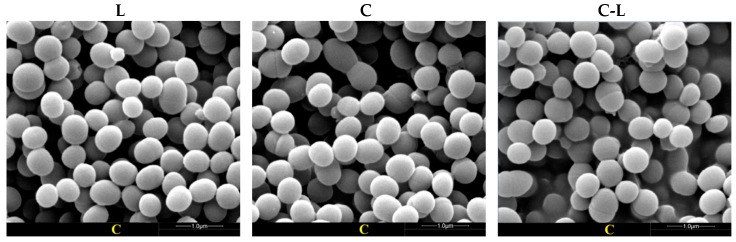
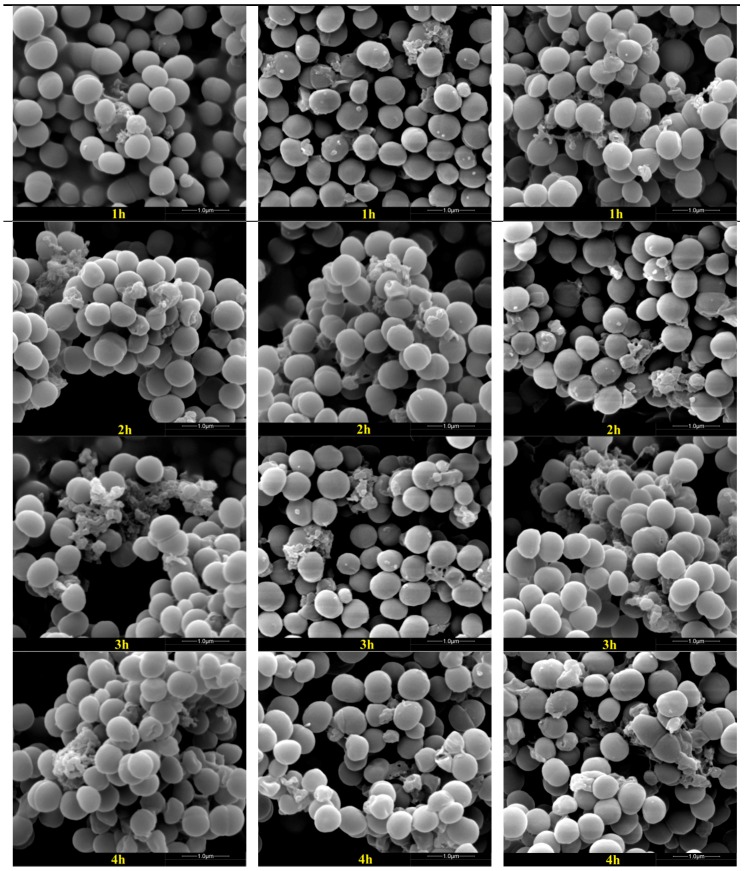
Scanning electron microscopy (SEM) allowed us to directly observe the external surface of the bacteria after peptide treatment. The antibacterial properties of C, L, and C-L were studied by observing the integrity of the treated bacterial cell surface structure. As shown in Figure 2 and Figure 3, some debris was observed in the surface of E. coli CVCC 245 and S. aureus ATCC 25923 after treatment with AMPs (C, L, and C-L) at MICs for 1 h, and more cellular debris and surface structure damage were observed after treatment for 2, 3, and 4 h, however, the untreated E. coli and S. aureus showed bright and smooth surfaces (Figure 2 and Figure 3). Furthermore, varying degrees of collapse or disfiguration were observed in bacterial cells treated with peptides for 2 h or more.
Figure 2.
Effects of L, C, and C-L on E. coli CVCC 245 morphology and integrity. The first, second, and third columns were bacteria treated with 1× minimal inhibitory concentrations (MICs) L, C, and C-L, respectively. The lines from top to bottom were bacteria untreated (C group) and treated on 1, 2, 3, and 4 h.
Figure 3.
Effects of L, C, and C-L on S. aureus ATCC 25293 morphology and integrity. The first, second, and third columns were bacteria treated with 1 × MICs L, C, and C-L, respectively. The lines from top to bottom were bacteria untreated (C group) and treated on 1, 2, 3, and 4 h, respectively.
2.5. Synergistic Assay
To test the efficacy of synergism, C-L was used to treat pathogens in combination with several antibiotics. As shown in Table 5, fractional inhibitory concentration (FIC) indexes of the combination groups “C-L + thiamphenicol” and “C-L + penicillin G” against E. coli CVCC 245 were higher than those of the “C-L + chloramphenicol”, “C-L + neomycin sulfate”, and “C-L + kanamycin” groups (p < 0.01), while the FIC index of the “C-L + penicillin G” group against S. aureus ATCC 25923 was the highest among all the groups (p < 0.01). FIC indexes ≤0.5 were observed in the combination of C-L with chloramphenicol (0.375), thiamphenicol (0.188), and neomycin sulfate (0.250) against S. aureus ATCC 25923 and with neomycin sulfate (0.313) against E. coli CVCC 245; 0.5 < FIC index ≤ 1 was observed in the combination of C-L with kanamycin (0.750) against S. aureus ATCC 25923 and chloramphenicol (1.00) against E. coli CVCC 245, respectively. Neither a synergistic nor antagonistic effect was observed in other combinations (FIC values ranged from 1 to 2).
Table 5.
Synergistic interaction of C-L with five antibiotics.
| Combination Group | Fractional Inhibitory Concentration (FIC) Index | p-Value | ||||
|---|---|---|---|---|---|---|
| C-L + Chloramphenicol | C-L + Thiamphenicol | C-L + Neomycin Sulfate | C-L + Penicillin G | C-L + Kanamycin | ||
| E. coli CVCC 245 | 1.00 ± 0.141 c | 1.50 ± 0.001 a | 0.313 ± 0.001 d | 1.50 ± 0.003 a | 1.25 ± 0.002 b | <0.01 |
| S. aureus ATCC 25923 | 0.375 ± 0.005 c | 0.188 ± 0.002 e | 0.250 ± 0.004 d | 1.13 ± 0.005 a | 0.750 ± 0.003 b | <0.01 |
a,b,c,d,e Means with different superscripts within the same row differ (p < 0.05).
3. Discussion
AMPs have been considered to be potential antimicrobial alternatives to traditional antibiotics. The antimicrobial mechanism of AMPs is totally different from traditional antibiotics. Antibiotics interfered with the inner biosynthesis of proteins, RNA, DNA, peptidoglycan, and folic acid [1], however, AMPs were reported to be less prone to drug resistance because their mechanisms were largely related to the interaction with bacterial cell membranes [34,35].
Over the decades, many efforts have been made to elevate the potency of AMPs against pathogenic agents and eliminate their undesirable cytotoxicity to eukaryotic cells. Hybridizing different AMPs is one of the successful approaches to obtain novel AMPs with increased antimicrobial activity but minimized cytotoxicity [3,10,16,17,18]. As reported previously, C had been frequently used as the parental peptide in many hybrid peptide designs due to its high α-helical conformation and broad antimicrobial activity [10,12,16]. However, almost no study about L in hybrid peptides was reported, even though it is a well characterized AMP with potential antibacterial, antiviral, and anticancer activities [23,24]. In the present study, the hybrid peptide C-L was designed by combining the N-terminal amphipathic α-helix fragment of C and the core antimicrobial fragment of L. Antibacterial and TKC data demonstrated that the hybrid peptide C-L was more active against both gram-positive and gram-negative bacteria than parental peptides (C and L). Additionally, C-L did not exhibit cytotoxicity, which was in agreement with studies of other hybrid peptides, such as Me (1–13)-LL37 (17–30) [9], Mdc-Hly [3], HP (2–9)-M (1–12) [36], CA (1–8)-ME (1–12) [17,18], CA (1–8)-MA (1–12) [10,17], and CA (1–7)-ME (2–9) [14].
Generally, the antimicrobial activity of AMPs depended on their structural and physicochemical properties. In the present study, C-L had a higher net charge, α-helical content, and hydrophobicity than parental AMPs (C and L). It was believed that almost all cationic peptides kill bacteria by initial electrostatic interacting with anionic membrane, followed by penetrating into the lipid bilayer, forming channels or pores, and consequently leading to cell lysis and death [37,38]. C and L had been observed to exert their antibacterial activity by forming pores in lipid bilayers with transmembrane helices via anion selective channel and carpet-type mechanisms [19,26,39,40,41]. During the process of sterilization, the higher positive charges were reported to correlate with stronger activity by augmenting the bonding force between the AMPs and the bacterial cell membranes [29,30]. The α-helices structure allows AMPs to potently interact with negatively charged membrane [31,32]. Therefore, in present research, the increased antibacterial activity of the hybrid peptide C-L may be attributed to changes in structural and physicochemical properties (higher net charge and α-helical content). Moreover, as a resulting benefit from the no hemolysis feature provided by the first eight residues of C [12,13,14,15], C-L was able to selectively disrupt bacterial surface structure while causing little injury to sheep blood cells.
In the present study, some cellular debris was observed on the surface of the bacterial cells treated with AMPs (C, L and C-L), indicating that the peptides could cause disruption of bacterial cells surface structure. Though in our study S. aureus ATCC 25923 and E. coli CVCC 245 were almost completely killed by C, L, and C-L within 5 h of incubation, C-L was found to be more effective than C or L in the first 3 h. The three peptides mentioned above provide protection against a wide variety of pathogens and show great potential to regulate intestine microflora as a promising candidate for antibiotics. However, excessive proteolysis may limit the therapeutic application of exogenous L in the gut. Incubation of L with 1 ng/mL trypsin at 37 °C for 3 h resulted in approximately 50% activity loss of the initial amount of L, and with 10 and 100 ng/mL resulted in almost 100% inactivation [42]. According to the preliminary assessment in our ongoing study (unpublished), similar sensitivity of C and C-L to trypsin was found when compared with L. Given this situation, C-L would have a better effect in the process of regulating intestine microflora than C and L because of its higher efficiency in a shorter amount of time.
Various AMPs and antibiotics were tested for synergistic effects against different bacterial strains [11,43] (e.g., the combination of nisin with daptomycin, ciprofloxacin or vancomycin resulted in increased antibacterial activity against both methicillin-resistant and methicillin-susceptible S. aureus strains [44]; the combination of CA (1–7)-ME (2–9) with β-lactam or ciprofloxacin resulted in increased antibacterial activity against Acinetobacter baumannii ATCC 19606 and Acinetobacter baumannii 04–01 [14,45]). In the present study, the FIC values calculated from the checkerboard experiments revealed that C-L acted synergistically with chloramphenicol, thiamphenicol, or neomycin sulfate against S. aureus ATCC 25923, and with neomycin sulfate against E. coli CVCC 245. The results were in agreement with previous synergy studies between AMPs and antibiotics [11,14]. Although the mechanism of this positive interaction remains unclear, we concluded the potential reasons were as follows: on the one hand, antibiotics promoted AMPs to stride over the outer membrane and then act on an intracellular basis, on the other hand, AMPs could penetrate the cell membrane and allow the antibiotic to act more effectively.
4. Materials and Methods
4.1. Bacterial Strains and Antibiotics
Six pathogenic bacterial strains were purchased from China Veterinary Culture Collection (CVCC, Beijing, China). The strains were E. coli K88, E. coli CVCC 245, S. aureus CVCC 26003, S. aureus ATCC 25923, Micrococcus luteus (M. luteus) CVCC 28001, and Listeria monocytogenes (L. mono.) CVCC 1599. Neomycin sulfate, chloramphenicol, thiamphenicol, kanamycin, and penicillin G were all purchased from Sigma (San Francisco, CA, USA).
4.2. Design, Biological Information Analysis, and Synthesis of Hybrid Peptides
Hybrid peptides derived from selected parental peptides were designed using APD2 [46]. Protein primary structure characteristics (including net charge, helical residue, and hydrophobic residue) were analyzed and predicted by Jpred 4 [47] and SSpro [33] online. The selected hybrid peptide and parental peptides were synthesized by 9-fluorenylmethoxycarbonyl solid-phase synthesis chemistry and purified by a reverse-phase semi-preparative high performance liquid chromatography (SBS, Shenzhen, China), and then stored at −80 °C until analysis.
4.3. Determination of Minimal Inhibitory Concentrations (MICs)
The antibacterial activities of the peptides were characterized by MICs. Bacterial strains at exponential phase were diluted to the concentration of 5 × 105 CFU/mL with Mueller-Hinton (MH) broth medium, and 180 µL culture was dispensed into each well of a 96-well microtiter plate. Susceptibility tests were performed by two-fold standard broth microdilutions of the test peptides (C-L, C, and L) according to the Clinical and Laboratory Standards Institute guidelines. Aliquots (20 µL) of peptide dilution were mixed with 180 µL bacterial suspensions, and the mixture was assayed with Bio-Rad 3700 plate reader by monitoring optical density at 600 nm after 16–18 h incubation at 37 °C. Each analysis was performed in triplicate. The lowest concentration (highest dilution) required to prevent the growth of bacteria was regarded as the MICs [2].
4.4. Hemolytic Assay
The hemolytic activities were evaluated by measuring the peptide concentrations that caused 50% hemolysis of sheep erythrocytes at 540 nm (A540 nm) [17,48]. Sheep erythrocytes with 10 mM phosphate buffered saline (PBS)/0.1% (v/v) Triton X-100 (Sigma) added were used as negative/positive control. Experiments were performed in triplicate.
4.5. Antibacterial Studies
Indicator strains S. aureus ATCC 25923 and E. coli CVCC 245 were cultured overnight and then diluted with MH to 108 CFU/mL. The bacterial suspensions were mixed with C, L, and C-L (total volume, 200 µL; final concentrations of 1 × MICs) respectively, incubated at 180 rpm and 37 °C. At 0, 1, 2, 3, 4, and 5 h, an aliquot (10 µL) of the culture was collected, diluted, and plated on an MH plate. Colonies were counted at 24 h and the original concentration was calculated. Each analysis was performed in triplicate.
4.6. SEM Analysis of Bacterial Cells
E. coli CVCC 245 and S. aureus ATCC 25923 cell suspensions (1 × 108 CFU/mL) were incubated with C-L (final concentrations of 1 × MICs, respectively) at 37 °C, 180 rpm. Four milliliters of mixture were taken at 0, 2, and 4 h and centrifuged at 10,000× g for 10 min—the supernatant was discarded. The bacterial cells were washed with PBS three times, fixed with 2.5% glutaraldehyde at 4 °C overnight, washed twice with PBS again, and postfixed for 2 h with 1% OsO4. The bacterial pellets were dehydrated with a grades series of ethanol and dried cells were coated with gold and observed under a scanning electron microscope (Quanta 200, FEI, Hillsboro, OR, USA).
4.7. Synergy Assays
Studies on synergistic effects between antibiotics and C-L on controlling pathogens (E. coli CVCC 245 and S. aureus ATCC 25923) were performed with the checkerboard titration method. The drugs (C-L and antibiotics) were set in a series of concentrations (ranged from 1/16× MICs to 3× MICs). Experiments were performed in triplicate. The FIC index for combinations was calculated according to the equation: FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drug A and drug B in the combination, respectively, MICA and MICB are the MICs of drug A and drug B, respectively, and FICA and FICB are the FICs of drug A and drug B, respectively. The FIC index was interpreted as follows: ≤0.5, synergy; 0.5 to 1.0, addition; 1.0 to 4.0, indifference; and ≥4.0, antagonism [49].
4.8. Statistical Analyses
Experiment data were analyzed by one-way ANOVA with general liner model procedure of SPSS (version 20.00, SPSS Institute Inc., Chicago, IL, USA). Statistically significant effects were further analyzed and means were compared using Duncan’s multiple range test. Statistical significance was determined at p < 0.05.
5. Conclusions
In conclusion, a novel hybrid peptide C-L with antibacterial activity that could cause disruption of cell surface structure was designed and tested. The hybrid peptide C-L did not show cytotoxicity to sheep blood cells. Due to the synergistic effect of hydrophobic N-terminal fragment of cecropin C and the core antimicrobial fragment of L, peptide C-L showed excellent antibacterial properties. The results indicated that the hybrid peptide C-L could serve as a promising candidate for pharmaceutical agents.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 31272476) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110008110002).
Author Contributions
Xu-Biao Wei and Ru-Juan Wu performed the experiments and wrote the paper; Da-Yong Si and Xiu-Dong Liao conceived and designed experiments; Lu-Lu Zhang analyzed the data; Ri-Jun Zhang guided the experiments. All authors helped to prepare the paper and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kim L. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 2.Hwang I.S., Hwang J.S., Hwang J.H., Choi H., Lee E., Kim Y., Lee D.G. Synergistic effect and antibiofilm activity between the antimicrobial peptide coprisin and conventional antibiotics against opportunistic bacteria. Curr. Microbiol. 2013;66:56–60. doi: 10.1007/s00284-012-0239-8. [DOI] [PubMed] [Google Scholar]
- 3.Lu X.M., Jin X.B., Zhu J.Y., Mei H.F., Ma Y., Chu F.J., Wang Y., Li X.B. Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl. Microbiol. Biotechnol. 2010;87:2169–2176. doi: 10.1007/s00253-010-2606-3. [DOI] [PubMed] [Google Scholar]
- 4.Brogden K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 5.Bowles D.J. Defense-related proteins in higher plants. Annu. Rev. Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- 6.Brown K.L., Hancock R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Vandamme D., Landuyt B., Luyten W., Schoofs L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cell. Immunol. 2012;280:22–35. doi: 10.1016/j.cellimm.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Kyungik L., Song Y.S., Kyoungho K.S., Saeng L., Kyung H., Kim Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004;33:712–719. doi: 10.1016/j.bbrc.2004.08.144. [DOI] [PubMed] [Google Scholar]
- 9.Wu R.J., Wang Q., Zheng Z.J., Zhao L.M., Shang Y.J., Wei X.B., Liao X.D., Zhang R.J. Design, characterization and expression of a novel hybrid peptides melittin (1–13)-LL37 (17–30) Mol. Biol. Rep. 2014;41:4163–4169. doi: 10.1007/s11033-013-2900-0. [DOI] [PubMed] [Google Scholar]
- 10.Shin S.Y., Rang J.H., Hahm K.S. Structure-antibacterial, antitumor and hemolytic activity relationships of cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1999;53:82–90. doi: 10.1111/j.1399-3011.1999.tb01620.x. [DOI] [PubMed] [Google Scholar]
- 11.Naghmouchi K., Baah J., Hober D., Jouy E. Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 2013;57:2719–2725. doi: 10.1128/AAC.02328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacalum M., Radu M. Cationic antimicrobial peptides cytotoxicity on mammalian cells: An analysis using therapeutic index integrative concept. Int. J. Pept. Res. Ther. 2015;21:47–55. doi: 10.1007/s10989-014-9430-z. [DOI] [Google Scholar]
- 13.Marassi F.M., Opella S.J., Juvvadi P., Merrifield R.B. Orientation of Cecropin A helices in phospholipid bilayers determined by solid-state NMR spectroscopy. Biophys. J. 1999;77:3152–3155. doi: 10.1016/S0006-3495(99)77145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacometti A., Cirioni O., Kamysz W., Amato G., Silvestri C., del Prete M.S., Lukasiak J., Scalise G. Comparative activities of cecropin A, melittin, and cecropin A–melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides. 2003;24:1315–1318. doi: 10.1016/j.peptides.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Pavon N., Buelna-Chontal M., Hernandez-Esquivel L., Hernandez S., Chavez E., Conde R., Lanz-Mendoza H. Mitochondrial inactivation by Anopheles albimanus cecropin 3: Molecular mechanisms. Peptides. 2014;53:202–209. doi: 10.1016/j.peptides.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Lee D.G., Shin S.Y., Maeng C.Y., Hahm K.S. CecropinA-melittin hybrid peptide exerts its antifungal effects by damaging on the plasma membranes of Trichosporon beigelii. Biotechnol. Lett. 1998;20:211–214. doi: 10.1023/A:1005357314588. [DOI] [Google Scholar]
- 17.Shin S.Y., Lee M.K., Kim K.L., Hahm K.S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1997;50:279–285. doi: 10.1111/j.1399-3011.1997.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 18.Saugar J.M., Rodriguez-Hernandez M.J., de la Torre B.G., Pachon-Ibanez M.E., Fernandez-Reyes M., Andreu D., Pachon J., Rivas L. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: Molecular basis for the differential mechanisms of action. Antimicrob. Agents Chemother. 2006;50:1251–1256. doi: 10.1128/AAC.50.4.1251-1256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boman H.G. Peptide antibiotics and their roles in innate immune. Annu. Rev. Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 20.Andreu D., Ubach J., Boman A., Wahlin B., Wade D., Merrifield R.B., Boman H.G. Shortened cecropin A-melittin hybrids: Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992;296:190–194. doi: 10.1016/0014-5793(92)80377-S. [DOI] [PubMed] [Google Scholar]
- 21.Hoskin D.W., Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Splith K., Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur. Biophys. J. 2011;40:387–397. doi: 10.1007/s00249-011-0682-7. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y.P., Gallo R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2008;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos R., Silva J.P., Rodrigues A.C., Costa R., Guardao L., Schmitt F., Soares R., Vilanova M., Domingues L., Gama M. Wound healing activity of the human antimicrobial peptide LL37. Peptides. 2011;32:146914–146976. doi: 10.1016/j.peptides.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008;283:32637–32643. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Li Y.F., Han H.Y., Miller D.W., Wang G.S. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 27.Hao G., Shi Y., Han J., Li Q., Tang Y., Le G. Design and analysis of structure-activity relationship of novel antimicrobial peptides derived from the conserved sequence of cecropin. J. Pept. Sci. 2008;14:290–298. doi: 10.1002/psc.926. [DOI] [PubMed] [Google Scholar]
- 28.Powers J., Hancock R. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Moraes L.G.M., Fazio M.A., Vieira R.F.F., Nakaie C.R., Miranda M.T.M., Schreier S. Conformational and functional studies of gomesin analogues by CD, EPR and fluorescence spectroscopies. Biochim. Biophys. Acta-Biomembr. 2007;1768:52–58. doi: 10.1016/j.bbamem.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Wood S.J., Park Y.A., Kanneganti N.P., Mukkisa H.R., Crisman L.L., Davis S.E. Modified cysteine-deleted tachyplesin (CDT) analogs as linear antimicrobial peptides: Influence of chain length, positive charge, and hydrophobicity on antimicrobial and hemolytic activity. Int. J. Pept. Res. Ther. 2014;20:519–530. doi: 10.1007/s10989-014-9419-7. [DOI] [Google Scholar]
- 31.Christensen B., Fink J., Merrifiel R.B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid-membranes. Proc. Natl. Acad. Sci. USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennison S.R., Wallace J., Harris F., Phoenix D.A. Amphiphilic α-helical antimicrobial peptides and their structure/function relationships. Protein Pept. Lett. 2005;12:31–39. doi: 10.2174/0929866053406084. [DOI] [PubMed] [Google Scholar]
- 33.SSpro. [(accessed on 17 June 2016)]. Available online: http://scratch.proteomics.ics.uci.edu/
- 34.Bonucci A., Balducci E., Pistolesi S., Pogni R. The defensin-lipid interaction: Insights on the binding states of the human antimicrobial peptide HNP-1 to model bacterial membranes. Biochim. Biophys. Acta-Biomembr. 2013;1828:758–764. doi: 10.1016/j.bbamem.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Sato H., Felix J.B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2006;1758:1245–1256. doi: 10.1016/j.bbamem.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 36.Kim H.K., Lee D.G., Park Y., Kim H.N., Choi B.H., Choi C.H., Hahm K.S. Antibacterial activities of peptides designed as hybrids of antimicrobial peptides. Biotechnol. Lett. 2002;24:347–353. [Google Scholar]
- 37.Epand R.M., Vogel H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta-Biomembr. 1999;1462:11–28. doi: 10.1016/S0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 38.Prenner E.J., Lewis R., Mcelhaney R.N. The interaction of the antimicrobial peptide gramicidins S with lipid bilater model and biological membranes. Biochim. Biophys. Acta-Biomembr. 1999;1462:201–221. doi: 10.1016/S0005-2736(99)00207-2. [DOI] [PubMed] [Google Scholar]
- 39.Durell S.R., Raghunathan G., Guy H.R. Modeling the ion channel structure of cecropin. Biophys. J. 1992;63:1623–1631. doi: 10.1016/S0006-3495(92)81730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G.S., Mishra B., Epand R.F., Epand R.M. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim. Biophys. Acta-Biomembr. 2014;1838:2160–2172. doi: 10.1016/j.bbamem.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thennarasu S., Tan A.M., Penumatchu R., Shelburne C.E., Heyl D.L., Ramamoorthy A. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys. J. 2010;98:248–257. doi: 10.1016/j.bpj.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gronberg A., Zettergren L., Agren M.S. Stability of the cathelicidin peptide LL-37 in a non-healing wound environment. Acta Derm-Venereol. 2011;91:511–515. doi: 10.2340/00015555-1102. [DOI] [PubMed] [Google Scholar]
- 43.Lin M.C., Hui C.F., Chen J.Y., Wu J.L. Truncated antimicrobial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus. Peptides. 2011;44:139–148. doi: 10.1016/j.peptides.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Dosler S., Gerceker A.A. In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy. 2011;57:511–516. doi: 10.1159/000335598. [DOI] [PubMed] [Google Scholar]
- 45.Dosler S., Mataraci E. In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides. 2013;49:53–58. doi: 10.1016/j.peptides.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 46.APD. [(accessed on 17 June 2016)]. Available online: http://aps.unmc.edu/AP/main.php.
- 47.Jpred. [(accessed on 17 June 2016)]. Available online: http://www.compbio.dundee.ac.uk/jpred4/index.html.
- 48.Tian Z.G., Dong T.T., Teng D., Yang Y.I., Wang J.H. Design and characterization of novel hybrid peptides from LFB15(W4,10), HP(2–20), and cecropin A based on structure parameters by computer-aided method. Appl. Microbiol. Biotechnol. 2009;82:1097–1103. doi: 10.1007/s00253-008-1839-x. [DOI] [PubMed] [Google Scholar]
- 49.Krogstad D.J., Moellering R.C.J.R. Antimicrobial combinations. In: Lorian V., editor. Antibiotics in Laboratory Medicine. 2nd ed. Williams & Wilkin; Easton, MD, USA: 1986. pp. 537–595. [Google Scholar]