Abstract
The glutamatergic system may be involved in the effects of neuroprotectant therapies. Echinacoside, a phenylethanoid glycoside extracted from the medicinal Chinese herb Herba Cistanche, has neuroprotective effects. This study investigated the effects of echinacoside on 4-aminopyridine-evoked glutamate release in rat cerebrocortical nerve terminals (synaptosomes). Echinacoside inhibited Ca2+-dependent, but not Ca2+-independent, 4-aminopyridine-evoked glutamate release in a concentration-dependent manner. Echinacoside also reduced the 4-aminopyridine-evoked increase in cytoplasmic free Ca2+ concentration but did not alter the synaptosomal membrane potential. The inhibitory effect of echinacoside on 4-aminopyridine-evoked glutamate release was prevented by ω-conotoxin MVIIC, a wide-spectrum blocker of Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels, but was insensitive to the intracellular Ca2+ release-inhibitors dantrolene and 7-chloro-5-(2-chloropheny)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157). Furthermore, echinacoside decreased the 4-aminopyridine-induced phosphorylation of protein kinase C, and protein kinase C inhibitors abolished the effect of echinacoside on glutamate release. According to these results, we suggest that the inhibitory effect of echinacoside on evoked glutamate release is associated with reduced voltage-dependent Ca2+ entry and subsequent suppression of protein kinase C activity.
Keywords: echinacoside, glutamate release, cerebrocortical nerve terminals, voltage-dependent Ca2+ channels, protein kinase C
1. Introduction
Echinacoside is a major phenylenthanoid glycoside present in Herba Cistanche, a well-known traditional Chinese medicine used to treat forgetfulness, impotency, and chronic constipation [1]. Echinacoside possesses various bioactivities such as antioxidation, anti-inflammation, anticancer, hepatoprotection, and immune modulation [2,3,4]. Notably, echinacoside has neuroprotective effects; for example, it can protect against oxidative stress- or neurotoxin-induced neurotoxicity in primary rat cortical neurons, human neuroblastoma SH-SY5Y cells, and pheochromocytoma (PC12) cells [5,6,7,8]. Furthermore, echinacoside attenuates brain damage and improves cognitive function in animal models of Parkinson’s disease, Alzhermer’s disease, and middle cerebral artery occlusion [9,10,11,12]. However, the mechanism through which echinacoside induces neuroprotection is not fully understood.
Neuroprotection is a complex process of preserving neuronal structure and function upon toxic insults. Glutamate excitotoxicity reduction is considered a potential mechanism involved in brain neuroprotection. Glutamate, an excitatory amino acid neurotransmitter, has a crucial role in several brain functions [13]. However, overactivation of glutamate receptors under high glutamate concentrations causes intracellular Ca2+ overload, mitochondrial dysfunction, free radical production, and neuronal death [14,15]. This pathological process is implicated in numerous brain disorders including cerebral ischemia, traumatic brain injury, epilepsy, and neurodegenerative disease [16,17]. Hence, inhibitors blocking pathophysiological glutamatergic transmission are considered a potential neuroprotective drugs. Notable examples of these are glutamate receptor antagonists [18,19]; however, clinical trials for these drugs have failed because of less effectivity and undesired, or even cytotoxic side effects [20,21]. In addition to direct glutamate receptor blockade, glutamate release inhibition may be an effective strategy for neuroprotection. Several neuroprotectants (e.g., memantine and riluzole) can reduce glutamate release in rat brain tissues [22,23,24].
Considering the role of glutamate in excitotoxicity and the neuroprotective profile of echinacoside, the present study used isolated nerve terminals (synaptosomes) purified from the rat cerebral cortex to investigate the effect of echinacoside on glutamate release and further explored potential mechanisms. The isolated nerve terminal preparation is a well-established model for studying the presynaptic regulation of neurotransmitter release by drugs in the absence of any postsynaptic effects [25]. By using this model, we evaluated the effect of echinacoside on glutamate release, membrane potential, presynaptic Ca2+ influx, and protein kinase C activity. According to our review of the literature, this is the first report documenting the mechanism through which echinacoside inhibits endogenous glutamate release at the presynaptic level.
2. Results
2.1. Echinacoside Inhibits 4-Aminopyridine-Evoked Glutamate Release from Rat Cerebrocortical Nerve Terminals by Reducing Vesicular Exocytosis
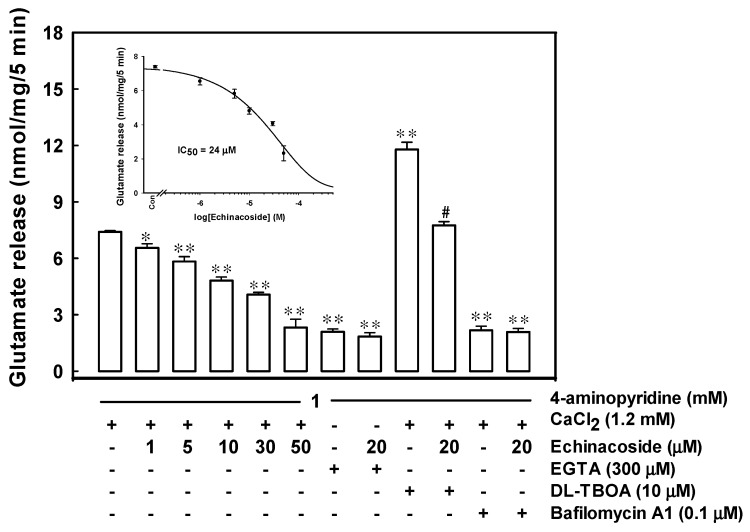
Figure 1 illustrates the concentration-dependent effect of echinacoside on 4-aminopyridine-evoked glutamate release from purified rat cerebrocortical synaptosomes. In synaptosomes incubated with 1 mM CaCl2, 1 mM 4-aminopyridine evoked a glutamate release of 7.4 ± 0.1 nmol/mg/5 min, which was reduced by 1, 5, 10, 30, and 50 µM echinacoside to 6.5 ± 0.2, 5.8 ± 0.3, 4.8 ± 0.2, 4.1 ± 0.1, or 2.3 ± 0.4 nmol/mg/5 min, respectively (F(5,24) = 67.1, p = 0.000). The IC50 value for echinacoside-mediated inhibition of 4-aminopyridine-evoked glutamate release, derived from a dose-response curve, was 24 µM. Moreover, the glutamate release evoked by 1 mM 4-aminopyridine in an extracellular Ca2+-free solution containing 300 µM ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) was 2.1 ± 0.2 nmol/mg/5 min (F(2,12) = 310.65, p = 0.000), and this Ca2+-independent component of 4-aminopyridine-evoked glutamate release was unaffected by 20 µM echinacoside (1.8 ± 0.2 nmol/mg/5 min; p = 0.58; Figure 1). In synaptosomes treated with 0.1 µM bafilomycin A1, a vesicular transporter inhibitor [26], 4-aminopyridine-evoked glutamate release was reduced significantly (2.2 ± 0.2 nmol/mg/5 min; F (2,12) = 249.518, p = 0.000). In the presence of bafilomycin A1, 20 µM echinacoside failed to significantly inhibit the release of glutamate (2.1 ± 0.2 nmol/mg/5 min; p = 0.94; Figure 1). By contrast, 10 µM dl-threo-beta-benzyl-oxyaspartate (dl-TBOA, a glutamate reuptake inhibitor) [27], increased 4-aminopyridine-evoked glutamate release to 11.8 ± 0.4 nmol/mg/5 min (t(8) = −11.31, p = 0.000). Even in the presence of dl-TBOA, 20 µM echinacoside inhibited 4-aminopyridine-evoked glutamate release significantly (7.7 ± 0.2 nmol/mg/5 min; F(2,12) = 87.23, p = 0.000; Figure 1).
Figure 1.
Echinacoside inhibits 4-aminopyridine-evoked glutamate release from rat cerebrocortical nerve terminals via the Ca2+-dependent exocytotic component. Glutamate release was evoked by 1 mM 4-aminopyridine in the absence (control) or presence of echinacoside (1, 5, 10, 30, and 50 μM), 300 μM ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (omitting CaCl2), 300 μM EGTA (omitting CaCl2) and 20 μM echinacoside, 10 μM dl-threo-beta-benzyl-oxyaspartate (dl-TBOA), 10 μM dl-TBOA and 20 μM echinacoside, 0.1 μM bafilomycin A1, or 0.1 μM bafilomycin A1 and 20 μM echinacoside. Echinacoside was added 10 min before depolarization and, EGTA, dl-TBOA or bafilomycin A1, 20 min prior to this. Results are mean ± S.E.M. of 4–6 independent synaptosomal preparations. *, p < 0.01, **, p < 0.001 versus control group. #, p < 0.05 versus the dl-TBOA-treated group.
2.2. Echinacoside Reduces Cytosolic Ca2+ Concentration but Does Not Alter the Synaptosomal Membrane Potential
Synaptosome depolarization caused by 1 mM 4-aminopyridine increased Ca2+ concentration (p = 0.000; Table 1). The application of 20 µM echinacoside did not significantly affect basal Ca2+ concentration (t(8) = 0.06, p = 0.95) but significantly reduced the 4-aminopyridine-induced increase in Ca2+ concentration (t(10) = 6.16, p = 0.000). In addition, 1 mM 4-aminopyridine increased in 3’,3’,3’-dipropylthiadicarbocyanine iodide [DiSC3(5)] fluorescence (p = 0.000). The addition of 20 µM echinacoside did not alter the resting membrane potential (t(8) = 0.976, p = 0.36) or significantly change the 4-aminopyridine-mediated increase in DiSC3(5) fluorescence (t(8) = −0.014, p = 0.99; Table 1).
Table 1.
Effect of echinacoside on membrane potential and cytosolic Ca2+ levels from rat cortical synaptosomes.
| Membrane Potential (Fluorescence Units) | Cytosolic [Ca2+] (nM) | |||
|---|---|---|---|---|
| Basal | 4-Aminopyridine (1 mM) | Basal | 4-Aminopyridine (1 mM) | |
| Control | 5.6 ± 0.3 | 14.8 ± 0.4 | 144.6 ± 3.1 | 219.3 ± 6.9 |
| Echinacoside | 5.2 ± 0.3 | 14.8 ± 0.4 | 143.7 ± 3.5 | 176.5 ± 6.7 ** |
Each value is mean ± S.E.M. of independent experiments using synaptosomal preparations from 5–6 animals. **, p < 0.001 versus the control group.
2.3. Reduced Ca2+ Influx through the Cav2.2 (N-Type) and Cav2.1 (P/Q-Type) Channels May Be Associated with the Inhibition of 4-Aminopyridine-Evoked Glutamate Release by Echinacoside
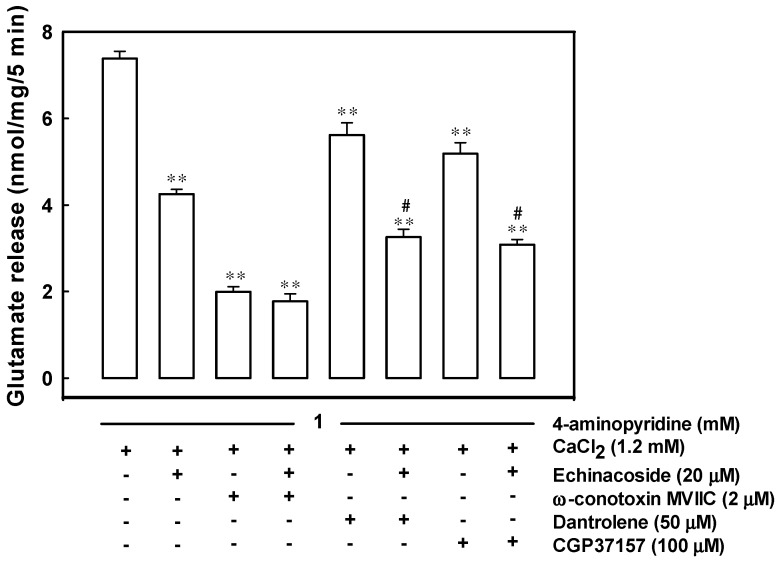
Figure 2 shows that 2 μM ω-conotoxin MVIIC, an N- and P/Q-type Ca2+ channel blocker, reduced 4-aminopyridine-evoked glutamate release from 7.4 ± 0.2 to 2.0 ± 0.1 nmol/mg/5 min (t(9) = 25.35, p = 0.000). In the presence of ω-conotoxin MVIIC, the effect of 20 μM echinacoside on 4-aminopyridine-evoked glutamate release was nonsignificant (1.8 ± 0.2 nmol/mg/5 min; t(8) = 1.06, p = 0.32). Dantrolene (10 µM), an inhibitor of intracellular Ca2+ release from the endoplasmic reticulum [28], reduced 4-aminopyridine-evoked glutamate release (5.6 ± 0.3 nmol/mg/5 min; F(2,14) = 104.95, p = 0.000). However, in the presence of dantrolene, 20 µM echinacoside could still inhibit glutamate release significantly (3.3 ± 0.2 nmol/mg/5 min; p = 0.000). Similar results were observed using 100 µM 7-chloro-5-(2-chloropheny)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157), a membrane-permeable blocker of mitochondrial Na+/Ca2+ exchange. In the five examined synaptosomal preparations, 20 µM echinacoside combined with 100 µM CGP37157 reduced 4-aminopyridine-evoked glutamate release by 48.3% ± 5.2% (F(2,13) = 136.79, p = 0.000), similar to the inhibition by echinacoside alone (46.2% ± 2.3%; p = 0.89; Figure 2).
Figure 2.
Effects of ω-conotoxin MVIIC, dantrolene, and 7-chloro-5-(2-chloropheny)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157) on the echinacoside-mediated inhibition of 4-aminopyridine-evoked glutamate release. Glutamate release was evoked by 1 mM 4-aminopyridine in the absence (control) or presence of 20 μM echinacoside, 2 μM ω-conotoxin MVIIC, 2 μM ω-conotoxin MVIIC and 20 μM echinacoside, 10 μM dantrolene, 10 μM dantrolene and 20 μM echinacoside, 100 μM CGP37157, or 100 μM CGP37157 and 20 μM echinacoside. Echinacoside was added 10 min before depolarization and, ω-conotoxin MVIIC, dantrolene or CGP37157, 30 min prior to this. Results are mean ± S.E.M. of 5–6 independent synaptosomal preparations. **, p < 0.001 versus control group. #, p < 0.05 versus the dantrolene- or CGP37157-treated group.
2.4. Echinacoside Inhibits 4-Aminopyridine-Evoked Glutamate Release by Signaling through Protein Kinase C
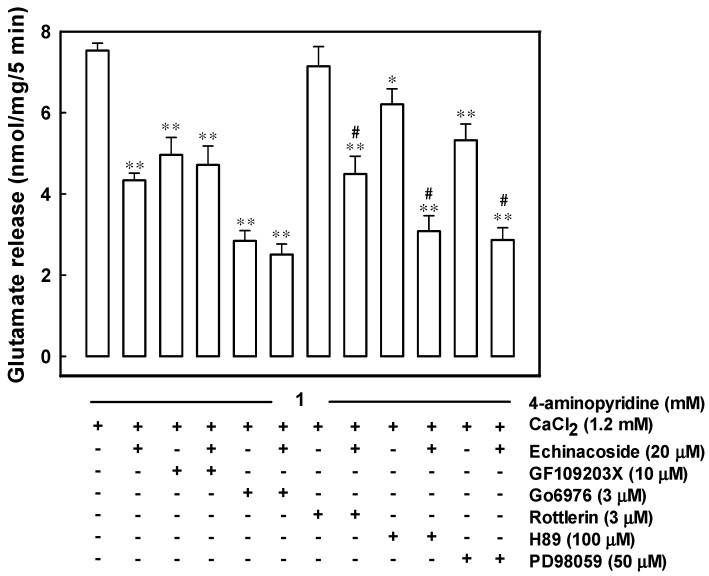
As illustrated in Figure 3, 10 μM 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X), a general protein kinase C inhibitor [29], reduced 4-aminopyridine-evoked glutamate release (F(2,13) = 19.46, p = 0.000). In the GF109203X-treated synaptosomes, 20 μM echinacoside reduced 4-aminopyridine-evoked glutamate release by only 5.5% ± 1.8% (p = 0.89), less than that by echinacoside alone (42.4% ± 2.3%; p = 0.000). Similar results were obtained with 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile (Go6976), a selective for Ca2+-dependent protein kinase C isoforms (α, βI, βII, γ) [29]. In the presence of 3 μM Go6976, 20 μM echinacoside reduced glutamate release by 11.9% ± 2.9% (p = 0.59), signifying a significant reduction compared with that by echinacoside alone (42.4% ± 2.3%; p = 0.000; Figure 3). By contrast, 3 μM rottlerin, a Ca2+-independent protein kinase Cδ inhibitor [30], did not significantly alter 4-aminopyridine (1 mM)-evoked glutamate release (p = 0.45). Nevertheless, in the presence of rottlerin, 20 μM echinacoside effectively caused an average inhibition of 37.1% ± 5.6% of the release (F(2,13) = 19.72, p = 0.000), similar to that by echinacoside alone (p = 0.41; Figure 3). In addition, the mitogen-activated protein kinase inhibitor 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one) (PD98059) (50 μM) and the protein kinase A inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H89) (100 μM) reduced 4-aminopyridine-evoked glutamate release (p = 0.000). Even in the presence of PD98059 or H89, 20 μM echinacoside effectively reduced the release (F(2,13) = 52.3, p = 0.000; Figure 3).
Figure 3.
Echinacoside-mediated inhibition of 4-aminopyridine-evoked glutamate release is blocked by protein kinase C inhibitors. Glutamate release was evoked by 1 mM 4-aminopyridine in the absence (control) or presence of 20 μM echinacoside, 10 μM 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X), 10 μM GF109203X and 20 μM echinacoside, 3 μM 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile (Go6976), 3 μM Go6976 and 20 μM echinacoside, 3 μM rottlerin, 3 μM rottlerin and 20 μM echinacoside, 100 μM N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H89), 100 μM H89 and 20 μM echinacoside, 50 μM PD98059, or 50 μM PD98059 and 20 μM echinacoside. Echinacoside was added 10 min before depolarization and, GF109203X, Go697, rottlerin, H89 or PD98059, 30 min prior to this. Results are mean ± S.E.M. of 5–6 independent synaptosomal preparations. *, p < 0.01, **, p < 0.001 versus control group. #, p < 0.05 versus the rottlerin-, H89-, or PD98059-treated group.
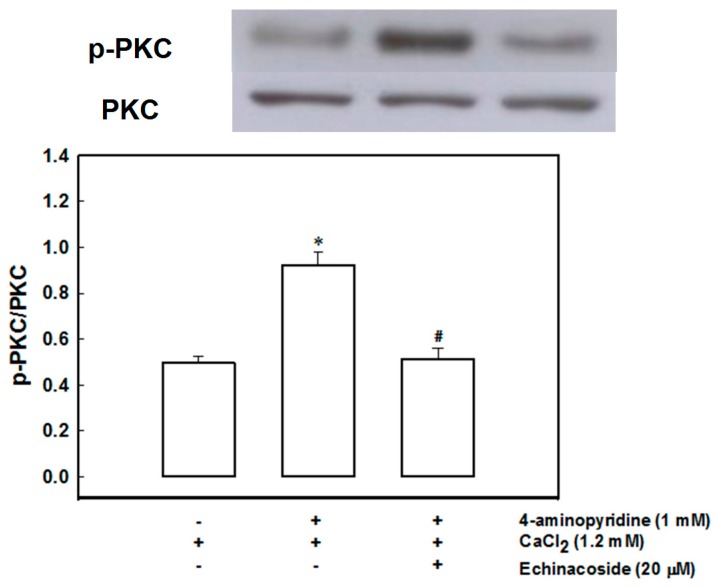
Figure 4 shows that 1 mM 4-aminopyridine increased the phosphorylation of protein kinase C in synaptosomes (t(4) = −6.871, p = 0.002). When synaptosomes were pretreated with 20 μM echinacoside for 10 min before the addition of 4-aminopyridine, 4-aminopyridine induced phosphorylation of protein kinase C considerably decreased (F(2,6) = 29.202, p = 0.001).
Figure 4.
Echinacoside decreases 4-aminopyridine-induced phosphorylation of protein kinase C (PKC). Phosphorylation of PKC was detected in synaptosomal lysates by Western blotting using anti-phospho-PKC (pan) antibody. Synaptosomes were incubated for 2 min in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer medium that contained 1.2 mM CaCl2 at 37 °C in the absence (control) or presence of 1 mM 4-aminopyridine, or 1 mM 4-aminopyridine and 20 µM echinacoside added 10 min before the addition of 4-aminopyridine. Results are the mean ± S.E.M. of five independent synaptosomal preparations. *, p < 0.01 versus control group. #, p < 0.05 versus the 4-aminopyridine-treated group.
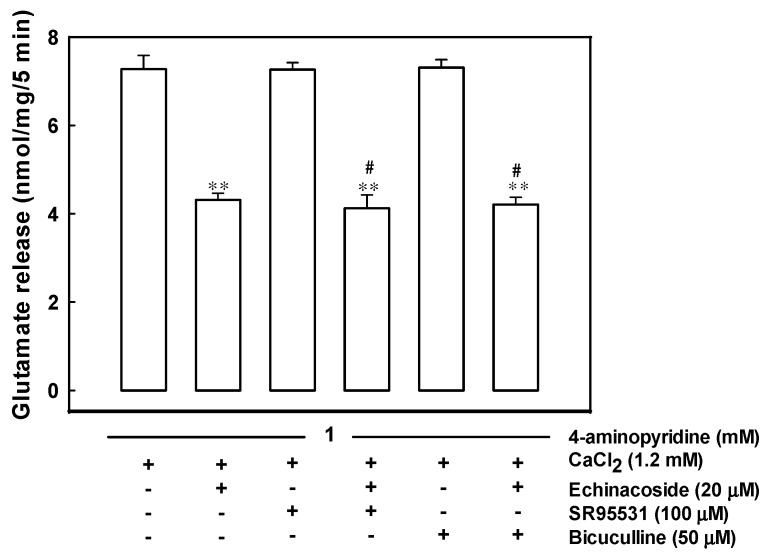
2.5. Echinacoside-Mediated Inhibition of Glutamate Release Does Not Involve a Gamma-Aminobutyric Acid Type A (GABAA) Receptor
In Figure 5, the effect of echinacoside on 4-aminopyridine-evoked glutamate release in the absence or presence of SR95531 (an antagonist of the GABAA receptor) was compared. In addition, 100 µM SR95531 did not significantly alter 4-aminopyridine (1 mM)-evoked glutamate release. In the SR95531-treated synaptosomes, application of 20 µM echinacoside resulted in a 43% inhibition on 4-aminopyridine-evoked glutamate release (F(2,12) = 42.63, p = 0.000), which was not significantly different from the inhibition produced by echinacoside alone (40%; p = 0.000). A similar result was obtained with another GABAA receptor antagonist, bicuculline (50 µM). The release measured in the presence of bicuculline and echinacoside was significantly different from that obtained in the presence of bicuculline alone (p = 0.000).
Figure 5.
Effect of the gamma-Aminobutyric acid A (GABAA) receptor antagonists SR95531 and bicuculline on the echinacoside-mediated inhibition of glutamate release. Glutamate release was evoked by 1 mM 4-aminopyridine in the absence (control) or in the presence of 20 µM echinacoside, 100 µM SR95531, 100 µM SR95531 and 20 µM echinacoside, 50 µM bicuculline, or 50 µM bicuculline and 20 µM echinacoside. Results are mean ± S.E.M. of 5 independent synaptosomal preparations. **, p < 0.001 versus control group. #, p < 0.05 versus the SR95531-, or bicuculline-treated group.
3. Discussion
In this study, echinacoside, an active compound in Herba Cistanche, inhibited 4-aminopyridine-evoked glutamate release in the rat cerebral cortex nerve terminals. The possible underlying mechanisms for the echinacoside-mediated inhibition of glutamate release are further investigated and discussed here.
3.1. Mechanisms Underlying Echinacoside-Mediated Inhibition of Glutamate Release
Glutamate release evoked by 4-aminopyridine comprises two components: a physiologically relevant Ca2+-dependent component, which is produced through exocytosis of synaptic vesicles containing glutamate; and a Ca2+-independent component, which originates from prolonged depolarization causing a membrane potential-mediated shift of the glutamate transporter steady-state toward the outward direction, thus affecting cytosolic glutamate efflux [31]. Here, we observed that echinacoside did not significantly inhibit 4-aminopyridine-evoked glutamate release in the presence of a Ca2+-free medium (Ca2+-independent release). Furthermore, the observed echinacoside-mediated inhibition of 4-aminopyridine-evoked glutamate release was effectively prevented by bafilomycin A1 (which depletes the glutamate content of synaptic vesicles), but not by dl-TBOA (which nonselectively inhibits all excitatory amino acid transporter subtypes). These results suggest that echinacoside affects the Ca2+-dependent exocytosis of glutamate release without affecting the Ca2+-independent cytosolic efflux of glutamate through the reversal of the nerve terminal plasma membrane glutamate transporter.
In synaptic terminals, Na+ channel inhibition or K+ channel activation stabilizes membrane excitability and consequently reduced the evoked Ca2+ entry and neurotransmitter release [32,33]. Therefore, the potential mechanism underlying echinacoside-mediated glutamate release inhibition involves a reduction in synaptosomal excitability. However, this possibility is untenable on the basis of two observations: (1) 4-aminopyridine-evoked membrane potential depolarization, measured with the membrane potential sensitive dye DiSC3(5) was unaffected by the addition of echinacoside; and (2) echinacoside did not affect the 4-aminopyridine-evoked Ca2+-independent glutamate release, a component of release that depends on only the membrane potential [31]. If the effect is not caused by synaptosomal excitability suppression, it may be manifested through a reduction in the activity of Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels coupled with glutamate exocytosis in the nerve terminals [34,35,36]. By using fura-2, we demonstrate that echinacoside significantly reduces the 4-aminopyridine-evoked increase in Ca2+ concentration. In addition, our data show that the inhibitory effect of echinacoside on 4-aminopyridine-evoked glutamate release decreased from 42.4% ± 2.3% to 12.1% ± 3.9% after exposure to a blocker of Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels. Furthermore, we observed that echinacoside continuted to significantly inhibit 4-aminopyridine-evoked glutamate release in the presence of intracellular Ca2+ release inhibitors. These results indicate that the simultaneous suppression of Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channel activity is the potentially mechanism underlying echinacoside-mediated glutamate release inhibition. However, the combined activation of Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channel activity could not block the action of echinacoside completely. Hence, other unidentified types of Ca2+ channels or other presynaptic pathways may be involved in the inhibition. For example, GABAA receptors are present at the presynaptic level, and their activation has been shown to inhibit Ca2+ influx and glutamate release [37]. In the present study, the GABAA receptor antagonists SR95531 and bicuculline did not block echinacoside-mediated inhibition of glutamate release, suggesting that GABAA receptors are not involved in the reduction of voltage-dependent Ca2+ channel activity and in the subsequent inhibition of glutamate release.
Ca2+ entry through voltage-dependent Ca2+ channels activates several protein kinases associated with glutamate release in nerve terminals including mitogen-activated protein kinase, protein kinase C, and protein kinase A. Here, we demonstrate that protein kinase C inhibitors efficiently antagonized the echinacoside-mediated inhibition of glutamate release; nevertheless, the mitogen-activated protein kinase inhibitor PD98059 or the protein kinase A inhibitor H89 was ineffective. Furthermore, the 4-aminopyridine-induced phosphorylation of protein kinase C decreased in synaptosomes after pretreatment with echinacoside at a concentration effective for inhibiting glutamate release. Therefore, the signaling pathway of the echinacoside-mediated glutamate release inhibition may involve protein kinase C. Protein kinase C is an important intracellular signaling system that is present at the presynaptic level and has a crucial role in neurotransmitter exocytosis. For example, several synaptic proteins involved in the synaptic vesicle trafficking or recruitment and exocytosis, such as the myristoylated alanine-rich C kinase substrate, are phosphorylated by protein kinase C [38,39]. This phosporylation process can be increased by depolarization-stimulated Ca2+ entry, which facilitates glutamate release [40]. Hence, we can reasonably speculate that the inhibitory effect of echinacoside on Ca2+ entry observed here may reduce protein kinase C activity and consequently glutamate release.
3.2. Therapeutic Implications
Excitotoxicity, a pathological process caused by excessive glutamate release and glutamate receptor activation, is the major cause of neuronal death in acute and chronic brain disorders such as stroke, traumatic brain injury, Parkinson’s, and Alzheimer’s diseases [13,41], and therapeutic strategies involving glutamate release inhibition may be promising neuroprotective strategies for treating such diseases. Echinacoside has been confirmed to penetrate the blood-brain barrier (BBB) and exhibits neuroprotective effects in various in vivo models of neurotoxicity [8,10,11,12,42]. Although the mechanism of these neuroprotective effects is not completely understood, several possible mechanisms have been reported including inflammatory response inhibition, mitochondrial function stabilization, antioxidation, free radical scavenging, and neurotrophic function mimicking [5,9,12,42]. In the current study, the ability of echinacoside to reduce glutamate release from nerve terminals may also partly explain its neuroprotective mechanism. However, whether this effect contributes to the apparent therapeutic potential of echinacoside in brain disorders associated with glutamate excitotoxicity warrants further research.
4. Materials and Methods
4.1. Chemicals
Fura-2-acetoxymethyl ester (Fura-2-AM) and 3’,3’,3’-dipropylthiadicarbocyanine iodide [DiSC3(5)] were purchased from Invitrogen (Carlsbad, CA, USA). ω-conotoxin MVIIC, rottlerin, 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X), 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile (Go6976) and N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H89) were purchased from Tocris Bioscience (Bristol, UK). Echinacoside, dantrolene, dl-threo-beta-benzyl-oxyaspartate (dl-TBOA), 7-chloro-5-(2-chloropheny)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157), 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one) (PD98059), ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) and all other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
4.2. Animals
Two-month old male Sprague–Dawley rats were used. Animals were housed under standardized environmental conditions (22 ± 1 °C; 50% relative humidity; 12 h light/dark cycle) and allowed unlimited access to food and water. The animals were killed by decapitation and the cerebral cortex rapidly removed at 4 °C. The experimental procedures were approved by the Fu Jen Institutional Animal Care and Utilization Committee (A10259), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to use a minimum number of animals necessary to produce reliable results.
4.3. Synaptosomal Preparations
Synaptosomes were purified from the cerebral cortex of rats on discontinuous Percoll gradients as described previously [43,44]. Briefly, the tissue was homogenized in medium containing 0.32 M sucrose (pH 7.4), the homogenate was centrifuged for 10 min at 3000× g (5000 rpm in a JA 25.5 rotor; Beckman Coulter, Inc., Miami, FL, USA) and 4 °C, and the supernatant was centrifuged again for 12 min at 14,500× g (11,000 rpm in a JA 25.5 rotor). The pellet was gently resuspended in 0.32 M sucrose (pH 7.4), and an aliquot of this synaptosomal suspension (2 mL) was placed onto a 3 mL Percoll discontinuous gradient containing 0.32 M sucrose, 1 mM EDTA, 0.25 mM dl-dithiothreitol, and 3%, 10%, and 23% Percoll (pH 7.4). After centrifugation at 32,500× g (16,500 rpm in a JA 20.5 rotor) for 7 min at 4 °C, the synaptosomes were recovered from between the 10% and the 23% Percoll bands, and they were diluted in a final volume of 30 mL of HEPES buffer medium (140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1 mM MgCl2·6H2O, 1.2 mM Na2HPO4, 10 mM glucose, and 10 mM HEPES (pH 7.4)). Following further centrifugation at 27,000× g (15,000 rpm in a JA 25.5) for 10 min, the synaptosome pellet was resuspended in 3 mL of HEPES buffer medium, and the protein content was determined using a Bradford assay. Finally, 0.5 mg of the synaptosomes suspension was diluted in 10 ml of HEPES buffer medium and centrifuged at 3000× g (5000 rpm in a JA 20.1 rotor) for 10 min. The supernatant was discarded, and the pellets containing the synaptosomes were stored on ice and used within 4–6 h.
4.4. Glutamate Release
Glutamate release was assayed by on-line fluorimetry as described previously [45,46]. Synaptosomal pellets were resuspended in HEPES buffer medium (0.5 mg/mL) and preincubated at 37 °C for 10 min in the presence of 16 µM bovine serum albumin to bind any free fatty acids released from synaptosomes during preincubation. A 2-mL aliquot of the synaptosomes was transferred to a stirred cuvette containing 2 mM NADP+, 50 units of glutamate dehydrogenase, and 1.2 mM CaCl2, and the fluorescence of NADPH was measured in a Perkin-Elmer LS-55 spectrofluorimeter (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) at excitation and emission wavelengths of 340 and 460 nm, respectively. As synaptosomes are not amenable to electrical stimulation, the potassium channel blocker 4-aminopyridine was used to stimulate glutamate release. 4-aminopyridine destabilizes the membrane potential and is thought to cause repetitive spontaneous Na+ channel-dependent depolarization that closely approximates in vivo depolarization of the synaptic terminal that leads to the activation of voltage-dependent Ca2+ channels and neurotransmitter release [47]. Data were obtained at 2 s intervals. A standard of exogenous glutamate (5 nmol) was added at the end of each experiment. The value of the fluorescence change produced by the standard addition was used to calculate the released glutamate as nanomoles of glutamate per milligram of synaptosomal protein (nmol/mg). Release values quoted in the text are levels attained at steady-state after 5 min of depolarization (nmol/mg/5 min). Cumulative data were analyzed using Lotus 1-2-3 spreadsheets (IBM, White Plains, NY, USA) and MicroCal Origin (OriginLab Corporation, Northampton, MA, USA).
4.5. Plasma Membrane Potential
The plasma membrane potential was determined with a membrane-potential-sensitive dye, DiSC3(5) [48]. Synaptosomes were resuspended in HEPES buffer medium, and 2 mL aliquots were transferred to a stirred cuvette containing 5 µM DiSC3(5) at 37 °C in a Perkin–Elmer LS-55 spectrofluorometer (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). After allowing the mixture to equilibrate for 3 min, the fluorescence was determined at excitation and emission wavelengths of 646 and 674 nm, respectively. Data were collected at 2 s intervals. Cumulative data were analyzed using MicroCal Origin (OriginLab Corporation, Northampton, MA, USA) and expressed in fluorescence units.
4.6. Cytosolic Ca2+ Concentration ([Ca2+]C)
The [Ca2+]C was measured with the Ca2+ indicator fura-2. Synaptosomes (0.5 mg/mL) were preincubated in HEPES buffer medium containing 5 µM fura-2 and 0.1 mM CaCl2, for 30 min at 37 °C in a stirred test tube. After fura-2 loading, synaptosomes were centrifuged in a microcentrifuge for 30 s at 3000× g (5000 rpm). The synaptosomal pellets were resuspended in HEPES buffer medium, and the synaptosomal suspension was stirred in a thermostatted cuvette in a Perkin-Elmer LS-55 spectrofluorometer (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). CaCl2 (1 mM) was added after 3 min and further additions were made after an additional 10 min. Fluorescence data were accumulated at excitation wavelengths of 340 and 380 nm (emission wavelength 505 nm) at 2 s intervals. [Ca2+]C (nM) was calculated using calibration procedures [49] and equations described previously [50]. Cumulative data were analyzed using MicroCal Origin (OriginLab Corporation, Northampton, MA, USA).
4.7. Western Blotting
Synaptosomes were homogenized in a lysis buffer (10 mM HEPES buffer, pH 7.4), 1% Triton X-100, and protease inhibitor mixture. Lysates were clarified by centrifugation, and protein concentration was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. The membranes were blocked with Tris-buffered saline that contained 5% low-fat milk and incubated with appropriate primary antibody (phospho-protein kinase C (pan), 1:3000, NOVUS Biologicals Inc., Beverly, MA, USA) overnight at 4 °C. After three washes in Tris-buffered saline, the membrane was then treated with the secondary horseradish peroxidase-conjugated antibody (1:3000) for 1 h at room temperature. The membranes were then washed at least three times with Tris-buffered saline and visualized using the enhanced chemiluminescence system (Amersham, Buckinghamshire, UK). An aliquot of samples was loaded and probed with anti-PKC antibody for detection of PKC as a loading control. The level of expression or phosphorylation was assessed by band density, which was quantified by densitometry. Densitometric quantification of bands was analyzed using Syngene software (Synoptics, Cambridge, UK).
4.8. Statistical Analysis
Data were obtained from a single synaptosomal preparation and were not independent of one another. To test the significance of the effect of a drug versus control, a two-tailed Student’s t-test was used. When an additional comparison was required (such as whether a second treatment influenced the action of echinacoside), a one-way ANOVA followed by Tukey’s test was used. Analysis was completed via software SPSS (17.0; SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± S.E.M.; significance was evaluated at p < 0.05 for all statistical measures.
5. Conclusions
This is the first study demonstrating that echinacoside inhibits glutamate release from rat cerebrocortical synaptosomes by reducing Ca2+ influx through Cav2.2 and Cav2.1 channels, and this release inhibition is likely dependent on the suppression of the protein kinase C pathway, at least in part. The present finding is valuable because it provides a novel insight into the mechanisms of action of echinacoside in the brain.
Acknowledgments
This work was supported by a grant from the Ministry of Science and Technology (MOST 103-2320-B-030-001 MY3).
Author Contributions
Tzu Yu Lin and Su Jane Wang conceived and designed the experiments; Cheng Wei Lu performed the experiments; Cheng Wei Lu and Shu Kuei Huang analyzed the data; Su Jane Wang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tu P.F., Wang B., Deyama T., Zhang Z.G., Lou Z.C. Analysis of phenylethanoid glycosides of Herba cistanchis by RP-HPLC. Acta Pharm. Sin. 1997;32:294–300. [PubMed] [Google Scholar]
- 2.Dalby-Brown L., Barsett H., Landbo A.K., Meyer A.S., Molgaard P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. J. Agric. Food Chem. 2005;53:9413–9423. doi: 10.1021/jf0502395. [DOI] [PubMed] [Google Scholar]
- 3.Dapas B., Dall’Acqua S., Bulla R., Agostinis C., Perissutti B., Invernizzi S., Grassi G., Voinovich D. Immunomodulation mediated by a herbal syrup containing a standardized Echinacea root extract: A pilot study in healthy human subjects on cytokine gene expression. Phytomedicine. 2014;21:1406–1410. doi: 10.1016/j.phymed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 4.He W.J., Fang T.H., Tu P.F. Research progress on pharmacological activities of echinacoside. China J. Chin. Mater. Med. 2009;34:476–479. [PubMed] [Google Scholar]
- 5.Deng M., Zhao J.Y., Tu P.F., Jiang Y., Li Z.B., Wang Y.H. Echinacoside rescues the SHSY5Y neuronal cells from TNFalpha-induced apoptosis. Eur. J. Pharmacol. 2004;505:11–18. doi: 10.1016/j.ejphar.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Koo K.A., Sung S.H., Park J.H., Kim S.H., Lee K.Y., Kim Y.C. In vitro neuroprotective activities of phenylethanoid glycosides from Callicarpa dichotoma. Planta Med. 2005;71:778–780. doi: 10.1055/s-2005-871213. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y.H., Xuan Z.H., Tian S., Du G.H. Echinacoside Protects against 6-Hydroxydopamine-Induced Mitochondrial Dysfunction and Inflammatory Responses in PC12 Cells via Reducing ROS Production. Evid. Based Complement. Altern. Med. 2015;2015:189239. doi: 10.1155/2015/189239. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Zhu M., Lu C., Li W. Transient exposure to echinacoside is sufficient to activate Trk signaling and protect neuronal cells from rotenone. J. Neurochem. 2013;124:571–580. doi: 10.1111/jnc.12103. [DOI] [PubMed] [Google Scholar]
- 9.Geng X., Tian X., Tu P., Pu X. Neuroprotective effects of echinacoside in the mouse MPTP model of Parkinson's disease. Eur. J. Pharmacol. 2007;564:66–74. doi: 10.1016/j.ejphar.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 10.Wei L.L., Chen H., Jiang Y., Tu P.F., Zhong M., Du J., Liu F., Wang L., Liu C.Y. Effects of echinacoside on histio-central levels of active mass in middle cerebral artery occlusion rats. Biomed. Environ. Sci. 2012;25:238–244. doi: 10.3967/0895-3988.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.R., Lin H.C., Su M.H. Reversal by aqueous extracts of Cistanche tubulosa from behavioral deficits in Alzheimer’s disease-like rat model: Relevance for amyloid deposition and central neurotransmitter function. BMC Complement. Altern. Med. 2014;14:1006. doi: 10.1186/1472-6882-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q., Gao J., Li W., Cai D. Neurotrophic and neurorescue effects of Echinacoside in the subacute MPTP mouse model of Parkinson’s disease. Brain Res. 2010;1346:224–236. doi: 10.1016/j.brainres.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Meldrum B.S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 14.Lee D., Shim M.S., Kim K.Y., Noh Y.H., Kim H., Kim S.Y., Weinreb R.N., Ju W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014;55:993–1005. doi: 10.1167/iovs.13-12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi D.W. Calcium and excitotoxic neuronal injury. Ann. N. Y. Acad. Sci. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- 16.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers. Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 17.Sattler R., Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- 18.Schauwecker P.E. Neuroprotection by glutamate receptor antagonists against seizure-induced excitotoxic cell death in the aging brain. Exp. Neurol. 2010;224:207–218. doi: 10.1016/j.expneurol.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeganeh F., Nikbakht F., Bahmanpour S., Rastegar K., Namavar R. Neuroprotective effects of NMDA and group I metabotropic glutamate receptor antagonists against neurodegeneration induced by homocysteine in rat hippocampus: In vivo study. J. Mol. Neurosci. 2013;50:551–557. doi: 10.1007/s12031-013-9996-5. [DOI] [PubMed] [Google Scholar]
- 20.Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol. Ther. 1999;81:163–221. doi: 10.1016/S0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 21.Muir K.W. Glutamate-based therapeutic approaches: Clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.González J.C., Egea J., del Carmen Godino M., Fernandez-Gomez F.J., Sánchez-Prieto J., Gandía L., García A.G., Jordán J., Hernández-Guijo J.M. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca2+ signalling in hippocampal neurons. Eur. J. Neurosci. 2007;26:2481–2495. doi: 10.1111/j.1460-9568.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- 23.Lu C.W., Lin T.Y., Wang S.J. Memantine depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebral cortex nerve terminals: An NMDA receptor-independent mechanism. Neurochem. Int. 2010;57:168–176. doi: 10.1016/j.neuint.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang S.J., Sihra T.S. Noncompetitive metabotropic glutamate 5 receptor antagonist (E)-2-methyl-6-styryl-pyridine (SIB1893) depresses glutamate release through inhibition of voltage-dependent Ca2+ entry in rat cerebrocortical nerve terminals (synaptosomes) J. Pharmacol. Exp. Ther. 2004;309:951–958. doi: 10.1124/jpet.103.064881. [DOI] [PubMed] [Google Scholar]
- 25.Dunkley P.R., Jarvie P.E., Heath J.W., Kidd G.J., Rostas J.A. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- 26.Araque A., Li N., Doyle R.T., Haydon P.G. SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlop J. Glutamate-based therapeutic approaches: Targeting the glutamate transport system. Curr. Opin. Pharmacol. 2006;6:103–107. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Zucchi R., Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol. Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 29.Fan Y., Li J., Zhang Y.Q., Jiang L.H., Zhang Y.N., Yan C.Q. Protein kinase C delta mediated cytotoxicity of 6-Hydroxydopamine via sustained extracellular signal-regulated kinase 1/2 activation in PC12 cells. Neurol. Res. 2014;36:53–64. doi: 10.1179/1743132813Y.0000000267. [DOI] [PubMed] [Google Scholar]
- 30.Gschwendt M., Müller H.J., Kielbassa K., Zang R., Kittstein W., Rincke G., Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls D.G., Sihra T.S., Sanchez-Prieto J. Calcium-dependent and-independent release of glutamate from synaptosomes monitored by continuous fluorometry. J. Neurochem. 1987;49:50–57. doi: 10.1111/j.1471-4159.1987.tb03393.x. [DOI] [PubMed] [Google Scholar]
- 32.Nicoll R.A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988;241:545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- 33.Wu L.G., Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/S0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 34.Turner T.J., Dunlap K. Pharmacological characterization of presynaptic calcium channels using subsecond biochemical measurements of synaptosomal neurosecretion. Neuropharmacology. 1995;34:1469–1478. doi: 10.1016/0028-3908(95)00133-Q. [DOI] [PubMed] [Google Scholar]
- 35.Millan C., Sanchez-Prieto J. Differential coupling of N- and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci. Lett. 2002;330:29–32. doi: 10.1016/S0304-3940(02)00719-X. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez E., Sanchez-Prieto J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur. J. Neurosci. 1997;9:2009–2018. doi: 10.1111/j.1460-9568.1997.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 37.Long P., Mercer A., Begum R., Stephens G.J., Sihra T.S., Jovanovic J.N. Nerve Terminal GABAA Receptors Activate Ca2+/Calmodulin-dependent Signaling to Inhibit Voltage-gated Ca2+ Influx and Glutamate Release. J. Biol. Chem. 2009;284:8726–8737. doi: 10.1074/jbc.M805322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner J.R., Angle J.M., Black E.D., Joyal J.L., Sacks D.B., Madara J.L. PKC-dependent regulation of transepithelial resistance: Roles of MLC and MLC kinase. Am. J. Physiol. 1999;277:C554–C562. doi: 10.1152/ajpcell.1999.277.3.C554. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan P.F., Walker J.H., Peers C. The regulation of neurotransmitter secretion by protein kinase C. Mol. Neurobiol. 1998;18:125–155. doi: 10.1007/BF02914269. [DOI] [PubMed] [Google Scholar]
- 40.Coffey E.T., Sihra T.S., Nicholls D.G., Pocock J.M. Phosphorylation of synapsin I and MARCKS in nerve terminals is mediated by Ca2+ entry via an Aga-GI sensitive Ca2+ channel which is coupled to glutamate exocytosis. FEBS Lett. 1994;353:264–268. doi: 10.1016/0014-5793(94)01061-7. [DOI] [PubMed] [Google Scholar]
- 41.Obrenovitch T.P., Urenjak J. Altered glutamatergic transmission in neurological disorders: From high extracellular glutamate to excessive synaptic efficacy. Prog. Neurobiol. 1997;51:39–87. doi: 10.1016/S0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D., Li H., Wang J.B. Echinacoside inhibits amyloid fibrillization of HEWL and protects against Aβ-induced neurotoxicity. Int. J. Biol. Macromol. 2015;72:243–253. doi: 10.1016/j.ijbiomac.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 43.Lin T.Y., Lu C.W., Wang C.C., Lu J.F., Wang S.J. Hispidulin inhibits the release of glutamate in rat cerebrocortical nerve terminals. Toxicol. Appl. Pharmacol. 2012;263:233–243. doi: 10.1016/j.taap.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Chang C.Y., Lin T.Y., Lu C.W., Huang S.K., Wang Y.C., Chou S.S., Wang S.J. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;2015:157–169. doi: 10.1016/j.neuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Nicholls D.G., Sihra T.S. Synaptosomes possess an exocytotic pool of glutamate. Nature. 1986;321:772–773. doi: 10.1038/321772a0. [DOI] [PubMed] [Google Scholar]
- 46.Wang C.C., Kuo J.R., Wang S.J. Dimebon, an antihistamine drug, inhibits glutamate release in rat cerebrocortical nerve terminals. Eur. J. Pharmacol. 2014;734:67–76. doi: 10.1016/j.ejphar.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 47.Tibbs G.R., Barrie A.P., van Mieghem F.J., Mc-Mahon H.T., Nicholls D.G. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: Effects on cytosolic free Ca2+ and glutamate release. J. Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- 48.Akerman K.E., Scott L.G., Heikkila J.E., Heinonen E. Ionic dependence of membrane potential and glutamate receptor-linked responses in synaptoneurosomes as measured with a cyanine dye, DiSC2(5) J. Neurochem. 1987;48:552–559. doi: 10.1111/j.1471-4159.1987.tb04128.x. [DOI] [PubMed] [Google Scholar]
- 49.Sihra T.S., Bogonez E., Nicholls D.G. Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J. Biol. Chem. 1992;267:1983–1989. [PubMed] [Google Scholar]
- 50.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]