Abstract
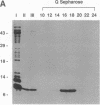
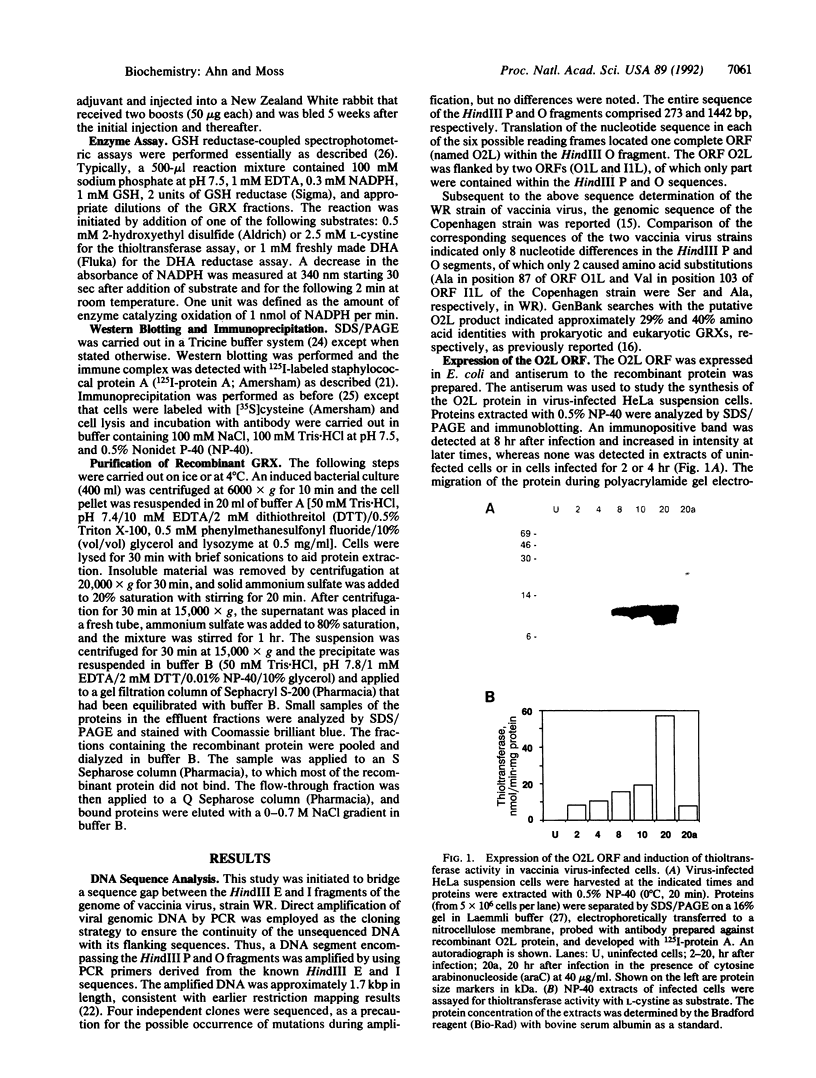
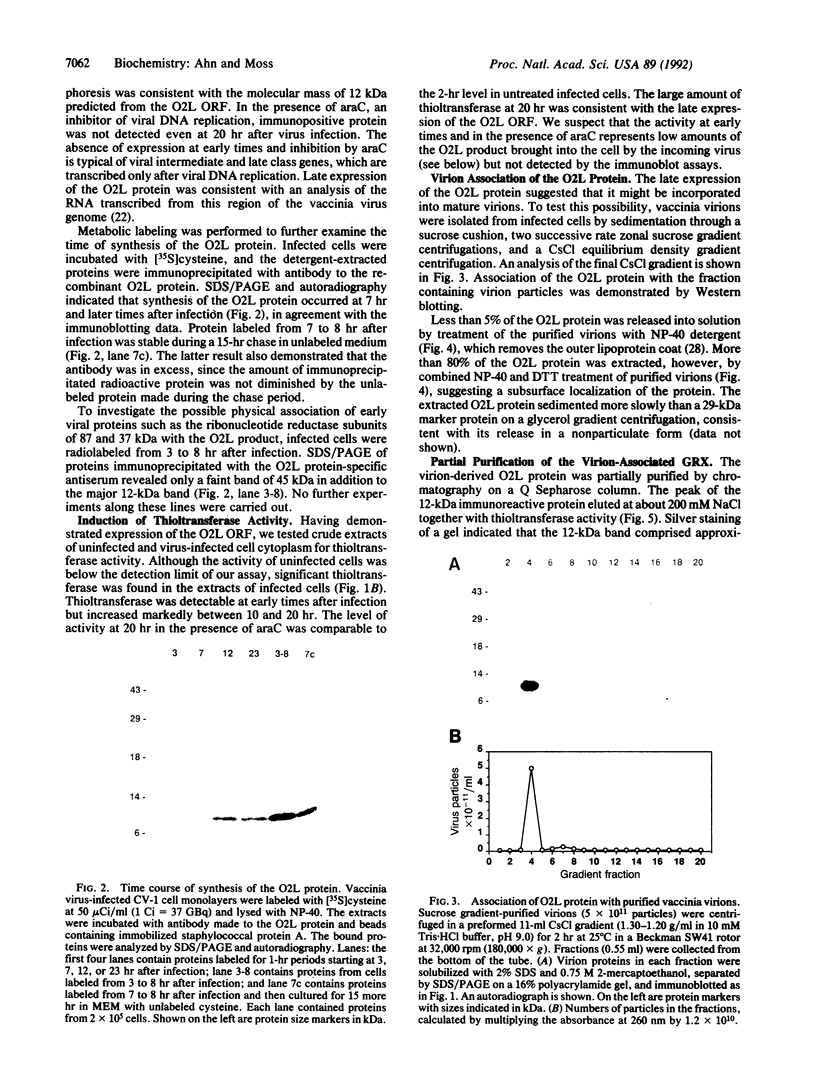
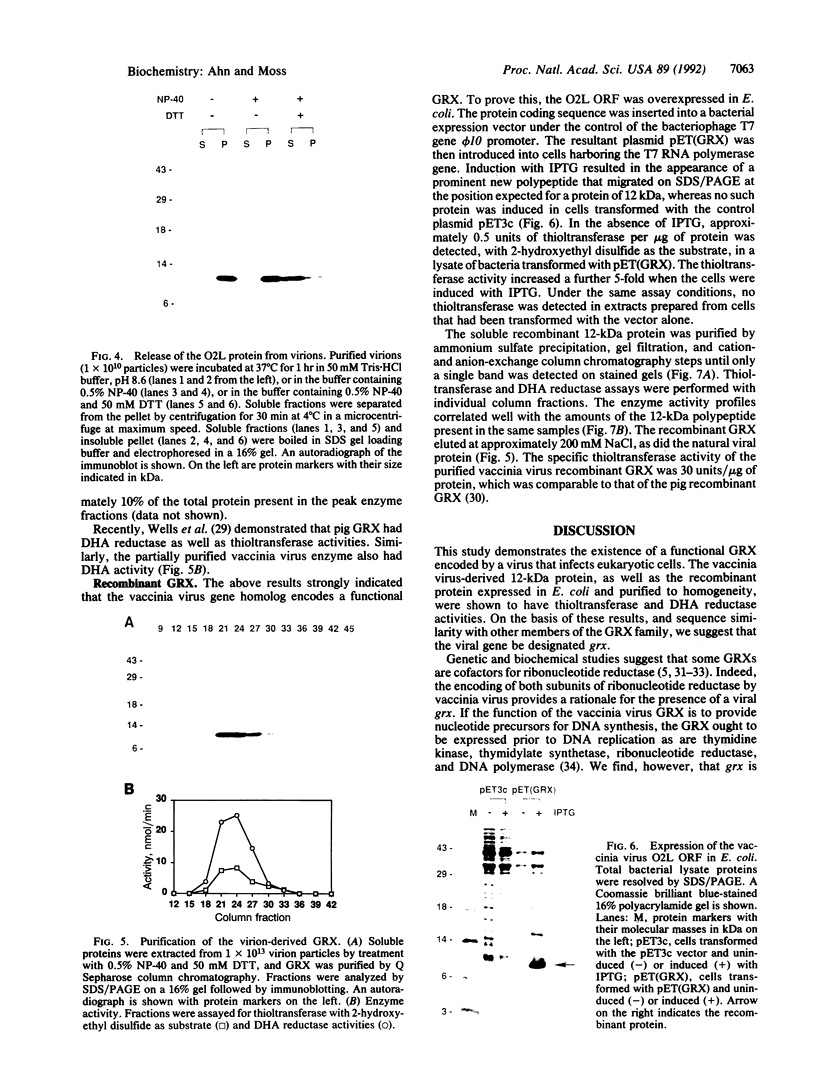
Glutaredoxins (GRXs), also known as thioltransferases, use glutathione as a cofactor for reduction of disulfides in prokaryotes and eukaryotes. We demonstrate that the vaccinia virus O2L open reading frame encodes a functional GRX, as predicted by Johnson et al. [Johnson, G. P., Goebel, S. J., Perkus, M. E., Davis, S. W., Winslow, J. P. & Paoletti, E. (1991) Virology 181, 378-381] from sequence homology. The 12-kDa protein product of the O2L open reading frame was synthesized after viral DNA replication, coincident with a major increase in cytoplasmic glutathione-dependent thioltransferase activity. The protein was associated with purified vaccinia virions and was not released by treatment with a nonionic detergent unless dithiothreitol was added. The virion-derived protein, as well as a recombinant form expressed in Escherichia coli, exhibited thioltransferase and dehydroascorbate reductase activities indicative of a functional GRX. The postreplicative synthesis of vaccinia virus GRX and its association with virions suggest that the enzyme may have novel roles in the virus growth cycle.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Gershon P. D., Jones E. V., Moss B. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol Cell Biol. 1990 Oct;10(10):5433–5441. doi: 10.1128/mcb.10.10.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Rosel J., Cole N. B., Moss B. Identification and expression of rpo19, a vaccinia virus gene encoding a 19-kilodalton DNA-dependent RNA polymerase subunit. J Virol. 1992 Feb;66(2):971–982. doi: 10.1128/jvi.66.2.971-982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Sundquist A. R. Evolution of glutathione metabolism. Adv Enzymol Relat Areas Mol Biol. 1991;64:1–53. doi: 10.1002/9780470123102.ch1. [DOI] [PubMed] [Google Scholar]
- Gan Z. R., Polokoff M. A., Jacobs J. W., Sardana M. K. Complete amino acid sequence of yeast thioltransferase (glutaredoxin). Biochem Biophys Res Commun. 1990 May 16;168(3):944–951. doi: 10.1016/0006-291x(90)91120-h. [DOI] [PubMed] [Google Scholar]
- Gan Z. R., Wells W. W. Purification and properties of thioltransferase. J Biol Chem. 1986 Jan 25;261(3):996–1001. [PubMed] [Google Scholar]
- Gan Z. R., Wells W. W. The primary structure of pig liver thioltransferase. J Biol Chem. 1987 May 15;262(14):6699–6703. [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent enzyme reactions of the phage T4 ribonucleotide reductase system. J Biol Chem. 1978 Oct 25;253(20):7424–7430. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979 May 10;254(9):3672–3678. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J Biol Chem. 1979 May 10;254(9):3664–3671. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Ohlsson I., Grankvist M. L. Thiroedoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978 Jan 25;253(2):430–436. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hopper S., Johnson R. S., Vath J. E., Biemann K. Glutaredoxin from rabbit bone marrow. Purification, characterization, and amino acid sequence determined by tandem mass spectrometry. J Biol Chem. 1989 Dec 5;264(34):20438–20447. [PubMed] [Google Scholar]
- Hög J. O., von Bahr-Lindström H., Jörnvall H., Holmgren A. Cloning and expression of the glutaredoxin (grx) gene of Escherichia coli. Gene. 1986;43(1-2):13–21. doi: 10.1016/0378-1119(86)90003-x. [DOI] [PubMed] [Google Scholar]
- Johnson G. P., Goebel S. J., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. Vaccinia virus encodes a protein with similarity to glutaredoxins. Virology. 1991 Mar;181(1):378–381. doi: 10.1016/0042-6822(91)90508-9. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren B., Parsell D., Fuchs J. A. Isolation and characterization of an Escherichia coli K-12 mutant deficient in glutaredoxin. J Bacteriol. 1988 Jan;170(1):308–315. doi: 10.1128/jb.170.1.308-315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Papayannopoulos I. A., Gan Z. R., Wells W. W., Biemann K. A revised sequence of calf thymus glutaredoxin. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1448–1454. doi: 10.1016/0006-291x(89)92272-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. The role of thioredoxin in filamentous phage assembly. Construction, isolation, and characterization of mutant thioredoxins. J Biol Chem. 1986 Nov 15;261(32):14997–15005. [PubMed] [Google Scholar]
- Schmitt J. F., Stunnenberg H. G. Sequence and transcriptional analysis of the vaccinia virus HindIII I fragment. J Virol. 1988 Jun;62(6):1889–1897. doi: 10.1128/jvi.62.6.1889-1897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Slabaugh M., Roseman N., Davis R., Mathews C. Vaccinia virus-encoded ribonucleotide reductase: sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J Virol. 1988 Feb;62(2):519–527. doi: 10.1128/jvi.62.2.519-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tengelsen L. A., Slabaugh M. B., Bibler J. K., Hruby D. E. Nucleotide sequence and molecular genetic analysis of the large subunit of ribonucleotide reductase encoded by vaccinia virus. Virology. 1988 May;164(1):121–131. doi: 10.1016/0042-6822(88)90627-7. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]
- Yang Y. F., Wells W. W. High-level expression of pig liver thioltransferase (glutaredoxin) in Escherichia coli. J Biol Chem. 1990 Jan 5;265(1):589–593. [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]