Abstract
Interference of the binding of programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) has become a new inspiring immunotherapy for resisting cancers. To date, the FDA has approved two PD-1 monoclonal antibody drugs against cancer as well as a monoclonal antibody for PD-L1. More PD-1 and PD-L1 monoclonal antibody drugs are on their way in clinical trials. In this review, we focused on the mechanism of the PD-1/PD-L1 signaling pathway and the monoclonal antibodies (mAbs) against PD-1 and PD-L1, which were approved by the FDA or are still in clinical trials. And also presented is the prospect of the PD-1/PD-L1 immune checkpoint blockade in the next generation of immunotherapy.
Keywords: Immunotherapy, PD-1 (Programmed cell death protein 1), PD-L1 (Programmed death-ligand 1), monoclonal antibodies (mAbs)
1. Introduction
In 2012, 14.1 million new cancer cases were diagnosed worldwide and 8.2 million cancer patients passed away and more than 32.6 million people were living with cancer (within 5 years of diagnosis). About 57% (approximately 8 million) of new arising cancer cases, 65% (approximately 5.3 million) deaths of cancer and 48% (approximately 15.6 million) of the 5-year prevalent cancer cases have taken place in underdeveloped areas [1]. Surgery, radiotherapy and chemotherapy have been the main approaches for treating cancer in previous decades. Since Dana R. Leach first proposed an immune checkpoint blockade in 1996 [2], the first immune checkpoint inhibitor was approved by the FDA in 2011. By 2013, the cover report of science showed the new weapon of immunotherapy against cancer and the new therapeutic approach of using immune checkpoint inhibitors as anticancer agents was a landmark innovation. Undoubtedly, PD-1(Programmed cell death protein 1)/PD-L1(Programmed death-ligand 1) became the brightest star in 2015, after president Carter’s tumor was cured using anti-PD-1 antibody.
At least two signaling pathways need to be stimulated for the activation of cytotoxic T lymphocytes (CTLs) in secondary lymphoid tissues: Firstly, the binding occurs between peptide-MHC (major histocompatibility complex) complex with the availability of antigen presenting cells (APCs) and T cell receptors (TCRs). Then, the B7 molecules on APC interact with the CD28 receptors of T cells. It is noticeable that some experimental data suggests that a third necessary signal (such as IL-12, Type I IFN and adjuvant) plays a very important role in determining the fate of naïve CD8 T cells, tolerance or full activation [3]. Although less well studied, there is evidences of naïve CD4 T cells requiring a cytokine-dependent ′signal 3′ for a productive response to Ag and may be complementary to IL-1 [4]. Cellular immunity mediated by T cell is strictly supervised and controlled by a check/balance system performed though many stimulatory and inhibitory molecules. The inhibitory receptors, also called immune checkpoints, regulate CTLs activation and effector function to maintain self-tolerance and limit bystander tissue damage as an indirect consequence of immune response against pathogenic invasion [5]. Targeting PD-1 and PD-L1, the immune checkpoint blockade agents could reactivate cytotoxic T cells to eliminate tumor cells.
This review will introduce PD-1, PD-L1 as well as the signaling pathway. It will also highlight the clinical development and research progress of the anti-PD-1 and anti-PD-L1 mAbs for managing categories of cancers. Except that, we interpret the aptamer that may become the next generation of PD-1/PD-L1 inhibitors and update the progress of tolerance mechanisms on PD-1/PD-L1 antibodies.
2. PD-1 (Programmed Cell Death Protein 1) and PD-L1 (Programmed Death-ligand 1) Signaling Pathway
2.1. PD-1
The Pd-1 gene, classified to the immunoglobulin gene superfamily was isolated by Ishida Y using the subtractive hybridization technique in 1992 [6]. Like other inhibitory co-receptors, PD-1 has been detected on T cells, Tregs, exhausted T cells, B cells, activated monocytes, dendritic cells (DCs), natural killer (NK) cells and natural killer T (NKT) cells [7,8,9]. The PD-1 molecule consists of an intracellular domain which has potential phosphorylation sites located with immune tyrosine-based inhibitory motif (ITIM) and immune receptor inhibitory tyrosine-based switch motif (ITSM) and an extracellular IgV domain. Consequently there is a hydrophobic transmembrane region bridging crossing the cytomembrane [8]. Early studies have shown that an activated switch motif (ITSM) is required for the inhibitory effect of PD-1 on active T cells [10]. Its ITIM and ITSM also bind to the inhibitory phosphatase SHP-2 [11].
2.2. PD-L1
Two ligands, programmed death ligand-1 (PD-L1, CD274 or B7-H1) and programmed death ligand-2 (PD-L2, CD273 or B7-DC) [12], share 37% sequence homology [13,14,15]. PD-L1 belongs to type I transmembrane protein which was composed of extracellular domains (IgV-like domain, IgC-like domain, signal sequence), transmembrane domain and intracellular domains. PD-L1 constitutively express upon antigen presenting cells, non-lymphoid organs and non-hematopoietic cells such as heart, lung, placenta and liver [8]. Widely expressed PD-L1 is involved in self-tolerance, such as protecting peripheral tissues from excess of inflammation and autoimmune pathologies [16].
PD-L1 was induced by various pro-inflammatory cytokines like IFN-γ (interferon-γ), TNF-α (tumor necrosis factor-α), VEGF (vascular endothelial growth factor), GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-10. Activated T helper cells were responsible for IFN-γ and TNF-α and tumor stromal cells produced VEGF and GM-CSF. Furthermore up regulated PD-L1 expression in tumor cells facilitate immune suppression in tumor microenvironment [16] which has been called “adaptive immune resistance” [17]. In human cholangiocytes, PD-L1 expression was induced by IFN-γ and the MicroRNA -513 which complementary to 3′-untranslated region of PD-L1 mRNA, also could regulate PD-L1 translation. In other words, the miRNA could mediate gene silencing in the cholangiocyte regulation which respond to IFN-γ [18]. While in human glioma, PD-L1 expression would be increased if the tumor suppressor phosphatase and tensin homolog (PTEN), were dysfunctional and the phosphatidylinositol-3-OH kinase (PI(3)K) pathway were in turn activated [19]. In contrast, PI3K could increase translation of PD-L1 mRNA and cause the high expression of PD-L1 protein [20]. IFN-γ inducible PD-L1 expression was also dependent on NF-κB [21]. Except for binding PD-1, PD-L1 also binds to CD80 to deliver an inhibitory signal inducing T cell tolerance [22].
2.3. PD-1 and PD-L1 Pathway
Under normal physiological conditions, PD-1 which acts as immune checkpoint could interact with its two ligands, PD-L1 and PD-L2, and plays a very important part in lowering the immune system though suppression of T-cells function, upregulating regulatory T cells (Treg), which in turn reduces autoimmunity and promotes self-tolerance [23,24]. After binding of PD-L1 or PD-L2, the recruitment of tyrosine phosphatases will begin and then generates an inhibitory signal blocking the downstream effects of PI3K/Akt pathway leading to cell cycle arrest and suppressed T-cell activation [10,25].
Varieties of cancer cells have been detected through PD-L1 expression including melanoma, multiple myeloma, leukemia, glioblastoma as well as gastric, renal cell, bladder, hepatocellular, cutaneous, breast and NSCLC (Non-Small Cell Lung Cancer) [26,27,28,29,30,31,32,33], whereas PD-1 have been highly detected on tumor-infiltrating lymphocytes (TILs) [34,35]. Apart from PD-L1 displaying on camera solid tumors, PD-L2 (as well as PD-L1) is conservatively expressed in a few subsets of B cell lymphomas [36]. When cancer cells are attacked by the immune system, they start to overexpress PD-L1 and PD-L2, for impacting T-cells efficiency. After that, T cells will be suppressed successfully, leading to immune escape [37].
In diverse forms of tumor microenvironment, T-cell viability suppressed by PD-1 and its ligand PD-L1 though various mechanisms. It has been demonstrated that overexpression of PD-L1 on tumor associated macrophages, DCs, MDSCs and tumor cells positively correlated with the exhaustion of TILs in the tumor [38]. PD-L1 could induce Treg cell (iTreg cell) development by the down-regulating of phospho-Akt, mTOR, S6 and ERK2 accompanied with PTEN up-regulating. These signaling molecules play a critical role in iTreg cell development. As a consequence, PD-L1 will inhibit T cell activation though the formation and holding of iTreg cells [9]. On the other hand, earlier researcher Dong H and colleagues have proven that PD-1 inhibits PI3K activation inducing cell death in activated T cells resulting in the down regulation of anti-apoptotic protein Bcl-xL [39]. What is more, PD-1 could also inhibit the T-cell receptor delivering downstream, which is required for production growth stimulatory IL-2, resulting in cell cycle arrest and blocking T cell proliferation [40].
3. Programmed Death 1 Inhibitors
In 1996, Jim Allison’s group found that anti-CTLA-4 could boost anti-tumor response of T cell, which proved the immune checkpoint blocking in tumor therapy for the first time [2]. Through 10 years’ tough clinical research, the FDA finally approved the ipilimumab in 2011. Except for the different efficacy profile, the immune-related adverse events (irAEs), which were studied in-depth and accepted by the FDA, were regarded as an example of a new immunotherapy safety [41] which drove the clinical trials of the PD1 and PD-L1 antibody. Because of the lessons learned from ipilimumab and tremelimumab, nivolumab and pembrolizumab was marketed without difficulty in 2014.
3.1. Nivolumab
Nivolumab (BMS-936558 or MDX1106b) is a human IgG4 antibody against PD-1, lacking detectable antibody-dependent cellular toxicity (ADCC). It is manufactured by Bristol-Myers Squibb Company Princeton and has been approved by the FDA for the use of unresectable or metastatic melanoma, metastatic NSCLC and advanced renal cell carcinoma (Table 1).
Table 1.
The Indication of Nivolumab.
| Cancers | Single Agent | Combination with Ipilimumab |
|---|---|---|
| Melanoma | BRAF V600 wild-type unresectable or metastatic melanoma | BRAF V600 wild-type unresectable or metastatic melanoma |
| Unresectable or metastatic, BRAF V600 mutation-positive melanoma and disease progression following ipilimumab and a BRAF inhibitor | ||
| NSCLC | Metastatic non-small cell lung cancer in patients with progression on or after platinum-based chemotherapy | |
| Renal cancer | Advanced renal cell carcinoma in patients who have received prior antiangiogenic therapy |
3.1.1. Unresectable or Metastatic Melanoma
Nivolumab as a Single Agent for Melanoma
A multi-center, double-blind, randomized (1:1) clinic trial data of patients with BRAF V600 wild-type unresectable or metastatic melanoma is shown in the Table 2. A much higher overall survival rate of 1 year OS in the nivolumab group has been reached, 72.9% comparing to 42.1% in dacarbazine. Progression-Free-Survival (PFS) was also statistically significant in contrast to dacarbazin [42].
Table 2.
Efficacy Results of Nivolumab as a Single Agent for Melanoma.
| Efficacy | Nivolumab (n = 210) | Dacarbazine (n = 208) |
|---|---|---|
| Overall Survival | ||
| Events (%) | 50 (24) | 96 (46) |
| Median, months (95% CI) | Not Reached | 10.8 (9.3, 12.1) |
| Hazard ratio (95% CI) | 0.42 (0.30, 0.60) | |
| p-Value | <0.0001 a | |
| Progression-Free Survival | ||
| Events (%) | 108 (51) | 163 (78) |
| Median, months (95% CI) | 5.1 (3.5, 10.8) | 2.2 (2.1, 2.4) |
| Hazard ratio (95% CI) | 0.43 (0.34, 0.56) | |
| p-Value | <0.0001 a | |
| Objective Response Rate (95% CI) | 34% (28, 41) | 9% (5, 13) |
| Complete response rate | 4% | 1% |
| Partial response rate | 30% | 8% |
a p-Value is compared with the allocated alpha of 0.0021 for this interim analysis.
Nivolumab in Combination with Ipilimumab for Melanoma
A total of 109 randomized (2:1) patients, previously untreated with unresectable or metastatic melanoma participated the double-blind clinic trial. They received either ipilimumab or combined with nivolumab. In all 43 patients, 21% (9) had a response within the duration time from three to seven months. Unfortunately at end of trail, some progressed, others died or received other therapy. Among the remaining thirty four patients, fourteen had at least six months and less than nine months in duration of ongoing responses while the remaining 20 patients had a long duration of ongoing responses of more than nine months [43]. In contrast to single-agent ipilimumab, the combination group had a statistically significant increased ORR (overall response rate) in this study (Table 3).
Table 3.
Efficacy Results of Nivolumab in Combination with Ipilimumab for Melanoma.
| Endpoint | Nivolumab Plus Ipilimumab a (n = 72) | Ipilimumab (n = 37) |
|---|---|---|
| Objective Response Rate (95% CI) | 60% (48, 71) | 11% (3, 25) |
| Difference in ORR (95% CI) | 49 (31, 61) | |
| p-Value | <0.001 | |
| CR (%) | 17% | 0 |
| PR (%) | 43% | 11% |
| Progression-Free Survival | ||
| Number of events | 27 | 23 |
| Median PFS (months) (95% CI) | 8.9 (7.0, NA) | 4.7 (2.8, 5.3) |
| Hazard ratio (95% CI) | 0.40 (0.22, 0.71) | |
| p-Value | <0.002 | |
NA: not available; a Nivolumab 1 mg/kg in combination with ipilimumab 3 mg/kg every 3 weeks for 4 doses, followed by nivolumab 3 mg/kg as a single agent every 2 weeks until disease progression or unacceptable toxicity.
3.1.2. Metastatic Non-Small Cell Lung Cancer
Second-Line Treatment of Metastatic Squamous NSCLC
The clinic trial named CheckMate017 phase 3, studied 272 patients suffering from metastatic squamous NSCLC and followed their progression during or after platinum doublet-based chemotherapy. The median overall survival rate was 9.2 months with nivolumab versus 6.0 months with docetaxel. The overall survival rate was 42% with nivolumab versus 24% with docetaxel at 1 year (Table 4).
Table 4.
Efficacy Results of Nivolumab as Second-Line Treatment of Metastatic Squamous NSCLC.
| Efficacy | Nivolumab (n = 135) | Docetaxel (n = 137) |
|---|---|---|
| Prespecified Interim Analysis | ||
| Events (%) | 86 (64%) | 113 (82%) |
| Median survival in months (95% CI) | 9.2 (7.3, 13.3) | 6.0 (5.1, 7.3) |
| p-Value a | 0.00025 | |
| Hazard ratio (95% CI) b | 0.59 (0.44, 0.79) | |
a p-Value is derived from a log-rank test stratified by region and prior to paclitaxel use; the corresponding O’Brien-Fleming efficacy boundary significance level is 0.0315; b Derived from a stratified proportional hazards model.
Second-Line Treatment of Metastatic Non-Squamous NSCLC (Non-Small Cell Lung Cancer)
Nivolumab had been expanded to NSCLC by the FDA on October 9, 2015 based on the CheckMate 057 trial [44] with 582 patients enrolled. Nivolumab improved OS from 9.4 months (docetaxel 95% CI: 8.0–10.7 months) to 12.2 months (nivolumab 95% CI: 9.7–15.0 months) with a hazard ratio (HR) of 0.73 (p = 0.0015) (Table 5).
Table 5.
Efficacy Results of Nivolumab as Second-Line Treatment of Metastatic Non-Squamous NSCLC (non-small cell lung cancer).
| Efficacy | Nivolumab (n = 292) | Docetaxel (n = 290) |
|---|---|---|
| Overall Survival | ||
| Deaths (%) | 190 (65%) | 223 (77%) |
| Median (months) (95% CI) | 12.2 (9.7, 15.0) | 9.4 (8.0, 10.7) |
| p-Value a,b | 0.0015 | |
| Hazard ratio (95% CI) c | 0.73 (0.60, 0.89) | |
| Objective Response Rate | 56 (19%) | 36 (12%) |
| (95% CI) | (15, 24) | (9, 17) |
| p-Value d | 0.02 | |
| Complete response | 4 (1.4%) | 1 (0.3%) |
| Partial response | 52 (18%) | 35 (12%) |
| Median Duration of response (months) | 17 | 6 |
| Progression-Free Survival | ||
| Disease progression or death (%) | 234 (80%) | 245 (84%) |
| Median (months) | 2.3 | 4.2 |
| p-Value a | 0.39 | |
| Hazard ratio (95% CI) c | 0.92 (0.77, 1.11) | |
a Based on stratified log-rank test; b p-value is compared with .0408 of the allocated alpha for this interim analysis; c Based on a stratified proportional hazards model; d Based on the stratified Cochran-Mantel-Haenszel test.
3.1.3. Renal Cell Carcinoma
A phase 3 study in 821 renal-cell carcinoma patients who have experienced previous treatment was completed in comparing nivolumab(OS 25.0 months) with everolimus(OS 19.6 months). OS benefit was significantly confirmed even if the expression of PD-L1 could not be detected. The hazard ratio for death was 0.73 (98.5% CI, 0.57 to 0.93; p = 0.002) when nivolumab was combined with everolimus [45]. Table 6 displays further information.
Table 6.
Efficacy Results of Nivolumab for Renal Cell Cancers.
| Efficacy | Nivolumab (n = 410) | Everolimus (n = 411) |
|---|---|---|
| Overall Survival | ||
| Events (%) | 183 (45) | 215 (52) |
| Median survival in months (95% CI) | 25.0 (21.7, NE) | 19.6 (17.6, 23.1) |
| Hazard ratio (95% CI) a | 0.73a (0.60, 0.89) | |
| p-Value b | 0.0018 b | |
| Confirmed Objective Response Rate (95% CI) | 21.5% (17.6, 25.8) | 3.9% (2.2, 6.2) |
| Median duration of response in months (95% CI) | 23.0 (12.0, NE) | 13.7 (8.3, 21.9) |
| Median time to onset of confirmed response in months (min, max) | 3.0 (1.4, 13.0) | 3.7 (1.5, 11.2) |
NE; not estimatble; a Hazard ratio is obtained from a Cox proportional-hazards model stratified by MSKCC risk group, number of prior anti-angiogenic therapies and region with treatment as the sole covariate; b p-value is obtained from a two-sided log-rank test stratified by MSKCC risk group, number of prior antiangiogenic therapies and region. The corresponding O’Brien-Fleming efficacy boundary significance level is 0.0148.
3.1.4. Adverse Reactions
After the immune checkpoints were blocked, the balance between the autoimmunity and immune tolerance were broken as well. Newly generated dysimmune toxicities created immune-meditated adverse reactions (IMARs) caused by the new immunotherapy. For instance, immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, rash, encephalitis and other immune-mediated adverse reactions were observed as IMARs. Immune-mediated pneumonitis, colitis, hepatitis, nephritis and renal dysfunction, meant patients required the use of corticosteroids and had no clear alternative etiology, which can occur with nivolumab treatment. Immune-mediated edocrinopathies and rash mainly occurred in combination with ipilimumab. A total of 8,490 patients received nivolumab as a single agent or in combination with ipilimumab in all clinical trials. Fortunately, less than 1.0% of them were confirm as having encephalitis. As well as less than 1.0% of patients were regarded as having severe in fusion when using nivolumab as a single-agent. In only one patient (0.3%) did fatal limbic encephalitis occur, after receiving nivolumab after 7.2 months of exposure. Others were administered with corticosteroids. In addition, the fetus could be harmed when pregnant woman received nivolumab treatment, based on data from animal studies.
In clinical trials, the most common adverse reactions experienced (≥20%) in melanoma were fatigue, musculoskeletal pain, rash and pruritus when nivolumab acted as a single-agent. Patient symptoms when Nivolumab was used in combination with ipilimumab were rash, pruritus, headache, vomiting and colitis.
The most common adverse reactions (≥20%) in patients with metastatic NSCLC were fatigue, musculoskeletal pain, decreased appetite, cough and constipation, based on clinical trials experience.
Just like other therapeutic proteins, nivolumab also has the potential of immunogenicity. 73 patients (11.4%) was tested positive for anti-nivolumab antibodies because of the treatment and using an electrochemiluminescent (ECL) assay in all 639 patients. In five patients (0.8%), anti-nivolumab neutralizing antibodies were detected as well. In combinational therapy with ipilimumab, 23 patients were tested positive for treatment-arising anti-nivolumab antibodies though ECL assay and neutralizing antibodies, anti-nivolumab were inspected at one patient.
3.1.5. Recruiting Clinical Trials of Nivolumab
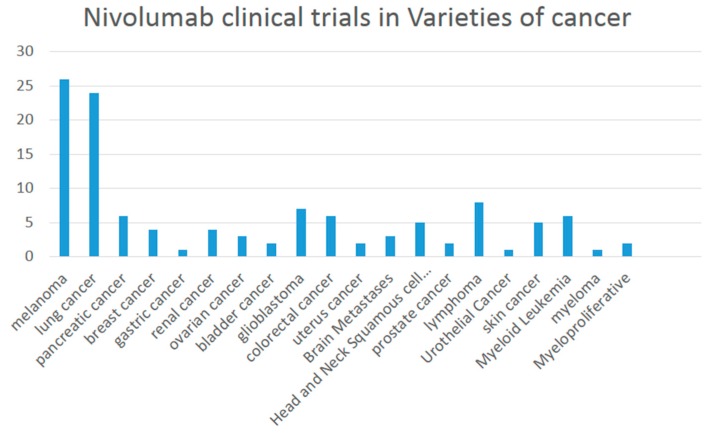
There are 121 studies found in ClinicalTrials.gov searching for “Nivolumab | Recruiting”. A variety of melanoma are involved including lung cancer, breast cancer, bladder cancer and renal cancer (Figure 1) when combined or compared with multiple medicines such as Ipilimumab, Pembrolizumab, Dabrafenib, Trametinib, Placebo, Omaveloxolone Capsules, sunitinib, BMS-936558 (MDX1106-04), Fotemustine and HyperAcute®-Melanoma (HAM) Immunotherapy.
Figure 1.
Nivolumab clinical trials in a variety of cancers.
3.2. Pembrolizumab
Pembrolizumab (MK-3475, lambrolizumab, KEYTRUDA) is an highly specific anti-PD-1 humanized IgG4 κ isotype antibody that contains a mutation at C228P designed to prevent Fc-mediated cytotoxicity. It can disrupt the engagement of PD-1 and PD-L1, resulting in tumor recognition by cytotoxic T cells.
It is approved by the FDA for the treatment of the patients suffering with unresectable or metastatic melanoma and patients with metastatic NSCLC. Accordingly, the PD-L1 expression level has to be detected in determining whether the patients receive pembrolizumab or not. Otherwise, their disease continued to progress on or after chemotherapy platinum. In the case of patients with EGFR or ALK, genomic aberrations had disease progression on other prior FDA-approved therapy before receiving pembrolizumab.
3.2.1. Pembrolizumab in Melanoma
Ipilimumab-Naive Melanoma
The safety and efficacy of pembrolizumab were well demonstrated in Ipilimumab-Naive Melanoma. The affirmed 6-month PFS rates were 47.3% for the patients receiving pembrolizumab every 2 weeks contrasting with 46.4% for every 3 weeks and 26.5% for ipilimumab, with 12-month survival rates: 74.1%, 68.4% and 58.2%, respectively. The improving response rate, regardless of the interval of pembrolizumab being administered; whether every 2 weeks (33.7%) or every 3 weeks (32.9%), as compared with ipilimumab (11.9%). The response rates were ongoing in 89.4%, 96.7%, and 87.9% of patients, respectively. All the data supports the efficiencies were similar in two pembrolizumab patients groups. The adverse events rates referring to treatment of grade 3 to 5 severity were lower in the pembrolizuma patients (13.3% and 10.1%) than in the ipilimumab patients (19.9%) [46,47] (Table 7). These clinical trials suggests there are statistically significant improvements in OS and PFS for patients receiving pembrolizumab in Ipilimumab-Naive Melanoma.
Table 7.
The Clinical Study of Pembrolizumab for Ipilimumab-Naive Melanoma (Trial 6).
| Efficacy | Pembrolizumab 10 mg/kg Every 3 Weeks n = 277 | Pembrolizumab 10 mg/kg Every 2 Weeks n = 279 | Ipilimumab 3 mg/kg Every 3 Weeks n = 278 |
|---|---|---|---|
| OS | |||
| Death (%) | 92 (33%) | 85 (30%) | 112 (40%) |
| Hazard ratio * (95% CI) | 0.69 (0.52, 0.90) | 0.63 (0.47, 0.83) | – |
| p-Value (stratified log-rank) | 0.004 | <0.001 | – |
| PFS by BICR | |||
| Events (%) | 157 (57%) | 157 (56%) | 188 (68%) |
| Median in months (95% CI) | 4.1 (2.9, 6.9) | 5.5 (3.4, 6.9) | 2.8 (2.8, 2.9) |
| Hazard ratio * (95% CI) | 0.58 (0.47, 0.72) | 0.58 (0.46, 0.72) | – |
| p-Value (stratified log-rank) | <0.001 | <0.001 | – |
| Best Overall Response by BICR | |||
| ORR % (95% CI) | 33% (27, 39) | 34% (28, 40) | 12% (8, 16) |
| Complete response % | 6% | 5% | 1% |
| Partial response % | 27% | 29% | 10% |
Hazard ratio * (Pembrolizumab compared to ipilimumab) based on the stratified Cox proportional hazard model.
Ipilimumab-Refractory Melanoma
A safety and efficacy phase II trial of pembrolizumab were evaluated in Ipilimumab-Refractory Melanoma. In this trial by active comparator arms, the pembrolizumab group had significantly improved PFS and ORR but not OS (although OS data are immature), when compared with BRAF/MEK inhibiting chemotherapy in ipilimumab-refractory patients with BRAF-mutation positive [47,48,49,50,51] (Table 8). After an analysis of 220 deaths, there was no statistically significant difference between pembrolizumab and chemotherapy, regardless of the dosage of pembrolizumabpembrolizumab, 2 mg/kg or 10 mg/kg (Table 8).
Table 8.
Efficacy Results of Pembrolizumab in Ipilimumab-Refractory Melanoma.
| Efficacy | Pembrolizumab 2 mg/kg Every 3 Weeks n = 180 | Pembrolizumab 10 mg/kg Every 3 Weeks n = 181 | Chemotherapy n = 179 |
|---|---|---|---|
| Progression-Free Survival | |||
| Number of events, n (%) | 129 (72%) | 126 (70%) | 155 (87%) |
| Progression, n (%) | 105 (58%) | 107 (59%) | 134 (75%) |
| Death, n (%) | 24 (13%) | 19 (10%) | 21 (12%) |
| Median in months (95% CI) | 2.9 (2.8, 3.8) | 2.9 (2.8, 4.7) | 2.7 (2.5, 2.8) |
| p-Value (stratified log-rank) | <0.001 | <0.001 | – |
| Hazard ratio * (95% CI) | 0.57 (0.45, 0.73) | 0.50 (0.39, 0.64) | – |
| Objective Response Rate | |||
| ORR, n% (95% CI) | 21% (15, 28) | 25% (19, 32) | 4% (2, 9) |
| Complete response % | 2% | 3% | 0% |
| Partial response % | 19% | 23% | 4% |
Hazard ratio * (KEYTRUDA compared to chemotherapy) based on the stratified Cox proportional hazard model.
3.2.2. Pembrolizumab in Non-Small Cell Lung Cancer
Altogether, 280 patients were involved in a multi-center, open-label multi-cohort, activity-estimating study. The sub-group defining was retrospectively analyzed using an analytically validated assay for PD-L1 expression TPS (tumor proportion score). Of a total of 280 patients, 61 were defined as highly expressed for PD-L1 with partial response, consequently the confirmed ORR reached to 41% (Table 9). In 25 ORR patients, 21 (84%) had duration response, as well as 11 patients (44%) who had ongoing response to ≥6 months [52].
Table 9.
Efficacy Results of Pembrolizumab for NSCLC.
| Endpoint | N = 61 |
|---|---|
| Overall Response Rate | |
| ORR %, (95% CI) | 41% (29,54) |
| Complete response | 0% |
| Partial response | 41% |
In a separate subgroup of 25 patients with limited follow-up with PD-L1 expression, TPS greater than or equal to 50% receiving pembrolizumab at a dose of 2 mg/kg every 3 weeks in Trial 1, activity was also observed. The ORR and duration of response were similar regardless of schedule (every 2 weeks or every 3 weeks) and thus the data below are pooled.
3.2.3. Adverse Reactions
In clinical trials research, the adverse reactions reported in ≥20% of patients were fatigue, pruritus, rash, constipation, diarrhea, nausea with decreased appetite and fatigue, dyspnea and cough in NSCLC.
In total, 2117 patients with melanoma, 1567 NSCLC 550 patients, pembrolizumab caused some immune-mediated adverse reaction such as immune-mediated pneumonitis, colitis, hepatitis, endocrinopathies, nephritis and renal dysfunction. The exact percentage is shown in (Table 10).
Table 10.
Adverse Reactions of Pembrolizumab.
| Immune-Mediated Adverse Reactions | Melanoma | NSCLC |
|---|---|---|
| Pneumonitis | 2.00% | 3.50% |
| Colitis | 2.00% | 0.70% |
| Hepatitis | 1.00% | – |
| Endocrinopathies | 0.80% | 0.20% |
| Hyperthyroidism | 3.30% | 1.80% |
| Hypothyroidism | 8.10% | 6.90% |
| Type 1 Diabetes Mellitus | 0.1% | |
| Nephritis | 0.40% | – |
Similar to other therapeutic proteins, the immunogenicity risk for pembrolizumab was observed in clinical studies. The anti-pembrolizumab antibody were detected in 1 (0.3%) of 392 patients which were verified positively in the neutralizing assay.
3.2.4. Clinical Trials on Recruiting
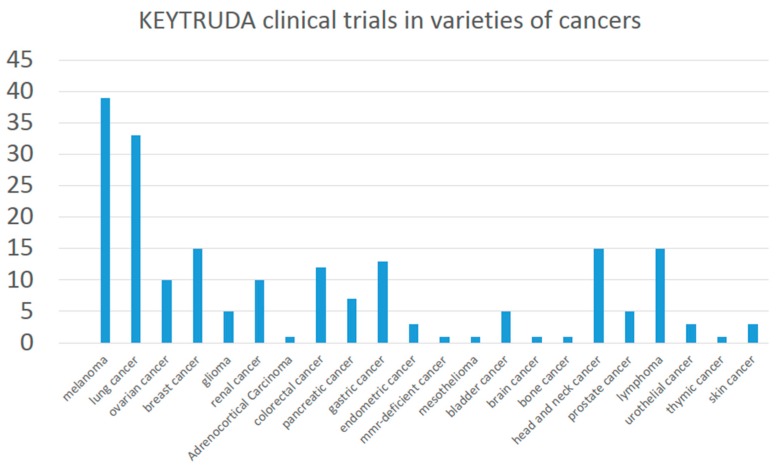
There were 178 studies in the ClinicalTrials.gov with recruited participants distributed throughout the world. The 178 clinical trials used pembrolizumab in many varieties of cancer, including melanoma, lung, ovarian, breast, glioma, renal, adrenocortical carcinoma, colorectal, pancreatic, gastric, endometrial, mesothelioma, bladder cancer and so on (Figure 2).
Figure 2.
Pembrolizumab clinical trials in varieties of cancer.
3.3. Pidilizumab
Pidilizumab targeting PD-1 is derived from BAT, a the mouse monoclonal antibody, and it was humanized to IgG 1κ [53]. In preclinical studies, CT-011 and BAT had successfully suppressed the tumor growth within melanoma, lymphoma, lung, colon and breast tumors and furthermore it extended the survival of tumor-bearing mice, both NK and T cell involved [54,55,56,57]. The Phase I study has affirmed the safety and pharmacokinetic of pidilizumab in advanced ematologic malignancies. Fortunately, there was no observed treatment or infusion-related serious adverse events [58]. Pharmacokinetic analyses show the Cmax (maximum concentration) and the AUC (area under the curve) of CT-011 in serum increasing dose with a median of t1/2 from 217 to 410 h. The peripheral blood CD4+ lymphocytes rose unremittingly until the 21st. day after CT-011 treatment.
Researchers designed a single group, open-label phase 2 trial, for assessing the safety and activity of the combination between Pidilizumab and Rituximab in relapsed Follicular Lymphoma patients. It was demonstrated that no autoimmune or therapy-related grade 3/4 toxicities were observed. Anemia (14 patients) and fatigue (13 patients) were the most frequent grade 1 adverse symptoms and 5 patients were defined as having respiratory infection with a grade 2 adverse event. Overall response rates were 66% (19/29), 15 being complete response rates. In total, 25/29 (86%) of the patients had tumor regression within 18.8 months (95% CI: 14.7 months to not reach) of median progression-free survival. Nineteen responders had 20.2 months (95% CI: 13.9 months to not reached) of median response duration [53].
The clinical activity of PD-1 blockade was confirmed in diffused large B-cell lymphoma (DLBCL) using a phase II trial (NCT00532259). After autologous hematopoietic stem-cell transplantation for DLBCL, the overall response rate of pidilizumab treatment has been reported as reaching 51% which presents a promising strategy of PD-1 blockade therapy. It is demonstrated in DLBCL for the first time. A total of 613 adverse events took place in 69 (96%) patients and 135 were regarded as treatment-related. Neutropenia (19%) and thrombocytopenia (8%) became the most common grade 3 to 4 adverse events but the neutropenia could be managed by growth factor treatment and remaining in asymptomatic. Sadly, one patient died due to herpes zoster infection after the third dose of pidilizumab, but is not related to this study treatment. At least 32% (23/72) of patients suffered one serious adverse event each with three undergoing a related serious adverse event. The evidence of significant autoimmune toxicity, infusion reactions and treatment related mortality have not yet been found [59].
Pidilizumab was studied in another two Phase 2 clinic trials on aggressive and indolent lymphomas which appeared in clinical activity of PD-1/PD-L1 positive lymphocytes. Then Michael B etc. initiated a large Phase 2 study to assess the safety and efficacy of pidilizumab in metastatic melanoma (MM) patients. It resulted in a substantial 12 month survival rate in heavily pretreated patients and was very well tolerated. It appears comparable to other anti- PD-1 MAbs in 12 months survival [60].
There were also two clinical trials but these were suspended due to a Pidilizumab (CT-011) licensing transfer and Pharmaceutical Companies decision. Two further clinical trials with Chronic Hepatitis C Genotype and Hepatocellular Carcinoma were terminated. The study of PD-1 blockading in combination with DC/AML vaccine and subsequent chemotherapy to induce remission is being sought. A Stage III-IV diffuse large B-cell lymphoma trial is requesting volunteer patients as well.
3.4. AMP-224
The curative effects of nivolumab and pembrolizumab are very encouraging [61]. However, targeting the PD-1 has the potential to prevent differentiated development [62,63]. Amplimmune and GlaxoSmithKline are assessing the safety and efficiency of a new arising PD-1 targeting agent, AMP-224. It is a fusion protein consist of the extracellular domain of PD-L2 and the Fc region of human IgG [64]. Contrasting to nivolumab and pembrolizumab, AMP-224 does not just perform as a blockading agent. It is hypothesized that AMP-224 could deplete PD-1 highly expressed T-cells, which referred to as exhausted effector cells, ADCC or CDC. Following restoration of the T-cell cohort with normal function may reestablish immune ability [2,3]. AMP-224 was well-tolerated up to its maximum administered dose of 30 mg/kg, with manageable infusion reactions in the majority of patients. The trial is ongoing, including monitoring for clinical activity [65].
Preclinical studies utilizing a murine AMP-224 in syngeneic murine tumor models show anti-tumor activity as a single agent which could be enhanced after the combination of low dose cyclophosphamide (unpublished data Amplimmune, Inc, Gaithersburg, MD, USA).
A pilot research of AMP-224 combined with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer was reported at the 2015 Gastrointestinal Cancers Symposium. A few other preclinical studies have reported an increase in peripheral anti-tumor immune activity, consequently with radiation for “abscopal effect”. The PD-L1 expression of tumor cells could also been induced by radiation, so the aim of this study is to assess whether the radiation therapy enhanced anti-tumor immunity of anti-PD-1 therapy (with AMP-224) or not. However, the clinical trial information is not yet complete [66].
3.5. MEDI0680
MEDI0680 (AMP-514) is a humanized IgG monoclonal antibody targeting human PD-1. It could also improve the cytotoxicity of T cells though inducing the internalization of PD-1 [67,68]. There are three ongoing clinic trails: NCT02271945 is a Phase 1b/2 open-label study to evaluate the safety/efficacy of MEDI-551 + AMP-514 in participants with relapsed or refractory aggressive B-cell lymphomas who have failed 1–2 prior lines of therapy; NCT02013804 is a dose-escalation study to test the safety, tolerability, PK, immunogenicity and anti-tumor activity in adult patients bearing solid tumors; NCT02118337 is a combinational trail to assess the safety and tolerability of AMP-514, with MEDI4736 (Anti-PD-L1 Antibody).
3.6. REGN2810
REGN2810 is a fully human hinge-stabilized IgG4 monoclonal Ab that binds to the extracellular domain of human PD-1 with high affinity and specificity inhibiting interaction of PD-1 with its ligands [69]. Elena Burova et al. generated a mouse with human PD-1 gene knock-in allowing direct testing of our anti-human PD-1 Ab. Human PD-1 knock-in mice express a hybrid protein containing the extracellular portion of human PD-1 with transmembrane and intracellular domains of mouse PD-1. We demonstrated functional PD-1/PD-L1 signaling and immune responses in this model and confirmed REGN2810 binding to hybrid PD-1 receptor on mouse T cells in vivo, following REGN2810 injections. Prophylactic and therapeutic treatments of subcutaneous syngeneic tumors with REGN2810 in human PD-1 knock-in mice resulted in a dose-dependent suppression of tumor growth [69,70].
Three phase I clinic trials are commencing at clinicaltrials.gov. NCT02383212 is an open-label, multi-center, ascending-dose escalation study of REGN2810, alone and in combination with other anti-cancer therapies in patients with advanced malignancies on recruiting. NCT02651662 is calling for participants in patients with lymphoma. It is an open-label, multi-center, dose escalation study of REGN2810 as single-agent. NCT02520245 has been designed to collect long-term follow-up information for patients who received REGN2810 in other clinical studies and to allow re-treatment for eligible patients not yet recruiting.
3.7. PDR001
PDR001 is a high-affinity, ligand-blocking, humanized anti-PD-1 IgG4 antibody that blocks the binding of PD-L1 and PD-L2 to PD-1. As a signal agent, NCT02678260, NCT02605967 and NCT02404441 are on phase I/II. NCT02404441 and is the “first-in-human” study of PDR001 to characterize the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and anti-tumor activity of PDR001 administered i.v. as a single agent, to adult patients with solid tumors. The purpose of NCT02678260 is to characterize the safety, tolerability, Pharmacokinetics (PK) and anti-tumor activity of PDR001 administered intravenous as a single agent to Japanese patients. The randomized controlled of NCT02605967 Phase II study is to assess the efficacy of PDR001 versus investigator's choice of chemotherapy in patients with advanced NPC. Another two agents (NCT02608268 and NCT02460224) were in combination with MBG453 or LAG525.
3.8. PD-1 Antibody in China
China has overtaken India becoming the largest biosimilar discovering country from Cortellis Competitive Intelligence, according to Thomson Reuters report in 2015. It mirror the investment environment’s expectation to Chinese biosimilars. There is no doubt that the development of biosimilar will make up for the massive unsatisfied clinic demand in diabetes, tumor and immunological diseases.
Nivolumab and pembrolizumab have submitted an application for clinic trials, but most are in assessing, except for a clinic trial of nivolumab (JXSL1300032). As expected, there are many ongoing PD-1 antibodies discoveries in China. According to incomplete statistics, two have reached clinical trials and a further two are in assessment (Table 11).
Table 11.
The New Drug Application of PD-1 Antibody in China.
| Acceptance Number | Drug | Date | Company | Progress |
|---|---|---|---|---|
| JXSL1600007 | Nivolumab | 17 February 2016 | Bristol-Myers Squibb | In Assessing |
| JXSL1500068 | 7 December 2015 | |||
| JXSL1300032 | 20 May 2013 | In Clinic | ||
| JXSL1600009 | Pembrolizumab | 29 February 2016 | Merck | In Assessing |
| JXSL1600005 | 16 February 2016 | |||
| JXSL1500074 | 31 December 2015 | |||
| JXSL1500058 | 30 September 2015 | |||
| JXSL1500040 | 28 July 2015 | |||
| JXSL1500020 | 25 May 2015 | |||
| CXSL1400138 | JS001-PD-1 | 21 January 2015 | ShangHai JunShi | In Clinic |
| CXSL1400153 | SHR-1210 | 19 January 2015 | ShangHai HengRui | |
| CXSL1500096 | BGB-317 | 11 December 2015 | BeiGene | In Assessing |
| CXSL1600016 | Genor PD-1 Antibody | 7 April 2016 | Genorbio |
4. Programmed Death Ligand 1 Inhibitors
PD-L1 constitutively express upon antigen presenting cells, non-lymphoid organs and non-hematopoietic cells such as heart, lung, placenta and liver [8]. Early studies, reported by many groups respectively, also have shown that PD-L1 is frequently expressed on human cancer cells which significantly correlate with the poor prognosis in various kinds of tumor (e.g., renal, gastric, urothelium, ovarian and melanoma) [71]. Thus using the anti-PD-L1 antibody could kill the tumor cell or block the PD-1/PD-L1 signal pathway to reactivate CTLs. The first therapeutic anti-PD-L1 antibody has been approved by the FDA on 18 May 2016 and immediately made headlines in the media.
4.1. BMS-936559
BMS-936559 is a PD-L1 specific, fully human, high-affinity, IgG4 (S228P) monoclonal antibody that inhibits the interaction of PD-L1 to PD-1 or CD80 [72]. NCT00729664 is a phase I clinic trial commenced in 2008 with the report was published in 2012. A total of 207 patients were included, with NSCLC (75), melanoma (55), colorectal cancer (18), renal-cell cancer (17), ovarian cancer (17), pancreatic cancer (14), gastric cancer (7) and breast cancer (4). The duration of therapy ranged from 2 to 111 weeks, with a median of 12 weeks. The researchers considered 9% of patients treatment related, Grade 3 or 4 toxic effects. An objective response, both complete and partial, was counted in 9/52 patients with melanoma; 2/17 patents with renal-cell cancer, 5/49 patients with NSCLC and 1/17 patient with ovarian cancer who were evaluated to have an objective response [72]. Of 8/16 patients, the response lasted for more than one year and at least a further year of follow-up. Another NCT02028403 trial was completed in safety and immune response of BMS-936559 of HIV-infected patients followed up with antiretroviral therapy. The purpose of NCT02576457 was to determine whether BMS-936559 is safe and has the desired pharmacologic activity in patients who have severe sepsis and are being sought for trial. However, there are two clinic trials (NCT01455103 and NCT01452334) that were withdrawn prior to enrollment.
4.2. Avelumab
In November 2014, Merck KGaA, Darmstadt, Germany and Pfizer proclaimed the avelumab would be co-developed and co-commercialized within a strategic alliance. Avelumab (MSB0010718C) is an investigational product consist of fully human anti-PD-L1 IgG1 monoclonal antibody. Though the blockading of PD-L1, avelumab is regarded as a function in the reactivation of T-cells and may induce ADCC with native Fc-region [73]. Altogether 16 clinical trials in three phases were active with 12 recruiting rounds (Table 12).
Table 12.
The Clinical Phase of Avelumab.
| Phase I | Phase II | Phase III |
|---|---|---|
| Solid Tumors | Merkel Cell Carcinoma | Non-Small Cell Lung Cancer |
| Renal Cancer | Non-Small Cell Lung Cancer | Renal Cell Cancer |
| Advanced Cancer | Gastric Cancer | |
| Non-Small Cell Lung Cancer | Ovarian Cancer | |
| Hodgkins Lymphoma | Urothelial Cancer | |
| Merkel Cell Polyomavirus Infection; Stage IV Merkel Cell Carcinoma | ||
4.3. MEDI4736
MEDI4736 is a human IgG1 κ monoclonal antibody with high affinity and specificity to PD-L1, which also been engineered to prevent ADCC. It significantly suppresses the growth of human tumors in a new xenograft model with co-implanted human T cells and is entirely dependent on the existence of human T cells [74,75]. Antibodies of the IgG1 isotype antibody could trigger cytotoxic effect or functions, such as ADCC activity and CDC (complement-dependent cytotoxicity) [76]. Fc (the fragment crystallizable) domain of the antibody molecule having a triple mutation of the IgG1 heavy chain, for reducing the interaction with the complement component C1q and the Fcγ receptors [74,76,77,78]. The absence of ADCC and CDC effector functions has been confirmed using cell-based functional assays [78,79]. Many anticipated clinic trials are ongoing (Table 13).
Table 13.
The Clinical Phase of MEDI4736.
| Phase I | Phase II | Phase III |
|---|---|---|
| Ovarian Cancers | Ovarian Cancers | Head and Neck Cancer |
| Breast | Breast | Non-Small Cell Lung Cancer |
| SCLC | SCLC | Breast Cancer |
| Gastric Cancers | Gastric Cancers | Bladder Cancer |
| Pancreatic Ductal Carcinoma | Pancreatic Ductal Carcinoma | Squamous Cell Lung Carcinoma |
| Non-Small Cell Lung Cancer | Malignant Mesothelioma | |
| Myelodysplastic Syndrome | Melanoma | |
| Advanced Solid Tumors | Hepatocellular Carcinoma | |
| Melanoma | Advanced Solid Tumors | |
| Gastric or Gastroesophageal Junction Adenocarcinoma | Glioblastoma | |
| Hepatocellular Carcinoma | Non-Small Cell Lung Cancer | |
| Head and Neck Cancer | Gastric or Gastroesophageal Junction Adenocarcinoma | |
| Colorectal Cancer | Colorectal Cancer | |
| Prostate Cancer | Esophageal Cancer | |
| Renal Cell Carcinoma | Sarcoma | |
| Malignant Mesothelioma | Mesothelioma | |
| Follicular Lymphoma | Lymphoma or Chronic Lymphocytic Leukemia | |
| Diffuse Large B-Cell Lymphoma | Myelodysplastic Syndromes | |
| Bladder Cancer | Oesophago-gastric Cancer |
4.4. MPDL3280A
MPDL3280A (also known as Atezolizumab, Tecentriq) is a phage-derived human IgG1 monoclonal antibody targeting PD-L1 and has shown promising anti-tumor activity [80]. Similarly to MEDI4736, MPDL3280A was also engineered with a mutation in the Fc domain (298 Asn to Ala) [81]. In a preclinical study in monkeys, MPDL3280A has shown pleasing results in patients with locally advanced or metastatic tumors [82,83]. Furthermore, on 18 May 2016, it became the first PD-L1 inhibitor post FDA approval of the treatment of urothelial carcinoma, the most common type of bladder cancer. The other 51 clinic trials were recruited around the world (Table 14).
Table 14.
The Clinical Phase of MPDL3280A.
| Phase I | Phase II | Phase III |
|---|---|---|
| Diffuse Large B-Cell Lymphoma, Lymphoma, Follicular | Non-Squamous Non-Small Cell Lung Cancer | |
| Renal Cell cancer | Non-Small Cell Lung Cancer | |
| Breast cancer | Bladder Cancer | Renal Cell Carcinoma |
| Bladder cancer | Advanced Non-Clear Cell Kidney Cancer | Metastatic Breast Cancer, Triple Negative Breast Cancer |
| Non-small cell lung cancer | Non-small cell lung cancer | Invasive Ductal Breast Carcinoma |
| Lymphoma | Lymphoma | Bladder Cancer |
| Malignant Melanoma | Colorectal Cancer | |
| Myelodysplastic Syndrome | Ovarian Neoplasms | |
| Multiple Myeloma | ||
| Prostate Cancer | ||
| Head and Neck Cancer | ||
| Colorectal Cancer | ||
5. Prospects
To date, three therapeutic antibodies targeting the PD-1/PD-L1 signal pathway have been approved by the FDA in the use of metastatic melanoma, NSCLC, renal cell cancer or urothelial carcinoma, with several others in clinical trials in preparation for release to the open market (Table 15) [84]. It is predicted that some will be approved.
Table 15.
The Monoclonal Antibodies of PD-1 and PD-L1.
| Target | Agent | Sponsor | Class | Clinical Testing Phase |
|---|---|---|---|---|
| PD-1 | Nivolumab | Bristol-Myers Squibb | Human IgG4 | FDA-approved for treatment of refractory unresectable melanoma , for metastatic NSCLC and advanced renal cell carcinoma |
| Pembrolizumab | Merck | Humanized IgG4 | FDA-approved for treatment of refractory unresectable melanoma and for metastatic NSCLC that expresses PD-1 | |
| CT-011 | CureTech | Humanized IgG1k | Phase 1–2 | |
| AMP-224 | Amplimmune | PD-L2 IgG2a fusion protein | Phase 1 | |
| MEDI0680 (AMP-514) | Amplimmune | PD-L2 fusion protein | Phase 1–2 | |
| REGN2810 | Regeneron | Human IgG4 | Phase 1 | |
| PDR001 | Novartis | Information not available | Phase 1–2 | |
| JS001-PD-1 | ShangHai JunShi | |||
| SHR-1210 | ShangHai HengRui | |||
| BMS-936559 | Bristol-Myers Squibb | Human IgG4 | Phase 1–2 | |
| PD-L1 | MEDI4736 | MedImmune/AstraZeneca | Humanized IgG1k | Phase 1–3 |
| MPDL3280A | Roche | Human IgG1k | FDA-approved for treatment of urothelial carcinoma | |
| MSB0010718C | Merck Serono | Human IgG1 | Phase 1–3 |
Varied response towards immunotherapy has resulted from the different immune backgrounds of patients. The immune background is dependent on many substances with the immunogenicity a person has in their immune system being determined in the womb. However, the gut microbiota plays a most important role in shaping the systemic immune responses [85,86,87]. Ayelet Sivan demonstrate the commensal Bifidobacterium could promote antitumor immunity and facilitate anti-PD-L1 activity in cancer [88]. So, it is possible to manipulate microbiota to modulate cancer immunotherapy. On the other hand, it has been a long journey for Ipilimumab due to the low responsible rate compared to small molecular drugs or other therapeutic antibodies. Similarly, the responsible rate of PD-1/PD-L1 antibodies were still low as immunotherapeutic antibodies. Most researchers view that the different performances in a variety of people are due to the individual immune system and tumor driven mutation. However, which gene has been a mystery, until recently. A researcher from University of California, Los Angeles found that highly mutational loads co-related with optimistic survival and the responding patients’ tumors are abundant with mutations in BRCA2. Similarly, mitogen-activated protein kinase (MAPK) inhibited therapy induces similar characteristics in melanoma, which indicate that non-genomic MAPK inhibitor resistance has the cross-resistance within anti-PD-1 therapy [89].
To address the immune-related adverse effects of mAbs and get more penetration in solid tumors, some peptides based on immune checkpoint blockers were discovered. A therapeutic peptide targeting PD-1/PD-L1 signal pathway for cancer immunotherapy, AUNP-12 (AUR-12/Aurigene-012) was co-developed by Aurigene Discovery Technologies and Pierre Fabre Laboratories and is currently undegoing preclinical study [84]. The sequence is still secret. Although it has shown valid antitumor activity, the druggability pharmacokinetic profile was too short. Some hydrolysis-resistant D-peptides were discovered as PD-L1 antagonists developed by using mirror-image phage display [90]. The highest affinity of Kd = 0.51 μM has shown inhibited tumor growth and prolonged animal survival.
Otherwise, there was one patent relating to small drug-like inhibitors. It was filed by Bristol-Myers Squibb in 2015 WO 2015/034820 A1 (priority to US 61/873,398). The compound and its ramification could inhibit the interactions between PD-1 and PD-L1 having IC50 values between 0.006 and 0.10 mM. It should be noted that Curis, Inc. (Nasdaq:CRIS, Lexington, MA, USA) announced the FDA has accepted the company’s Investigational New Drug (IND) application for CA-170 at 1 June 2016. CA-170 is the first orally available small molecule targeting and inhibiting the immune checkpoints, PD-L1 and V-domain Immunoglobulin Suppressor of T-cell Activation (VISTA).
Since the regulatory approval of ipilimumab in 2011, immuno-oncology has not only grown rapidly but also inspired the entire pharmaceutical industry. Dozens of new technologies and investments in immune-oncology have soared overnight, especially since Science magazine in 2013 reported cancer immunotherapy as the breakthrough of the year [91]. Between 2014 and 2015, immune-oncology technologies and business deals led to extreme valuations and the market is expected to reach US $35 billion by 2023 [92]. Axel Hoos also reviewed or forecast three generations of immuno-oncology drugs. Generation 1 encompasses the initiating antibodies of the immune-oncology era, such as ipilimumab and sipuleucel‑T (autologous dendritic cell therapy developed by Dendreon), which were approved respectively in 2011 and 2010 [93,94]; and then immune-oncology drugs rapidly develop to next generation agents against new targets and new emerging mechanisms, which are represented by the PD-1 and PD-L1 antibodies. A bi‑specific T cell adaptor (BITE), blinatumomab (Amgen) targeting CD19 + B cell malignancies and T cell, was also approved in 2015 [95,96,97]. Generation 3 will be various immune-oncology modalities combined with adaptive immunity and innate immunity. In other words, establishing the best combination of therapies and drugs is under development. Further development of PD-1/PD-L1 pathway inhibitors are expected to be a powerful weapon in the fight against cancers.
We expected more PD-1 (or PD-L1) inhibitors to show excellent results at immune checkpoints in reactivating the adaptive or innate immunity for defeating cancers. However, antibody drugs have some insurmountable disadvantages, such as high immunogenicity [98], high cost, low stability and low production [99]. Undoubtedly, the marketed two PD-1 antibodies have been reported with anti-antibodies which weaken the therapeutic effect. The anti-antibody of nivolumab (OPDIVO) has reached up to 12.6% (67/532) and so a new solution may be required. The aptamer with minimal immunogenicity [100], low cost [101], high production [101] and high stability [99] may became the key to solving the puzzle. More and more anti-tumor drugs will be investigated until we overcome cancer.
Abbreviations
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| FDA | Food and Drug Administration |
| mAbs | Monoclonal antibodies |
| DCs | Dendritic cells |
| NK | Natural killer |
| NKT | Natural killer T |
| ITIM | Immune tyrosine-based inhibitory motif |
| ITSM | Inhibitory tyrosine-based switch motif |
| Ig | Immune globin |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IL | Interleukin |
| PENT | Phosphatase and tensin homolog |
| Treg | Regulatory T cells |
| TIL | Tumor-infiltrating lymphocytes |
| ZAP | Zeta-chain-associated protein |
| CTLs | Cytotoxic T lymphocytes |
| APCs | Antigen presenting cells |
| MHC | Major histocompatibility complex |
| ADCC | Antibody-dependent cellular toxicity |
| NSCLC | Non-small cell lung cancer |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 3.Curtsinger J.M., Lins D.C., Mescher M.F. Signal 3 determines tolerance versus full activation of naive CD8 T cells: Dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtsinger J.M., Mescher M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceeraz S., Nowak E.C., Noelle R.J. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory t cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley J.L. PD-1 signaling in primary t cells. Immunol. Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y.F., Zhang G.M., Wang X.H., Zhang H., Yuan Y., Li D., Feng Z.H. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J. Immunol. 2004;173:4919–4928. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 13.Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 14.Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 15.Tseng S.Y., Otsuji M., Gorski K., Huang X., Slansky J.E., Pai S.I., Shalabi A., Shin T., Pardoll D.M., Tsuchiya H. B7-Dc, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarti N., Prieto V.G. Predictive factors of activity of anti-programmed death-1/programmed death ligand-1 drugs: Immunohistochemistry analysis. Transl. Lung Cancer Res. 2015;4:743–751. doi: 10.3978/j.issn.2218-6751.2015.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taube J.M., Anders R.A., Young G.D., Xu H., Sharma R., McMiller T.L., Chen S., Klein A.P., Pardoll D.M., Topalian S.L., et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong A.Y., Zhou R., Hu G., Li X., Splinter P.L., O′Hara S.P., LaRusso N.F., Soukup G.A., Dong H., Chen X.M. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-γ-induced B7-H1 expression in cholangiocytes. J. Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsa A.T., Waldron J.S., Panner A., Crane C.A., Parney I.F., Barry J.J., Cachola K.E., Murray J.C., Tihan T., Jensen M.C., et al. Loss of tumor suppressor pten function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 20.Crane C.A., Panner A., Murray J.C., Wilson S.P., Xu H., Chen L., Simko J.P., Waldman F.M., Pieper R.O., Parsa A.T. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gowrishankar K., Gunatilake D., Gallagher S.J., Tiffen J., Rizos H., Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS ONE. 2015;10:1151. doi: 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francisco L.M., Sage P.T., Sharpe A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fife B.T., Pauken K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki T., Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 26.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson R.H., Gillett M.D., Cheville J.C., Lohse C.M., Dong H., Webster W.S., Krejci K.G., Lobo J.R., Sengupta S., Chen L., et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Pro. Natl. Acad. Sci. USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boland J.M., Kwon E.D., Harrington S.M., Wampfler J.A., Tang H., Yang P., Aubry M.C. Tumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lung. Clin. Lung Cancer. 2013;14:157–163. doi: 10.1016/j.cllc.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Velcheti V., Schalper K.A., Carvajal D.E., Anagnostou V.K., Syrigos K.N., Sznol M., Herbst R.S., Gettinger S.N., Chen L., Rimm D.L. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Investig. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper W.A., Tran T., Vilain R.E., Madore J., Selinger C.I., Kohonen-Corish M., Yip P., Yu B., O′Toole S.A., McCaughan B.C., et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y., Zhang S.D., McCrudden C., Chan K.W., Lin Y., Kwok H.F. The prognostic significance of PD-L1 in bladder cancer. Oncol. Rep. 2015;33:3075–3084. doi: 10.3892/or.2015.3933. [DOI] [PubMed] [Google Scholar]
- 32.Kakavand H., Vilain R.E., Wilmott J.S., Burke H., Yearley J.H., Thompson J.F., Hersey P., Long G.V., Scolyer R.A. Tumor PD-L1 expression, immune cell correlates and PD-1+ lymphocytes in sentinel lymph node melanoma metastases. Mod. Pathol. 2015;28:1535–1544. doi: 10.1038/modpathol.2015.110. [DOI] [PubMed] [Google Scholar]
- 33.Nduom E.K., Wei J., Yaghi N.K., Huang N., Kong L.Y., Gabrusiewicz K., Ling X., Zhou S., Ivan C., Chen J.Q., et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-oncology. 2016;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., Rosenberg S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sfanos K.S., Bruno T.C., Meeker A.K., de Marzo A.M., Isaacs W.B., Drake C.G. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ansell S.M., Lesokhin A.M., Borrello I., Halwani A., Scott E.C., Gutierrez M., Schuster S.J., Millenson M.M., Cattry D., Freeman G.J., et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N. Engl. J. Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou W.P., Chen L.P. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 38.Duraiswamy J., Freeman G.J., Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard K.A., Fitz L.J., Lee J.M., Benander C., George J.A., Wooters J., Qiu Y., Jussif J.M., Carter L.L., Wood C.R., et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3ζ signalosome and downstream signaling to PKCθ. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 41.Hoos A., Ibrahim R., Korman A., Abdallah K., Berman D., Shahabi V., Chin K., Canetta R., Humphrey R. Development of ipilimumab: Contribution to a new paradigm for cancer immunotherapy. Semin. Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 43.Brahmer J., Reckamp K.L., Baas P., Crino L., Eberhardt W.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazandjian D., Suzman D.L., Blumenthal G., Mushti S., He K., Libeg M., Keegan P., Pazdur R. FDA approval summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 47.Deeks E.D. Pembrolizumab: A review in advanced melanoma. Drugs. 2016;76:375–386. doi: 10.1007/s40265-016-0543-x. [DOI] [PubMed] [Google Scholar]
- 48.Bagcchi S. Pembrolizumab for treatment of refractory melanoma. Lancet Oncol. 2014;15:e419. doi: 10.1016/S1470-2045(14)70348-1. [DOI] [PubMed] [Google Scholar]
- 49.Robert C., Ribas A., Wolchok J.D., Hodi F.S., Hamid O., Kefford R., Weber J.S., Joshua A.M., Hwu W.J., Gangadhar T.C., et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 50.Ivashko I.N., Kolesar J.M. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am. J. Health-Syst. Pharm. 2016;73:193–201. doi: 10.2146/ajhp140768. [DOI] [PubMed] [Google Scholar]
- 51.Ribas A., Puzanov I., Dummer R., Schadendorf D., Hamid O., Robert C., Hodi F.S., Schachter J., Pavlick A.C., Lewis K.D., et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sul J., Blumenthal G.M., Jiang X., He K., Keegan P., Pazdur R.U.S. Food and Drug Administration approval summary: Pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016 doi: 10.1634/theoncologist.2015-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westin J.R., Chu F., Zhang M., Fayad L.E., Kwak L.W., Fowler N., Romaguera J., Hagemeister F., Fanale M., Samaniego F., et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy B., Indjiia L., Rodionov G., Raiter A., Inbal A. Treatment with bat monoclonal antibody decreases tumor burden in a murine model of leukemia/lymphoma. Int. J. Oncol. 2001;19:897–902. doi: 10.3892/ijo.19.5.897. [DOI] [PubMed] [Google Scholar]
- 55.Hardy B., Niv Y., Fadaeev L., Raiter A. Bat mab induces lymphopoiesis in nude mice. Int. Immunol. 2005;17:615–619. doi: 10.1093/intimm/dxh244. [DOI] [PubMed] [Google Scholar]
- 56.Feinmesser M., Raiter A., Hardy B. Prevention of melanoma metastases in lungs of bat treated and peptide immunized mice. Int. J. Oncol. 2006;29:911–917. doi: 10.3892/ijo.29.4.911. [DOI] [PubMed] [Google Scholar]
- 57.Hardy B., Morgenstern S., Raiter A., Rodionov G., Fadaeev L., Niv Y. Bat monoclonal antibody immunotherapy of human metastatic colorectal carcinoma in mice. Cancer Lett. 2005;229:217–222. doi: 10.1016/j.canlet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 58.Berger R., Rotem-Yehudar R., Slama G., Landes S., Kneller A., Leiba M., Koren-Michowitz M., Shimoni A., Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 59.Armand P., Nagler A., Weller E.A., Devine S.M., Avigan D.E., Chen Y.B., Kaminski M.S., Holland H.K., Winter J.N., Mason J.R., et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J. Clin. Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atkins M.B., Kudchadkar R.R., Sznol M., McDermott D.F., Lotem M., Schachter J., Wolchok J.D., Urba W.J., Kuzel T., Schuchter L.M., et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. Am. Soc. Clin. Oncol. 2014;32:9001. [Google Scholar]
- 61.Trivedi M.S., Hoffner B., Winkelmann J.L., Abbott M.E., Hamid O., Carvajal R.D. Programmed death 1 immune checkpoint inhibitors. Clin. Adv. Hematol. Oncol. 2015;13:858–868. [PubMed] [Google Scholar]
- 62.Smothers F., Hoos A., Langermann S., Marshall S., May R., Fleming M.E. AMP-224, a fusion protein that targets PD-1. Ann. Oncol. [(accessed on 4 May 2016)]. Available online: http://tatcongress.org/wp-content/uploads/2014/05/l02.04-smothers-tat-2013-final.pdf.
- 63.Momtaz P., Postow M.A. Immunologic checkpoints in cancer therapy: Focus on the programmed death-1 (PD-1) receptor pathway. Pharm. Personal. Med. 2014;7:357–365. doi: 10.2147/PGPM.S53163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Infante J.R., Powderly J.D., Burris H.A., Kittaneh M., Grice J.H., Smothers J.F., Brett S., Fleming M.E., May R., Marshall S., et al. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J. Clin. Oncol. 2013;31:3044. [Google Scholar]
- 65.Lorusso P.M., Powderly J., Burris H.A., Kittaneh M., Grice J., Smothers J.F., Brett S., Fleming M., May R.J., Marshall S. Abstract LB-193: Phase I study of safety, tolerability, pharmacokinetics, and pharmacodynamics of AMP-224 (B7-DC Fc fusion protein) in a regimen containing cyclophosphamide (CTX) in patients with advanced solid tumors. Cancer Res. 2013;73:LB-193. doi: 10.1158/1538-7445.AM2013-LB-193. [DOI] [Google Scholar]
- 66.Duffy A.G., Makarova-Rusher O.V., Fioravanti S., Walker M., Venkatesan A., Abi-Jaoudeh N., Wood B.J., Citrin D.E., Greten T.F. A pilot study of AMP-224—A PD-1 inhibitor—In combination with stereotactic body radiation therapy (SBRT) in patients with metastatic colorectal cancer. J. Clin. Oncol. 2015;33:TPS788. [Google Scholar]
- 67.Hamid O., Chow L.Q.M., Tavakkoli F., Marshall S., Gribbin M.J., Karakunnel J.J., Gray J.E. Phase I, open-label study of MEDI0680, an anti-programmed cell death-1 (PD-1) antibody, in combination with medi4736, an anti-programmed cell death ligand-1 (PD-L1) antibody, in patients with advanced malignancies. J. Clin. Oncol. [(accessed on 5 May 2016)]. Available online: http://meetinglibrary.asco.org/content/145153-156.
- 68.Infante J.R., Goel S., Tavakkoli F., Marshall S., Robbins P.B., D′Angelo G., Gribbin M.J., Karakunnel J.J., Naing A. A phase I, multicenter, open-label, first-in-human study to evaluate MEDI0680, an anti-programmed cell death-1 antibody, in patients with advanced malignancies. J. Clin. Oncol. [(accessed on 5 May 2016)]. Available online: http://meetinglibrary.asco.org/content/145636-156.
- 69.Burova E., Allbritton O., Poueymirou W., Lai V., Waite J., Skokos D., Papadopoulos N., Murphy D., Lowy I., Ioffe E., et al. In vivo characterization of anti-PD-1 antibody REGN2810 in human PD-1 knock-in mice. Cancer Immunol. Res. 2016;4:B113. doi: 10.1158/2326-6074.CRICIMTEATIAACR15-B113. [DOI] [Google Scholar]
- 70.Elena Burova O.A., William P., Venus L., Janelle W., Dimitris S., Nicholas P., Drew M., Israel L., Ella I., Gavin T. Antitumor activity of REGN2810, a fully human anti-PD-1 monoclonal antibody, against MC38.Ova tumors grown in immune-competent humanized PD-1 mice. Cancer Res. 2015;75:266. doi: 10.1158/1538-7445.AM2015-266. [DOI] [Google Scholar]
- 71.Chikuma S. Basics of PD-1 in self-tolerance, infection, and cancer immunity. Int. J. Clin. Oncol. 2016;21:448–455. doi: 10.1007/s10147-016-0958-0. [DOI] [PubMed] [Google Scholar]
- 72.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyerinas B., Jochems C., Fantini M., Heery C.R., Gulley J.L., Tsang K.Y., Schlom J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E., Watkins A., Mullins S., Chodorge M., Andrews J., et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol. Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 75.Ibrahim R., Stewart R., Shalabi A. Pd-l1 blockade for cancer treatment: MEDI4736. Semin. Oncol. 2015;42:474–483. doi: 10.1053/j.seminoncol.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Oganesyan V., Gao C., Shirinian L., Wu H., Dall′Acqua W.F. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr. 2008;64:700–704. doi: 10.1107/S0907444908007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segal N.H., Antonia S.J., Brahmer J.R., Maio M., Blake-Haskins A., Li X., Vasselli J., Ibrahim R.A., Lutzky J., Khleif S. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J. Clin. Oncol. 2014;32:3002. [Google Scholar]
- 78.Khleif S., Lutzky J., Segal N., Antonia S., Blake-Haskin A., Stewart R. MEDI4736,an anti-PD-L1 antibody with modified fc domain: Preclinical evaluation and early clinical results from a phse 1 study in patients with advanced solidtumors. Eur. J. Cancer. 2013;49:S161. [Google Scholar]
- 79.Stewart R., Mulgrew K., Wang S. Blockade of PD-L1 mediated immunosupression for cancer therapy-medi4736, monoclonal antibody discovery and preclinical development. J. Immunother. 2013;35:2. [Google Scholar]
- 80.Deng R., Bumbaca D., Pastuskovas C.V., Boswell C.A., West D., Cowan K.J., Chiu H., McBride J., Johnson C., Xin Y., et al. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-pd-l1 monoclonal antibody, an immune checkpoint inhibitor. mAbs. 2016;8:593–603. doi: 10.1080/19420862.2015.1136043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Powles T., Eder J.P., Fine G.D., Braiteh F.S., Loriot Y., Cruz C., Bellmunt J., Burris H.A., Petrylak D.P., Teng S.L., et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 82.Herbst R.S., Jean-Charles S., Marcin K., Fine G.D., Omid H., Gordon M.S., Sosman J.A., Mcdermott D.F., Powderly J.D., Gettinger S.N. Predictive correlates of response to the anti-pd-l1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herbst R.S., Gordon M.S., Fine G.D., Sosman J.A., Soria J.-C., Hamid O., Powderly J.D., Burris H.A., Mokatrin A., Kowanetz M., et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Annu. Meet. Proc. 2013;31:3000. [Google Scholar]
- 84.Zhan M.M., Hu X.Q., Liu X.X., Ruan B.F., Xu J., Liao C. From monoclonal antibodies to small molecules: The development of inhibitors targeting the PD-1/PD-L1 pathway. Drug Discov. Today. 2016;21:1027–1036. doi: 10.1016/j.drudis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12:496–508. doi: 10.1016/j.chom.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAleer J.P., Kolls J.K. Maintaining poise: Commensal microbiota calibrate interferon responses. Immunity. 2012;37:10–12. doi: 10.1016/j.immuni.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.-L., et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hugo W., Zaretsky J.M., Sun L., Song C., Moreno B.H., Hu-Lieskovan S., Berent-Maoz B., Pang J., Chmielowski B., Cherry G., et al. Genomic and transcriptomic features of response to anti–PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang H.N., Liu B.Y., Qi Y.K., Zhou Y., Chen Y.P., Pan K.M., Li W.W., Zhou X.M., Ma W.W., Fu C.Y. Blocking of the PD-1/PD-L1 interaction by a d-peptide antagonist for cancer immunotherapy. Angew. Chem. 2015;127:11760–11764. doi: 10.1002/anie.201506225. [DOI] [PubMed] [Google Scholar]
- 91.Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 92.Hoos A. Development of immuno-oncology drugs—From CTLA4 to PD1 to the next generations. Nat. Rev. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 93.Kibel A.S. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:1966–1966. doi: 10.1016/j.yuro.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 94.Hodi F.S., O′Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Topp M.S., Kufer P., Gökbuget N., Goebeler M., Klinger M., Neumann S., Horst H.A., Raff T., Viardot A., Schmid M. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 96.Topp M.S., Gökbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S., Viardot A., Marks R., Diedrich H., Faul C. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 97.Topp M.S., Gökbuget N., Stein A.S., Zugmaier G., O’Brien S., Bargou R.C., Dombret H., Fielding A.K., Heffner L., Larson R.A. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 98.Gilboa E., Berezhnoy A., Schrand B. Reducing toxicity of immune therapy using aptamer-targeted drug delivery. Cancer Immunol. Res. 2015;3:1195–1200. doi: 10.1158/2326-6066.CIR-15-0194. [DOI] [PubMed] [Google Scholar]
- 99.Jayasena S.D. Aptamers: An emerging class of molecules that rival antibodies in diagnostics. Clin. Chem. 1999;45:1628–1650. [PubMed] [Google Scholar]
- 100.Ellington A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banerjee J. Antibodies are challenged. Indian J. Med. Sci. 2010;64:144–147. [PubMed] [Google Scholar]