Abstract
Nutrients play a fundamental role as regulators of the activity of enzymes involved in liver metabolism. In the general population, the action of nutrients may be affected by gene polymorphisms. Therefore, individualization of a diet for individuals with fatty liver seems to be a fundamental step in nutritional strategies. In this study, we tested the nutrient-induced insulin output ratio (NIOR), which is used to identify the correlation between the variants of genes and insulin resistance. We enrolled 171 patients, Caucasian men (n = 104) and women (n = 67), diagnosed with non-alcoholic fatty liver disease (NAFLD). From the pool of genes sensitive to nutrient content, we selected genes characterized by a strong response to the NIOR. The polymorphisms included Adrenergic receptor (b3AR), Tumor necrosis factor (TNFα), Apolipoprotein C (Apo C III). Uncoupling Protein type I (UCP-1), Peroxisome proliferator activated receptor γ2 (PPAR-2) and Apolipoprotein E (APOEs). We performed three dietary interventions: a diet consistent with the results of genotyping (NIOR (+)); typical dietary recommendations for NAFLD (Cust (+)), and a diet opposite to the genotyping results (NIOR (−) and Cust (−)). We administered the diet for six months. The most beneficial changes were observed among fat-sensitive patients who were treated with the NIOR (+) diet. These changes included improvements in body mass and insulin sensitivity and normalization of blood lipids. In people sensitive to fat, the NIOR seems to be a useful tool for determining specific strategies for the treatment of NAFLD.
Keywords: NAFLD, NAFLD diet, insulin sensitivity, NIOR, reduction of body mass, fat reduction, liver fat
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most frequently diagnosed liver diseases in the industrialized world—approximately 20%–30% of nations’ populations are affected by it [1,2]. With the increase in obesity, NAFLD has become a major risk factor for cirrhosis (and other diseases, e.g., cardiovascular diseases) [3]. Multiple trials have demonstrated that weight loss reduces histological steatosis (intrahepatic fat content) and the amount of serum enzymes [4].
One of the key causes of NAFLD is an improper diet based on caloric oversupply, the excessive intake of fats, and, at the same time, the low intake of grains, fruits, vegetables, proteins and ω-3 fatty acids [2]. This pattern of nutrition leads to the development of hyperinsulinemia, insulin resistance and obesity [2,5,6,7]. Therefore, on the one hand, nutrition is a major cause of NAFLD, but on the other, it presents an effective form of treatment [5,8,9].
In NAFLD, nutrition can be characterized by an appropriate choice of active nutrients that can play a regulatory role in metabolism. Nutrients regulate the activity of enzymes involved in metabolic processes, acting at the level of the proteome and metabolome and functioning as sensors that influence metabolic pathways [10,11,12]. Importantly, the same nutrient may have different influences on given people due to genetic polymorphisms found in the population [12]. The interactions between nutrients, genetic factors (polymorphism/mutations) and health are the subject matter of nutrigenomics [12]. This field of science aims to establish personalized nutrition strategies for the prevention and treatment of lifestyle diseases [12,13]. It can be assumed that if the action of nutrients is affected by polymorphisms, it is advisable to search for methods of individualizing a patient’s nutrition. Therefore, in this study, we focused on testing a tool that could be used for the individualization of nutrition in patients with NAFLD. The specific tool used in this study was the nutrient-induced insulin output ratio (NIOR), which was selected to determine the genotype-phenotype interaction [14]. The NIOR has already been used to identify a correlation between the variants of genes (associated with the metabolism of carbohydrates and fat) and the output of insulin and the development of diet-induced insulin resistance. Using the NIOR, we identified the carriers of the alleles of gene variants characterized by a reduced tolerance to fat or carbohydrates in the diet. The pool of genes associated with NIOR includes glucose-sensitive genes, such as genes for Adrenergic receptors (b3AR), Tumor necrosis factor (TNF-α) and Apolipoprotein C (APOC3) [14]. The variants of these genes are described in the literature as being responsible for an increased risk of developing insulin resistance (gene b3AR, rs 4994) [15], the induction and development of insulin resistance and metabolic syndrome (gene TNF-α, rs 1800629) [16] and severe forms of hyperlipidemia (gene APOC3, rs 5128) [17].
Fat-sensitive genes associated with NIOR include the genes of Uncoupling Protein type I (UCP-1, rs 1800592), Peroxisome proliferator-activated receptor γ 2 (PPAR-γ2, rs 18012820) and Apolipoprotein E (ApoE). Selected variants of these genes are responsible for the regulation of body weight and the concentration of plasma high density lipoprotein (Type 1 uncoupling protein (UCP1)) [18], an increased risk of metabolic syndrome by the regulation of energy homeostasis and glucose (Peroxisome proliferator activated receptor γ2 PPAR-γ2 gene) [19], the furthering of insulin-resistance, the development of hyperlipidemia and hypertriglyceridemia and the progression of coronary heart disease (APOE rs 405509, rs 7412 rs 429358) [17].
The aim of this study was to determine whether the NIOR can be useful in planning the individualized nutrition of patients with NAFLD and whether its use contributes to a more effective inhibition of NAFLD progression, defined as a reduced degree of hepatic steatosis and improved biochemical and anthropometric parameters.
2. Results
2.1. The Analysis of the Data Using Model 1
2.1.1. Changes in Anthropometric Parameters after Six Months Depending on the Type of Diet
The most beneficial changes in body composition were observed among patients treated with the NIOR (+) diet (Table 1). The body mass reduction, the reduction in waist circumference, and the reduction in fat mass were significant.
Table 1.
Anthropological and biochemical characteristics of the study participants’ blood parameters at baseline and after six months of the diet in Model 1, with p-values of the comparison between subjects within this same intervention before and after six months. a p < 0.0005 Wilcoxon test, comparison between baseline and the fourth visit in this same group; b p < 0.005 Wilcoxon test, comparison between baseline and the fourth visit in this same group; * Mann-Whitney U test, comparison between NIOR (+) and Cust (+); # Mann-Whitney U test, comparison between NIOR (+) and contrary diets NIOR (−)/Cust (−); & Mann-Whitney U test, comparison between Cust (+) and contrary diets Cust (−) and NIOR (−). BMI: Body mass index; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids, HA: hyaluronic acid.
| Parameters | Baseline | 24W | p Value | ||||
|---|---|---|---|---|---|---|---|
| CUST (+) | NOR (+) | CONTRA CUST (−) and NOR (−) | CUST (+) | NOR (+) | CONTRA CUST (−) and NOR (−) | ||
| Age | 52.12 ± 14.74 | 52.80 ± 12.37 | 51.87 ± 12.11 | 52.12 ± 14.74 | 52.80 ± 12.37 | 51.87 ± 12.11 | |
| Body mass (kg) | 94.70 ± 22.55 a | 89.01 ± 15.26 a | 92.20 ± 19.34 b | 87.59 ± 17.96 a | 82.21 ± 15.35 a,# | 89.63 ± 20.79 b,# | a p < 0.0005 |
| b p < 0.005 | |||||||
| BMI (kg/m2) | 32.10 ± 4.13 | 30.70 ± 3.64 | 32.27 ± 6.59 | 30.01 ± 2.84 | 28.29 ± 15.35 # | 28.29 ± 15.35 # | # p < 0.015 |
| Arm circumference (cm) | 33.30 ± 3.64 a | 31.66 ± 2.87 | 32.33 ± 3.86 | 31.85 ± 2.56 a,& | 30.75 ± 3.46 | 33.38 ± 4.53 & | a p < 0.0005 |
| & p < 0.04 | |||||||
| Waist circumference (cm) | 105.14 ± 14.69 a | 100.12 ± 11.75 b | 106.25 ± 14.45 b | 97.02 ± 10.63 a | 94.00 ± 11.97 b | 102.60 ± 16.02 b | a p < 0.0009 |
| b p < 0.005 | |||||||
| Hip circumference (cm) | 105.14 ± 14.69 a | 100.12 ± 11.75 b | 106.25 ± 15.73 b | 104.20 ± 14.89 a | 94.00 ± 13.33 b | 102.60 ± 17.04 b | a p < 0.0009 |
| b p < 0.005 | |||||||
| Fat mass (%) | 34.71 ± 5.78 b | 35.41 ± 5.67 a | 36.02 ± 14.09 | 31.31 ± 5.78 b,* | 29.74 ± 8.21 a,*,# | 35.09 ± 14.94 # | a p < 0.0006 |
| b p < 0.002 | |||||||
| # p < 0.007 | |||||||
| * p < 0.04 | |||||||
| Fat content (%) | 37.00 ± 6.63 b | 35.61 ± 10.96 b | 38.07 ± 7.79 | 36.34 ± 5.75 b,* | 31.03 ± 13.46 b,*,# | 38.95 ± 7.90 # | b p < 0.005 |
| # p < 0.005 | |||||||
| * p < 0.04 | |||||||
| Lean mass (%) | 60.02 ± 16.47 b | 53.48 ± 10.96 | 55.94 ± 7.80 b | 55.92 ± 14.17 b | 51.03 ± 13.46 | 53.66 ± 9.28 b | b p < 0.002 |
| AST (U/L) | 36.10 ± 25.83 b | 30.70 ± 13.99 b | 32.71 ± 10.46 b | 35.10 ± 29.60 b | 34.20 ± 26.31 b,# | 23.85 ± 5.21 b,# | b p < 0.035 |
| # p < 0.041 | |||||||
| ALT (U/L) | 54.00 ± 36.86 b | 46.70 ± 26.56 a | 47.92 ± 15.85 a | 44.40 ± 27.86 b | 39.40 ± 33.04 a | 32.57 ± 18.40 a | a p < 0.0006 |
| a p < 0.038 | |||||||
| Trigliceride (mg/dL) | 129.30 ± 36.30 b | 123.80 ± 52.82 | 238.42 ± 482.09 | 106.40 ± 56.24 b | 121.80 ± 90.52 | 204.78 ± 319.17 | b p < 0.04 |
| HDL (mg/dL) | 51.00 ± 12.22 b | 54.40 ± 12.83 | 51.57 ± 15.73 | 54.00 ± 16.39 b | 54.30 ± 12.82 | 52.14 ± 14.31 | b p < 0.025 |
| Insulin (mcU/L) | 15.80 ± 9.05 | 14.59 ± 8.20 b | 18.54 ± 19.69 b | 12.07 ± 8.62 | 9.58 ± 6.81 b | 9.63 ± 9.46 b | b p < 0.05 |
| HOMA–IR | 4.03 ± 2.31 | 3.76 ± 1.94 b | 5.44 ± 6.65 b | 3.08 ± 2.08 | 2.41 ± 1.74 b | 2.80 ± 3.00 b | b p < 0.05 |
| Hyaluronic acid (U/L) | 54.56 ± 29.57 | 45.47 ± 25.82b | 50.23 ± 31.76 | 45.93 ± 22.62 | 32.56 ± 16.28 b | 37.42 ± 22.21 | b p < 0.04 |
| Fatty liver Hamaguchi score | 2.13 ± 0.74 b | 2.47 ± 0.94 b | 2.11 ± 0.98 b | 1.2 ± 1.0 b | 1.12 ± 1.08 b,# | 1.11 ± 0.92 b,# | b p < 0.01 |
| # p < 0.04 | |||||||
Weight reductions were also recorded in the Cust (+) group, but in comparison to NIOR (+), the reduction in fat content was less significant (−3.40 ± 6.27, p < 0.002 vs. −0.66 ± 3.67, p < 0.02) (Table 1). In Cust (+) patients, negative changes associated with the loss of lean body mass and arm circumference were also recorded (Table 1).
Slight changes in body mass, waist circumference, and hip circumference were observed in the group contrary to NIOR (−) and Cust (−) (called CONTRA in Table 1).
The analysis of changes between these groups provided interesting results. The most significant changes were observed when NIOR (+) and NIOR (−) and Cust (−) were compared (CONTRA NIOR (−) and Cust (−)). Between these groups, there were significant differences in the reduction of body mass (−6.79 ± 4.79 kg, NIOR (+) vs. −2.56 ± 2.88 kg NIOR (−), p < 0.026), BMI (−2.41 ± 1.73 kg/m2 NIOR (+) vs. −0.83 ± 1.04 kg/m2 NIOR (−), p < 0.015), fat mass (−5.39 ± 6.19, p < 0.006 NIOR (+) vs. −0.136 ± 2.97 NIOR (−), p < 0.007) and fat content (−2.45 ± 7.01 NIOR (+) vs. −0.88 ± 3.00 NIOR (−), p < 0.005) (Table 1).
Between the groups Cust (+) and NIOR (+), we observed a significant difference in the reduction of fat mass (−3.40 ± 6.27 kg Cust (+) vs. −5.39 ± 6.19 kg NIOR (+), p < 0.04) (Table 1).
Between the groups Cust (+) and Cust (−), we found a difference in arm circumference change (−1.45 ± 1.60 cm Cust (+) vs. 1.05 ± 3.01 cm Cust (−), p < 0.04) (Table 1).
2.1.2. Changes in Biochemical Parameters after Six Months in Model 1
One of the most important objectives to achieve during nutritional therapy in patients with NAFLD is a reduction in insulin resistance [20]. This effect was measured by determining. The homeostatc model assessment HOMA IR and HOMA B (used to estimate the improved β-cell “function”) [21]. HOMA IR under normal physiological conditions is 1.0; higher values indicate peripheral insulin resistance or resistance of hepatic origin [22,23]. Patients in all groups were characterized by insulin resistance at the beginning (Table 1). The highest average HOMA IR value was observed for the NIOR (−) and Cust (−) groups. The reduction in HOMA IR in both of these groups reached −2.64 ± 4.57, p < 0.05. The initial HOMA IR in NIOR (+) patients was 3.76 ± 1.94. The recorded reduction in HOMA IR after six months was −1.34 ± 1.86, p < 0.05 (Table 1).
Additionally, the normalization of blood lipids (total cholesterol, triglyceride (TG), low density lipoprotein (LDL), high density lipoprotein (HDL) is an important element of nutritional therapy. Positive trends toward blood lipid normalization were observed in all types of diets (Table 1).
2.1.3. A Significant Reduction in the Degree of Fatty Liver Disease Was Observed in Patients with a Diet Selected According to NIOR
In the NIOR (+) group, the average reduction in the degree of fatty liver disease was −1.31 ± 1.01, p < 0.002. The difference in the reduction of fatty liver disease was significant between the NIOR (+) and NIOR (−) groups, p < 0.04—Mann-Whitney U test (Table 1).
2.2. The Data Analysis in Model 2
Individuals from Different Groups Who Had a Similar Range of Reduction in Body Weight Obtained Different Reductions in Hepatic Steatosis and Other Parameters
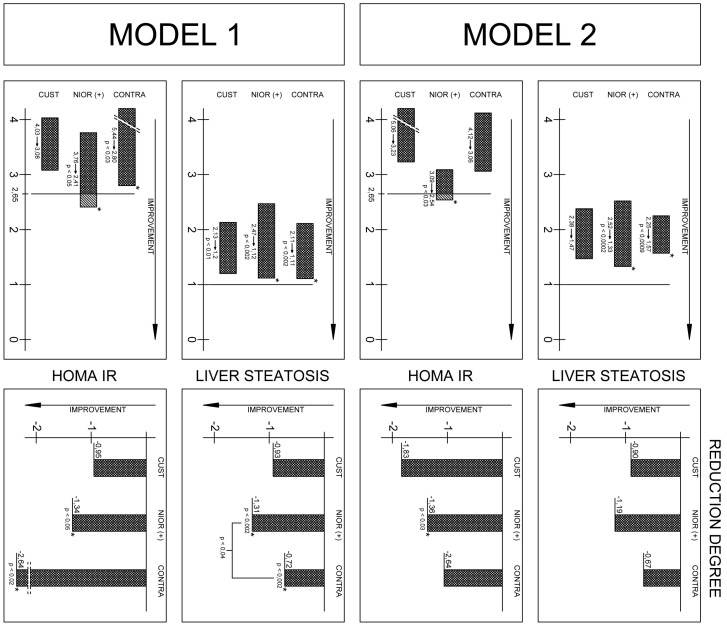
Only individuals in the NIOR (+) group showed improvement in the degree of hepatic steatosis (Figure 1, Table S1).
Figure 1.
Changes in biochemical blood parameters in Model 1 and 2Note: All data represent the mean (standard deviation).
The analysis of the differences between the groups showed that the reduction in hepatic steatosis in the NIOR (+) group significantly differed from that observed in the NIOR (−) group (Mann-Whitney U test, p < 0.04). A similar significant difference between groups was observed for hyaluronic acid, with levels differing significantly between the Cust (+) and NIOR (+) groups (−26.45 ± 17.72 NIOR (+) vs. −1.94 ± 5.4 Cust (−) and NIOR (−), Mann-Whitney U test, p < 0.005).
3. Discussion
Obesity and insulin resistance present a considerable challenge in the nutrition plans of patients with NAFLD [24,25]. Current dietary guidelines are based on epidemiological data showing a link between diets enriched in saturated fatty acids and in fructose and the development of insulin resistance [26]. However, the response to diet differs depending on individual variations in genetic and metabolic phenotypes. Therefore, it is important to personalize patients’ diets, taking into account their genetic predispositions [13,14].
One potentially interesting tool is the nutrient-induced insulin output ratio (NIOR). The NIOR makes it possible to categorize patients (gene variant carriers) into two groups: phenotypically sensitive to glucose or fat in the diet. The polymorphisms of genes associated with the NIOR have previously been associated with the severity of metabolic syndrome and susceptibility to the effects of nutrients [14,15,16,17,18,19]. In our work, we examined polymorphisms (linked with NIOR) according to their impact on the output of insulin after a meal [14]. The usefulness of NIOR as a potential tool to individualize diets was examined through the introduction of quantitative changes in nutrients (fat or simple carbohydrates), consistent with the results of genetic tests. To exclude the impact of polymorphisms themselves, some people were randomly assigned to a group in which the key nutrient contents were chosen in quantities contrary to the indications of genetic research.
The second important objective of this study was to create a nutritional plan that would be accepted by the respondents for an extended period of time. We succeeded in obtaining the results of a half-year-long diet, resulting in an acceptable reduction in the content of the tested nutrients in the diet.
We showed that a selection of nutrients consistent with the indications of the NIOR contributed to an effective reduction in hepatic steatosis in both Model 1 and Model 2. This is a very important result, as fat droplets accumulating in hepatocytes are considered the main hepatotoxic factor, inducing hepatic steatosis and fibrosis [27,28,29]. The reduction of lipid content in the liver, therefore, means a reduction in the intensity of fibrosis [27], which is marked by hyaluronic acid content in the blood [28,29]. Such a reduction in hyaluronic acid was recorded in all groups, but the largest decline in hyaluronic acid content was found in the NIOR (+) group, regardless of the research model (Table 1 and Figure 1).
Additionally, individual selections of nutrients based on the NIOR were intended to contribute to the reduction of fat mass (Table 1). The results seem to confirm the usefulness of NIOR for the efficient reduction in body fat mass and fat content when comparing the NIOR (+) and NIOR (−) groups. It seems that the reduction of fat mass and fat tissue was most effective in the group in which the amount of dietary fat or dietary sugar was adjusted to gene polymorphisms. Of note is that there was no significant effect of NIOR on the reduction of insulin resistance between groups. The HOMA IR ratio was effectively reduced in all groups, regardless of the type of diet (Table 1). Fats are components that play a crucial role in the progression of NAFLD [27,30,31,32,33,34]. The positive changes in the liver were the result of a decrease in the fat content of the diet, especially among fat-sensitive polymorphism carriers (Table 1 and Figure 1). Our study confirms the results of other authors, e.g., Marina et al. [31], who found that fat (in different contents in the diet—20% vs. 55% the total daily energy expenditure (TDEE) caused minor effects in the content of intra-abdominal fat and intrahepatic lipids. In another study (a short-term intervention), a three-week isocaloric low-fat diet (20% TDEE ) decreased intrahepatic lipids by 13%, whereas a high fat diet (55% TDEE) increased the amount of lipids in the liver by up to 17% [30]. Unfortunately, both studies were limited to a short period of observation [30,31].
Though our study was longer, it suffered from a significant limitation, which was the exclusion of variants sensitive to simple sugars (after six months, only one person remained—Figure 2). This was a substantial loss because simple sugars, especially fructose (a common nutrient in western diets), is reported to be associated with an increased risk of NAFLD [35,36,37]. Although the consumption of fructose is high and continues to be on the rise [38], there are still no conclusive results that indicate a connection between the high intake of fructose and NAFLD [35,37]. The available evidence is not sufficiently robust to draw conclusions regarding the effects of fructose, high fructose corn syrup (HFCS) or sucrose consumption on NAFLD [37].
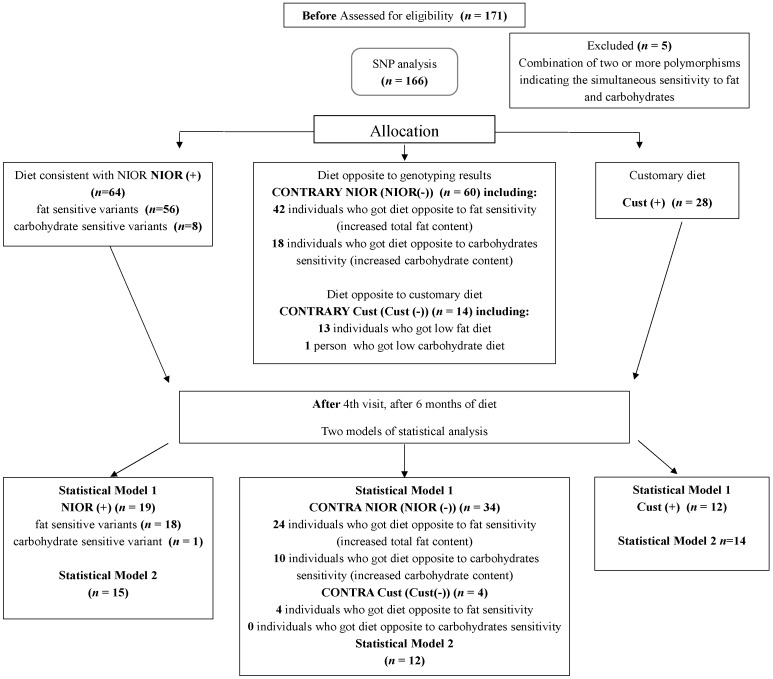
Figure 2.
Flowchart for the selection of individuals from the nutrient-induced insulin output ratio (NIOR) cohort. Participants entering subsequent phases of the study as well as dropouts out are indicated in the total. NIOR (+) represents individuals consuming a diet consistent with the results of genotyping; Cust (+), individuals consuming a diet comprising the typical dietary recommendations for non-alcoholic fatty liver disease (NAFLD); NIOR (−) and Cust (−), individuals consuming a diet contrary to the genotyping results.
It seems that the lack of clear associations between the consumption of simple sugars and hepatic steatosis can result from yet another important variable, i.e., gender. Research from 2014 shows that the severity of hepatic steatosis may be significantly influenced by feeding patterns associated with gender [27]. Unfortunately, our study cannot be included in the discussion in this area. Slightly more severe hepatic steatosis was shown in our analysis of diets before the initiation of the prescribed diet. The analysis of the FFQ results indicates a lack of a relationship between the consumption of products containing large amounts of sugars and the degree of hepatic steatosis among our respondents, regardless of gender (unpublished results). Understanding the specific interaction between nutrients and dietary needs and maintaining this balance is extremely important in providing treatment for NAFLD [39,40].
4. Materials and Methods
4.1. Patients
A group of 171 eligible participants, Caucasian men (n = 104) and women (n = 67) diagnosed with NAFLD, were prospectively enrolled in the study (Figure 2). Of the 171 total recruited patients, only 166 confirmed patients with NAFLD met the inclusion criteria. We conducted the measurements at the beginning of the study and at check points conducted at the first visit, after the first month, the second month and after six months—the final check point (Figure 2).
The exclusion criteria included the following: diabetes mellitus (DMII); infection with either HBV (Hepatitis B Virus) or HCV (Hepatitis C Virus); obesity (body mass index (BMI) >30 kg/m2); high levels of physical activity (>3000 kcal/week in leisure-time physical activity); changes in physical activity during the dietary intervention; use of statins; any condition that could limit the mobility of the participant; not being able to attend control visits; vegetarianism or a need for other special diets; the excessive consumption of alcohol (≥20 g in women and ≥30 g in men, per day); and other drug addiction.
Physical activity was assessed during the first visit and in subsequent appointments using the International Physical Activity Questionnaire (IPAQ) [41]. In this study we recommended moderate activity and we advised our patients not to change physical activity during the time of intervention. The degree of fatty liver disease was assessed by a trained physician according to the Hamaguchi score [42], using a high-resolution B-mode abdominal ultrasound scanner (Acuson X300, Simens, San Jose, CA, USA).
The study protocol was approved by the ethics committee of the Pomeranian Medical University (Szczecin, Poland, 25 01 2010 KB-0012/09/10) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The volunteers provided written informed consent before the study.
4.2. The Anthropometric Data
Anthropometric assessments were performed routinely during each of the four visits. The study included measurements of height (m), body weight (kg), skinfold thickness (mm), arm circumference (cm), waist circumference (cm) and hip circumference (cm). The measurements of body weight and height were obtained by means of medical scales with a stadiometer. Body mass index was calculated according to these measurements (BMI = body weight (kg)/square of height (m)) [24,43]. Using a medical tape measure, waist circumference was measured (midway between the bottom edge of the ribs and the iliac crest) as was hip circumference. Based on these measurements, WHR was calculated (WHR = waist circumference (cm)/hip circumference (cm)) [24]. A caliper was used to measure skinfold thicknesses: biceps, triceps, subscapular and abdominal skinfolds. In addition, in each subject, body composition was measured with a multifrequency bioimpedance meter, BIA-101 (Akern, Bioresearch SRL, PONASSIEVE, Florence, Italy).
4.3. Methods and Experimental Design
A randomized parallel controlled clinical trial with three dietary interventions was performed:
A diet consistent with the results of genotyping, called NIOR (+);
A diet with typical dietary recommendations for NAFLD, called Cust (+) [8];
A diet opposite to genotyping results, called (NIOR (−) and Cust (−) (CONTRA NIOR and Cust) (Figure 3).
Figure 3.
Baseline treatment characteristics. NIOR (+) represents individuals consuming a diet consistent with the results of genotyping; Cust (+), individuals consuming a diet comprising the typical dietary recommendations for NAFLD; NIOR (−) and Cust (−), individuals consuming a diet contrary to the genotyping results.
4.4. Allocation to Groups
The patients were randomly assigned to the NIOR (+) group. They represented:
-
(a)
a single polymorphism indicative of sensitivity to carbohydrates or fats
-
(b)
more than one polymorphism indicative of sensitivity to carbohydrates or to fats (e.g., two polymorphisms indicative of sensitivity to fat)
Only eight patients from the NIOR (+) group had polymorphisms indicative of sensitivity to carbohydrates. Unfortunately, these people dropped out of the study at various stages of the study. Only one carrier of sensitivity to carbohydrates completed the study (19 patients remained in the group).
Persons with a combination of two or more polymorphisms indicating simultaneous sensitivity to fat and carbohydrates were excluded from the study.
4.5. Dietary Intervention
4.5.1. General Recommendation
The diet was calculated individually according to the patient’s caloric needs. Individuals with a BMI indicating that they were overweight or obese received a reduced caloric diet of 500 kcal/day. People with a BMI within the normal range were given a normocaloric diet that allowed them to maintain their current body weight.
The total daily energy expenditure (TDEE) was calculated using the direct measurement of resting metabolic rate (RMR). RMR was measured during the first visit and in subsequent follow-up visits with a Fitmate apparatus (Pro, COSMED). The activity factor (AF) was determined in accordance with the generally accepted norm (TDEE = AF × RMR). The caloric content of the diet was adjusted during visits to the changing values of the patient’s TDEE.
All patients received weekly menus and guidelines on the timing of meals throughout the day, their composition and the size of the portions. Menus were prepared in the form of a daily plan for the seven days of the week and included guidance on the timing during the day of the five meal times.
The recommended sources of fat included vegetable fats, with a predominance of rapeseed oil and olive oil. It was permissible to use butter and margarine. Animal fats such as lard were excluded.
The recommended sources of carbohydrates included products with a low and medium glycemic index (GI). These included whole wheat bread, whole wheat pasta, cereal and brown rice. Sweets were excluded from the diet.
The recommended protein sources comprised poultry, fish (oily fish three times a week), fermented dairy products (two times a day), eggs (four to five times a week), lean cottage cheese, and cheese with a reduced fat content. Pork fat and offal products were excluded from the diet. The amount of fruit and vegetables recommended in the diets included three portions of vegetables and two portions of fruit. The amount of fluid intake was calculated to be 35 mL/kg of actual body weight.
4.5.2. Recommendations Based on the Nutrient-Induced Insulin Output Ratio (NIOR)
-
(a)
NIOR (+) patients received dietary recommendations with a reduced fat content (20% TDEE when NIOR polymorphisms showed sensitivity to fat) or reduced carbohydrate content (55% of TDEE, including <5% of sugars, when the polymorphisms showed sensitivity to simple carbohydrates).
-
(b)
Cust (+) patients received dietary advice with the following nutrient content: fat content at 30% of TDEE and carbohydrates at 55% of TDEE (including 10% of simple carbohydrates).
-
(c)
NIOR (−) patients, when they had “fat-sensitive” gene variants, received dietary recommendations that increased total fat content up to 30% of TDEE.
When participants had sugar-sensitive variants of genes, they received an increased amount of carbohydrates (10% simple carbohydrates).
Cust (−) patients were randomly assigned to groups with a reduced fat content or lower carbohydrate content.
4.5.3. Dietary Control
Nutrition patterns were analyzed with a Food Frequency Questionnaire (FFQ) and a 72 h food diary (including two working days and one day free of work) during the first visit. At all check points, the patients brought their completed 72 h food diary. The amounts consumed were recorded in household units, by volume or by measuring with a ruler. The dietary records were validated by a nutritionist according to a corresponding food table and nutrient database (Table 2).
Table 2.
Characteristics of dietary interventions. Nutrient-induced insulin output ratio (NIOR) (+) represents individuals consuming a diet consistent with the results of genotyping; Cust (+), individuals consuming a diet comprising the typical dietary recommendations for non-alcoholic fatty liver disease (NAFLD); NIOR (−) and Cust (−), individuals consuming a diet contrary to the genotyping results. * Group with a lower amount of carbohydrate (CHO) or fat.
| Content of Diet | NIOR (+) Variant Sensitive for Fat | NIOR (+) Variant Sensitive for Carbohydrate | Cust (+) | CONTRA NIOR NIOR (−) If Variant Was Sensitive for Fat | CONTRA NIOR NIOR (−) If Variant Was Sensitive for Carbohydrate | CONTRA Cust Cust (−) Randomly Selected to Group with Lower Amount of Fat of CHO * |
|---|---|---|---|---|---|---|
| Energy | Calculated individually | Calculated individually | Calculated individually | Calculated individually | Calculated individually | Calculated individually |
| Fat percent of total caloric in % | 20 | 30 | 30 | 30 | 20 | 20 or 30 * |
| Carbohydrates in % | 65 | 55 | 55 | 55 | 65 | 65 or 55 * |
| Simple carbohydrate in % | ≥10 | <5 | ≥10 | <5 | ≥10 | ≥10 or <5 * |
| Protein (%) | 15 | 15 | 15 | 15 | 15 | 15 |
| Fiber (g/day) | 30–35 | 30–35 | 30–35 | 30–35 | 30–35 | 30–35 |
| Fluid ( mL/kg) | 35 | 35 | 35 | 35 | 35 | 35 |
4.6. Laboratory Analyses
After overnight fasting, venous blood was collected into tubes containing anticoagulant Ethylenediaminetetraacetic acid (EDTA).
Blood samples were centrifuged at 3500 rpm for 10 min at 4 °C within 2 h of collection. Standard blood biochemical analyses were carried out at the University Hospital Laboratory (Szczecin, Poland). Hyaluronic acid was determined with an ELISA kit (Wuhan EIAab Science, A1710 Guangguguoji, Wuhan, China).
4.7. Genotyping
From the pool of genes sensitive to nutrient content, we selected genes that were characterized by a strong response to the oral glucose tolerance test after 75 g of glucose or after a high-fat meal. These included the b3-adrenergic receptor (b3AR), tumor necrosis factor (TNF-α) and apolipoprotein C III (apo CIII) [14].
From the pool of carbohydrate-sensitive genes, we selected Type 1 uncoupling protein (UCP-1), peroxisome proliferator-activated receptor γ 2 (PPAR-Y2) and apolipoprotein E (ApoE).
DNA from mononuclear peripheral blood was isolated using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA). Genotypes were determined by the real-time polymerase chain reaction using TaqMan® Genotyping 36 g Assays for polymorphisms, including b3AR rs4994 (Applied Biosystems Assay ID C___2215549_20); TNF-rs1800629 (C___7514879_10); Apo C III-rs5128 (C___8907537_1); Ucp-1-rs1800592 (C___8866368_20); PPAR-2-rs 1801282 (C___1129864_10); APOE-rs 405509 (C____905013_10); APOE-rs7412 (C_904973_10); and APOE-rs429358 (C___3084793_20). Fluorescence data were analyzed with allelic discrimination—7500 Software v 2.0.2 (Foster City, CA, USA).
4.8. Statistical Analysis
Statistica 7.1 software (Statsoft, Poznań, Poland) was used for the statistical analysis, and all results are expressed as the mean ± standard deviation. As the distribution, in most cases, deviated from normal (Shapiro-Wilk’s test), non-parametric tests were used: Wilcoxon tests were used for comparisons among groups and Mann-Whitney U tests were used for comparisons between groups. A p < 0.05 was considered significant.
Two Models of Statistical Analysis
Model 1 included the analysis of the results of the anthropometric and biochemical measurements with the criterion of the dietary recommendations that were adopted by the patients throughout the study (six months). The caloric value of the patients’ menus was estimated during checkups, which took place after one, two and six months, based on their 72 h diaries. The patients who were included in the statistical analysis followed the diet carefully (which was estimated based on menus in relation to the recommended caloric content ±200 kcal/day). Patients who exceeded that value at any stage of the study were excluded from the statistical analysis in Model 1.
Model 2 included the analysis of the anthropometric and biochemical results with the criterion of weight loss in the range of 8–10 kg over six months. We excluded patients who were characterized by normal weight at the beginning of the experiment from this analysis.
5. Conclusions
It seems that by introducing an individual nutrition and genotyping plan that takes into account the normal supply of calories, nutrients, proteins, and micro- and macronutrients, we are able to prevent problems that result from the progression of disease. Therefore, individualization, understood as the work of a dietitian with the patient, seems to be a therapeutic necessity, and the nutrient-induced insulin output ratio in people sensitive to fat seems to be n useful tool for determining specific strategies for patients with NAFLD.
Acknowledgments
Supported by a grant from the Narodowe Centrum Nauki (NCN), Nr N404 150539.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/7/1192/s1.
Author Contributions
Ewa Stachowska conceived and designed the experiments, wrote the paper; Karina Ryterska performed the experiments; Dominika Maciejewska performed the experiments (biochemistry); Marcin Banaszczak performed the experiments (biochemistry); Piotr Milkiewicz conceived and designed the experiments (medical support); Małgorzata Milkiewicz performed the experiments (biochemistry-PCR); Izabela Gutowska analyzed the data; Piotr Ossowski performed the experiments (biochemistry); Małgorzata Kaczorowska performed the experiments (nutrition); Dominika Jamioł-Milc performed the; experiments (nutrition); Anna Sabinicz performed the experiments (nutrition); Małgorzata Napierała performed the experiments (nutrition); Lidia Wądołowska contributed reagents/materials/analysis tools (nutritional analysis); Raszeja-Wyszomirska Joanna analyzed the data (medical support).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Argo C.K., Northup P.G., Al-Osaimi A.M., Caldwell S.H. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 4.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands JR., Fava J.L., Wing R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon H.J., Cha B.S. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J. Hepatol. 2014;6:800–811. doi: 10.4254/wjh.v6.i11.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan K., Bhalla V., El Regal M.E., A-Kader H.H. Nonalcoholic fatty liver disease: A comprehensive review of a growing epidemic. World J. Gastroenterol. 2014;20:12082–120101. doi: 10.3748/wjg.v20.i34.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiß J., Rau M., Geier A. Non-alcoholic fatty liver disease: Epidemiology, clinical course, investigation, and treatment. Dtsch. Ärzteblatt Int. 2014;111:447–452. doi: 10.3238/arztebl.2014.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kargulewicz A., Stankowiak-Kulpa H., Grzymisławski M. Dietary recommendations for patients with nonalcoholic fatty liver disease. Prz. Gastroenterol. 2014;9:18–23. doi: 10.5114/pg.2014.40845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrera F., George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin. Liver. Dis. 2014;18:91–112. doi: 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Keijer J., Hoevenaars F.P., Nieuwenhuizen A., van Schothorst E.M. Nutrigenomics of body weight regulation: A rationale for careful dissection of individual contributors. Nutrients. 2014;6:4531–4551. doi: 10.3390/nu6104531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J. Gastroenterol. 2014;20:16452–16463. doi: 10.3748/wjg.v20.i44.16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesketh J., Wybranska I., Dommels Y., King M., Elliott R., Pico C., Keijer J. Nutrient-gene interactions in benefit-risk analysis. Br. J. Nutr. 2006;95:1232–1239. doi: 10.1079/BJN20061749. [DOI] [PubMed] [Google Scholar]
- 13.Kang J.X. The coming of age of nutrigenetics and nutrigenomics. J. Nutr. Nutr. 2012;5 doi: 10.1159/000339375. [DOI] [PubMed] [Google Scholar]
- 14.Wybranska I., Malczewska-Malec M., Partyka L., Kiec-Wilk B., Kosno K., Leszczynska-Golabek I., Zdzienicka A., Gruca A., Kwasniak M., Dembinska-Kiec A. Evaluation of genetic predisposition to insulin resistance by nutrient-induced insulin output ratio (NIOR) Clin. Chem. Lab. Med. 2007;45:1124–1132. doi: 10.1515/CCLM.2007.142. [DOI] [PubMed] [Google Scholar]
- 15.Zafarmand M.H., van der Schouw Y.T., Grobbee D.E., de Leeuw P.W., Bots M.L. T64A polymorphism in β3-adrenergic receptor gene (ADRB3) and coronary heart disease: A case-cohort study and meta-analysis. J. Intern. Med. 2008;263:79–89. doi: 10.1111/j.1365-2796.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 16.Miranda J.L., Perez-Martinez P.P., Marin C.F., Fuentes F., Delgado J., Pérez-Jiménez F. Dietary fat, genes, and insulin sensitivity. J. Mol. Med. 2007;85:213–226. doi: 10.1007/s00109-006-0138-1. [DOI] [PubMed] [Google Scholar]
- 17.Henneman P., van der Sman-de Beer F., Moghaddam P.H., Huijts P., Stalenhoef AF., Kastelein J.J., van Duijn C.M., Havekes L.M., Frants R.R., van Dijk K.W., et al. The expression of type III hyperlipoproteinemia: Involvement of lipolysis genes. Eur. J. Hum. Genet. 2009;54:3043–3048. doi: 10.1038/ejhg.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamada T., Kotani K., Nagai N. Low-calorie diet-induced reduction in serum HDL cholesterol is ameliorated in obese women with the −3826 G allele in the uncoupling protein-1 gene. Tohoku J. Exp. Med. 2009;219:337–342. doi: 10.1620/tjem.219.337. [DOI] [PubMed] [Google Scholar]
- 19.Meirhaeghem A., Cottel D., Amouyel P., Dallongeville J. Association between peroxisome proliferator-activated receptor γ haplotypes and the metabolic syndrome in French men and women. Diabetes. 2005;54:3043–3048. doi: 10.2337/diabetes.54.10.3043. [DOI] [PubMed] [Google Scholar]
- 20.Marchesini G., Pagotto U., Bugianesi E., De Iasio R., Manini R., Vanni E., Pasquali R., Melchionda N., Rizzetto M. Low ghrelin concentrations in nonalcoholic fatty liver disease are related to insulin resistance. J. Clin. Endocrinol. Metab. 2003;88:5674–5679. doi: 10.1210/jc.2003-031094. [DOI] [PubMed] [Google Scholar]
- 21.Pfützner A., Derwahl M., Jacob S., Hohberg C., Blümner E., Lehmann U., Fuchs W., Forst T. Limitations of the HOMA-B score for assessment of β-cell functionality in interventional trials-results from the PIOglim study. Diabetes. Technol. Ther. 2010;12:599–604. doi: 10.1089/dia.2010.0019. [DOI] [PubMed] [Google Scholar]
- 22.Haffner S.M., Kennedy E., Gonzalez C., Stern M.P., Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–1141. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- 23.Eslamparast T., Eghtesad S., Poustchi H., Hekmatdoost A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J. Hepatol. 2015;7:204–212. doi: 10.4254/wjh.v7.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng R.D., Chen Z.R., Chen J.N., Lu Y.H., Chen J. Role of body mass index, waist-to-height and waist-to-hip ratio in prediction of nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2012;2012:362147. doi: 10.1155/2012/362147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudekula A., Rachakonda V., Shaik B., Behari J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS ONE. 2014;9:1192. doi: 10.1371/journal.pone.0111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deer J., Koska J., Ozias M., Reaven P. Dietary models of insulin resistance. Metabolism. 2015;64:163–171. doi: 10.1016/j.metabol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Jia Q., Xia Y., Zhang Q., Wu H., Du H., Liu L., Wang C., Shi H., Guo X., Liu X., et al. Dietary patterns are associated with prevalence of fatty liver disease in adults. Eur. J. Clin. Nutr. 2015;69:914–921. doi: 10.1038/ejcn.2014.297. [DOI] [PubMed] [Google Scholar]
- 28.Adams L.A. Biomarkers of liver fibrosis. J. Gastroenterol. Hepatol. 2011;26:802–809. doi: 10.1111/j.1440-1746.2010.06612.x. [DOI] [PubMed] [Google Scholar]
- 29.Rossi E., Adams L.A., Ching H.L., Bulsara M., MacQuillan G.C., Jeffrey G.P. High biological variation of serum hyaluronic acid and Hepascore, a biochemical marker model for the prediction of liver fibrosis. Clin. Chem. Lab. Med. 2013;51:1107–1114. doi: 10.1515/cclm-2012-0584. [DOI] [PubMed] [Google Scholar]
- 30.Van Herpen N.A., Schrauwen-Hinderling V., Schaart G., Mensink R.P., Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. JCEM. 2011;96:E691–E695. doi: 10.1210/jc.2010-2243. [DOI] [PubMed] [Google Scholar]
- 31.Marina A., von Frankenberg A.D., Suvag S., Callahan H.S., Kratz M., Richards T.L., Utzschneider K.M. Effects of dietary fat and saturated fat content on liver fat and markers of oxidative stress in overweight/obese men and women under weight-stable conditions. Nutrients. 2014;6:4678–4690. doi: 10.3390/nu6114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westerbacka J., Lammi K., Hakkinen A.M., Rissanen A., Salminen I., Aro A., Yki-Järvinen H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J. Clin. Endocrinol. Metab. 2005;90:2804–2809. doi: 10.1210/jc.2004-1983. [DOI] [PubMed] [Google Scholar]
- 33.Moore J.B., Gunn P.J., Fielding B.A. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6:5679–5703. doi: 10.3390/nu6125679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bémeur C., Butterworth R.F. Nutrition in the management of cirrhosis and its neurological complications. J. Clin. Exp. Hepatol. 2014;4:141–150. doi: 10.1016/j.jceh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin R., Welsh J.A., Le N.A., Holzberg J., Sharma P., Martin D.R., Vos M.B. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. 2014;6:3187–3201. doi: 10.3390/nu6083187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu S., Sievenpiper J.L., de Souza R.J., Cozma A.I., Mirrahimi A., Carleton A.J., Ha V., di Buono M., Jenkins A.L., Leiter L.A., et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung M., Ma J., Patel K., Berger S., Lau J., Lichtenstein A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014;100:833–849. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sluik D., Engelen A.I., Feskens E.J. Fructose consumption in the Netherlands: The Dutch National Food Consumption Survey 2007–2010. Eur. J. Clin. Nutr. 2015;69:475–481. doi: 10.1038/ejcn.2014.267. [DOI] [PubMed] [Google Scholar]
- 39.Veena J., Muragundla A., Sidgiddi S., Subramaniam S. Non-alcoholic fatty liver disease: Need for a balanced nutritional source. Br. J. Nutr. 2014;112:1858–1872. doi: 10.1017/S0007114514002591. [DOI] [PubMed] [Google Scholar]
- 40.Rinella M.E., Sanyal A.J. Management of NAFLD: A stage-based approach. Nat. Rev. Gastroenterol. Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- 41.Hagströmer M., Oja P., Sjöström M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/PHN2005898. [DOI] [PubMed] [Google Scholar]
- 42.Hamaguchi M., Kojima T., Itoh Y., Harano Y., Fujii K., Nakajima T., Kato T., Takeda N., Okuda J., Ida K., et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007;102:2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 43.Wells J.C. Commentary: The paradox of body mass index in obesity assessment: Not a good index of adiposity, but not a bad index of cardio-metabolic risk. Int. J. Epidemiol. 2014;43:672–674. doi: 10.1093/ije/dyu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.