Abstract
Perceptual learning, the improvement in performance with practice, reflects plasticity in the adult visual system. We challenge a standard claim that specificity of perceptual learning depends on task difficulty during training, instead showing that specificity, or conversely transfer, is primarily controlled by the precision demands (i.e., orientation difference) of the transfer task. Thus, for an orientation discrimination task, transfer of performance improvement is observed in low-precision transfer tasks, while specificity of performance improvement is observed in high-precision transfer tasks, regardless of the precision of initial training. The nature of specificity places important constraints on mechanisms of transfer in visual learning. These results contribute to understanding generalization of practiced improvements that may be key to the development of expertise and for applications in remediation.
Keywords: perceptual learning, transfer, specificity, precision, task difficulty
Introduction
Perceptual learning improves performance on visual tasks due to repeated practice or training (Fahle & Poggio, 2002). It occurs in visual search (Ahissar & Hochstein, 1996, 1997), texture discrimination (Karni & Sagi, 1991, 1993), motion perception (Liu & Weinshall, 2000), orientation discrimination of simple visual objects (Dosher & Lu, 1998, 1999; Petrov, Dosher, & Lu, 2005), and many other tasks typical of intelligent perceptual agents (Fine & Jacobs, 2000; McKee & Westheimer, 1978; Saarinen & Levi, 1995; Yu, Klein, & Levi, 2004).
Perceptual learning achieved in one task often fails to transfer to other, related, tasks or stimuli. Lack of transfer of learned attributes, or specificity, is seen as one significant property of perceptual learning. Specificity has been reported for retinal position, orientation, size, spatial frequency, and motion direction (Ball & Sekuler, 1982, 1987; Crist, Kapadia, Westheimer, & Gilbert, 1997; Fahle & Poggio, 2002; Fiorentini & Berardi, 1980, 1981; Schoups, Vogels, & Orban, 1995; Schoups, Vogels, Qian, & Orban, 2001). On the other hand, there are some situations in which perceptual learning may transfer quite well to other related tasks (Ahissar & Hochstein, 1997; Liu & Weinshall, 2000; Watanabe et al., 2002).
So, the degree of specificity, or conversely transfer, can vary widely and depends on the specifics of the training and transfer tasks (see below). Transfer—at least appropriate transfer to related tasks—may have great value in normal circumstances and in applications of perceptual learning in rehabilitation of impaired or developmentally delayed populations (e.g., Huang, Zhou, & Lu, 2008). Understanding the determinants of specificity and transfer remains one of the large outstanding questions in field. This paper challenges existing views that transfer is controlled by the difficulty of the training tasks. Instead, we argue for a task precision theory of specificity and transfer and focus on the role of precision (e.g., extent of orientation difference) primarily in the transfer, not the training, task.
The major claim in the literature is that transfer, or specificity, depends on the ‘difficulty’ of the training task (Ahissar & Hochstein, 1997; Liu & Weinshall, 2000; Rubin, Nakayama, & Shapley, 1997). It has been postulated that training in an easy task is easily transferred to other related tasks, while training in a difficult task is not easily transferred. For example, Ahissar and Hochstein (1997) investigated the transfer in a visual search task in which targets and distractors were of different orientations. Nearly full transfer occurred between ‘easy’ initial training and subsequent ‘easy’ transfer tests in which the orientations of targets differed from distractors by 30° and the orientations of targets and distractors were changed between the initial training and transfer tasks. In contrast, there was much less transfer, or more specificity, between two ‘difficult’ tasks in which the target and distractor orientations differed by 16°. In another example (Liu & Weinshall, 2000), learning transferred strongly from a training task to a transfer task, both requiring the discrimination between motion directions differing by 8°but in different general directions. There was almost no transfer from a training task to a transfer task that required the discrimination between motion directions differing by 4°. The claim in both cases was that the perceptual learning in an easy task is transferred, while perceptual learning in a difficult task is not.
Although these studies developed theories of transfer and specificity based on ‘task difficulty,’ the accompanying experiments in fact manipulated task precision (orientation difference). In a more general sense, we usually think of task difficulty as related to performance level—an easier task permits higher accuracy than a difficult one. Accuracy in visual discrimination can be a function of many stimulus factors, including contrast, duration, eccentricity, or the presence or absence of masking noise. Performance accuracy is also a function of the training state or expertise of the observer. Here, we suggest that the important stimulus factor determining transfer is in fact the precision of discrimination—not task difficulty per se.
In addition, the previous claims about task difficulty in initial training as the determinant of transfer (Ahissar & Hochstein, 1997; Liu & Weinshall, 2000) were based on experiments that did not independently manipulate the ‘difficulty’ (precision) of the training and transfer tasks. (In this paper, the training task refers to the initially trained task, and the transfer task refers to the task that is trained after the task switch.) While the results of the previous studies were consistent with the theoretical claims of the authors, it is unclear whether the initial training task, the transfer task, or both, controlled the extent of specificity or transfer since they did not implement a full factorial design (i.e., such as Doane, Sohn, Alderton, & Pelligrino, 1996 for mental rotation tasks).
The current study independently manipulates task precision of the training and transfer tasks, keeping the task difficulty (performance accuracy) constant. Our observers make basic orientation discrimination judgments. The initial training and subsequent transfer tasks differ from each other both in base angle and in retinal position. This is a direct analogy to Ahissar and Hochstein (1997), in which the location of the targets and the orientations of targets and distractors both changed at the point of task transfer. In addition, we also manipulated the presence or absence of external (masking) visual noise in the displays. There is now extensive evidence that perceptual learning may express distinct learning mechanisms in high and low visual noise (Dosher & Lu, 1998, 2005; Lu, Chu, & Dosher, 2006). Testing in both noisy and noiseless displays allows us to evaluate whether the transfer results differ in these two regimes.
The independent manipulation of task precision in initial learning and in subsequent transfer turns out to be critical—while previous emphasis was placed on the difficulty of initial training, the required precision of the transfer task is the controlling factor in transfer, and this is so in both noisy and noiseless displays. We consider the implication of these results for the reweighting and representational retuning hypotheses of perceptual learning.
Methods
Participants
Fourteen naive observers participated in the high-precision transfer test conditions and 16 naive observers participated in the low-precision transfer test conditions. One half of each group trained initially in the high-precision task and one half in the low-precision task. All subjects had normal to corrected-to-normal vision and provided written consent under the UC Irvine Institutional Review Board protocol.
Materials
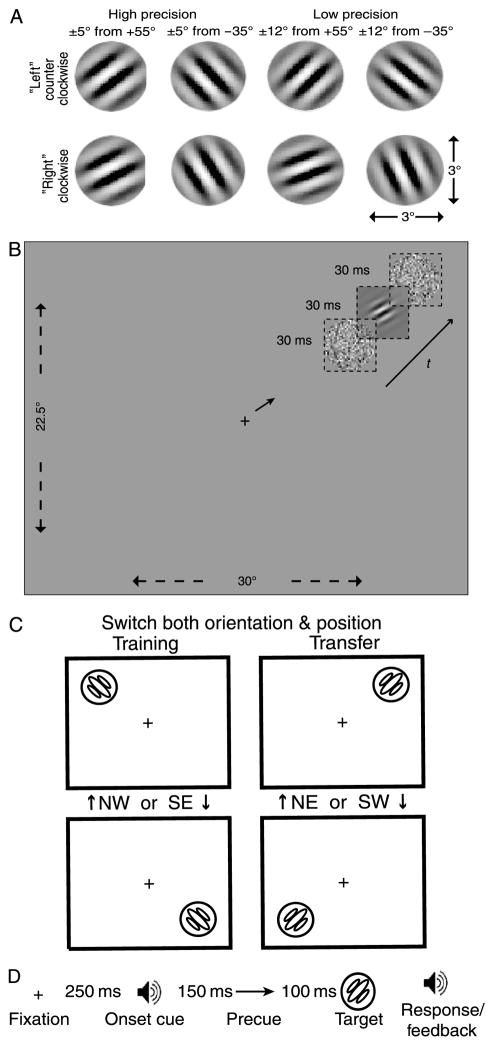
Figure 1 shows sample stimuli and the layout. The signal Gabor patch was 64 × 64 pixels (3° × 3° visual angle at a viewing distance of 58 cm):
| (1) |
with angle θ of −35° ± 5° or +55° ± 5° (High Precision) or −35° ± 12° or +55° ± 12° (Low Precision), spatial frequency f = 2 cpd, standard deviation of the Gaussian envelope σ = 0.4 degrees. The contrast c is the maximum contrast of the Gabor, and l0 is the mid-gray luminance. A 64 × 64 pixel noise image had individual 2 × 2 pixel noise elements with Gaussian-distributed contrasts about the mean value l0 and a standard deviation of 0.33 of 100% contrast. Signal and noise images were combined via temporal integration (15 ms per frame). The Gabor frames were ‘sandwiched’ between noise (or blank) frames (Figure 1B). A total of 640 external noise images were generated offline with samples selected at random for each trial.
Figure 1.
Sample stimuli and a trial sequence for the perceptual learning tasks. (A) Low-precision and high-precision Gabor targets were tilted ±12° or ±5°, respectively, from an implicit reference angle (−35° or +55°). (B) The display sequence included a fixation, a precue, and display sequence of external noise (if present) and the target, at 5.67° eccentricity. In this sample trial, the target, in high noise, is tilted clockwise of a +55°reference angle. Dotted squares represent frames over time. (C) On each trial, a target occurred in one of two diagonally opposite screen positions, selected randomly. If the first phase of training used the NW/SE diagonal, then the transfer tests used the NE/SW diagonal, and vice versa. (D) For each trial, the participant was presented with a fixation, followed by an onset cue (beep) 250 ms later, then followed by a “precue” arrow 400 ms and the target sequence. Responses were followed by auditory feedback on error trials.
Stimuli were displayed on a 15″ color monitor using the internal 10-bit video card of a Power Macintosh G3/267 (refresh rate 67 Hz, resolution 640 × 480 pixels, or 22.5°by 30°). Luminance calibration was performed both with psychophysical matching judgments and measurement with a Tektronix Lumacolor J17 photometer. Lookup tables were used to divide the luminance range (from 1 cd/m2 to 35 cd/m2) into 127 equidistant levels for the noise frames and [−c, +c] into 127 finer gray levels for the Gabor targets. A chin rest stabilized the observer’s head.
Procedure
Subjects discriminated between a Gabor tilted clockwise (“Right”) or counterclockwise (“Left”) from a reference angle of either −35° or +55° (Figure 1A). The implicit reference angles were fixed throughout each phase of the experiment. The stimuli could occur in one of four retinal positions in the NW, NE, SW, and SE corners of the screen, approximately 5.67° of visual angle from fixation (Figures 1B and 1C). Each block of training involved only two diagonally opposite positions. If the first phase of training used the NW/SE diagonal, then the transfer tests used the NE/SW diagonal, and vice versa. The reference angle and presentation diagonal were randomly assigned to subjects for initial training (4 sessions) and switched to the opposite reference angle and diagonal for the transfer tests (4 sessions).
The Gabor targets in the high-precision condition differed by ±5°, or δ = 10°, in orientation; those in the low-precision condition differed by ±12°, or δ = 24°. These values may be compared to the physiological orientation bandwidth (25–30°) of neurons in early visual cortex (De Valois, Yund, & Hepler, 1982). In addition, the Gabors appeared in external masking noise or with no noise. The no noise and high noise conditions were randomly intermixed within each testing block.
On each trial (Figure 1D), the participant fixated on a small cross at the center of the screen. An onset cue (beep) occurred 250 ms after the fixation cross. Another 250 ms later, the stimulus sequence (a noise frame, Gabor frame, and new noise frame, each for two refresh cycles) appeared for a total of 90 ms (15 ms/refresh). In no noise conditions, neutral clear frames replaced the white noise frames in the displays. A precue appeared 400 ms after fixation for the low-precision transfer groups; subsequent collateral testing indicated performance was unaffected by the precue. A negative feedback tone was presented after each error. The next trial began 750 ms after the key press response.
Observers completed 8 sessions of 1248 trials each, plus practice trials, or almost 10,000 trials. Two practice trials preceded the beginning of the staircase measurements, except for sessions 1 and 5, which had ten practice trials. Contrast thresholds were tracked through the remaining trials using randomly interleaved adaptive staircases (see below; Levitt, 1971). Each session had 4 blocks separated by brief rest periods.
Staircases
Two interleaved adaptive staircase procedures (Levitt, 1971) were used to estimate contrast thresholds for the Gabor orientation judgments. Signal contrast levels were reduced by 10% after either 3 or 2 consecutive correct responses and increased by 10% after each incorrect response. The 3/1 and 2/1 staircases track accuracies of 79.3% and 70.7% correct, respectively. Separate staircases for all stimulus parameters (including retinal position) were interleaved. There were 168 and 144 trials, respectively, for the 3/1 and 2/1 staircases for a total of 312 in each block. Reversals in staircase direction were determined from the sequence of responses. Threshold contrast levels were computed by averaging an even number of reversals for each staircase sequence, excluding the first four or five. Eliminating the beginning of the staircase excludes the early ‘level-finding’ trials that could bias the threshold estimates either low or high depending on the relation of the starting values to the true thresholds. Averaging an even number of reversals balances high- and low-point reversals, which also reduces bias. Based on examination of all staircase traces, a somewhat larger number of reversals were eliminated in blocks 1 and 5 in some individuals to deal with additional trials of range finding. An overall contrast threshold corresponding to 75% correct was estimated by averaging the thresholds of all staircases every two blocks for a total of 16 data points per observer. In addition to the explicit practice trials, the staircase estimation often began after about 100–120 trials over all interleaved staircases in the first session of the initial training task and the first session of the transfer task. This extent of general practice prior to calculation of the thresholds limits the contamination by early task familiarization.
Results
Threshold measures of perceptual learning
Perceptual learning is indexed by the improvements in thresholds as a function of practice for all conditions averaged over observers and retinal positions on one diagonal and over every two blocks of practice. The thresholds are also averaged over 3/1 and 2/1 staircases corresponding to 79.3% or 70.7% correct, yielding an expected asymptotic accuracy of 75% correct. After averaging, there are two measured thresholds in each external noise condition per session.
Figure 2A first shows the 75% contrast threshold data for the two groups of observers who transferred to a low-precision task and Figure 2B shows the data for the two groups who transferred to a high-precision task, and finally Figure 2C shows all four groups together.
Figure 2.
Perceptual learning measured as reductions in average contrast threshold at 75% correct during practice on an initial training task and during a subsequent transfer task. (A) Thresholds for the two groups that transferred to a low-precision task (±12°) following initial training on a low-or a high-precision training task for (top) no noise and (bottom) high external noise. (B) Thresholds for the two groups that transferred to a high precision task (±5°) for (top) no noise or (bottom) high noise. (C) All groups together (Black: Low → Low Precision, Orange: High → Low Precision, Red: Low → High Precision, Blue: High → High Precision). Error bars are two standard deviations of the mean, estimated using Monte Carlo simulations based on standard deviations from the mean reversals for each subject at each data point resampled 1000 times. Smooth curves are power function estimates for each condition and group.
The thresholds of each pair of two groups randomly assigned to the same initial learning conditions during the training phase were indistinguishable (all p > 0.2). This is expected, as the conditions were identical, only differing in the subjects randomly assigned to the groups. Although learning in low-precision, no-noise conditions appear to differ, these differences are not significant (over observers) either here (F(1,13) = 1.97, p ≈ 0.2) or in a series of independent validation tests.
Perceptual learning, i.e., reductions of contrast thresholds, occurred during initial training (four days, 8 threshold points) for all conditions; the magnitude of learning was larger in high external noise and/or for high-precision tasks. Learning in the initial phase of practice was tested using a paired t-test that compared the first threshold with the last threshold value prior to the task switch. Learning was significant for training in the low-precision task in no noise (t(6) = 2.73, p = 0.03, t(7) = 2.38, p = 0.05) and in high noise (t(7) = 5.97, p < 0.001, t(6) = 3.52, p = 0.01). Similarly, learning was significant in the high-precision task in no noise (t(6) = 2.71, p = 0.04 and t(7) = 7.99, p < 0.001) and in high noise (t(7) = 7.28, p < 0.001, t(6) = 8.680, p < 0.001). So, each initial training condition exhibited learning that could lead to subsequent transfer.
In addition, as expected, high-precision discriminations (±5°, δ = 10°) consistently had higher contrast thresholds than the corresponding low-precision discriminations (±12°, δ = 24°). The thresholds were higher at the beginning of training (average contrast of 0.65 versus 0.37 averaged over external noise, F(1,58) = 20.40, p < 0.0001) and transfer (average contrast of 0.48 versus 0.20, F(1,58) = 32.133, p < 0.0001) and were also significant for each external noise level individually. Also, as expected, external noise conditions required higher average contrast thresholds than no-noise conditions in initial training tasks (initial contrasts of 0.68 versus 0.34, F(1,58) = 33.30, p < 0.0001 averaged over precision) and in subsequent transfer tasks (initial contrasts of 0.48 versus 0.19, F(1,58) = 32.87, p < 0.0001) and were also significant for each precision level individually. These increases in contrast threshold for high-precision tasks and for external noise conditions validate the effectiveness of both manipulations.
Performance on the transfer task
Most importantly and surprisingly, the thresholds in the transfer task depended only on the precision of the transfer task. Statistical tests evaluated whether the two groups assigned to the same precision task during transfer, but following different initial training experiences, differed from one another. For the low-precision transfer task in no noise, F(1,14) = 2.323, p ≈ 0.15 (ns); for the low-precision transfer task in high noise, F(1,14) = 0.680, p ≈ 0.42 (ns); for the high-precision transfer task in no noise, F(1,12) = 0.033, p ≈ 0.86 (ns); and for the high-precision transfer task in high noise, F(1,14) = 0.065, p ≈ 0.83 (ns). The tests show that the two groups that differ in the initial training task but transfer to the same task precision do not differ significantly. This, in turn, indicates that the controlling factor in transfer performance is the precision of the transfer task.
Not only were performance thresholds during the first block of transfer equivalent regardless of the previous training but also the subsequent improvements with practice on the transfer task were the same regardless of the nature of the training task (see power function model below). The equivalence of the performance thresholds following initial training with different precision tasks is remarkable. These results clearly rule out models of transfer in which the precision of the initial training task determines the extent of transfer.
Specificity
Specificity to a stimulus attribute or location reflects the extent to which performance in the transfer phase is independent of performance in the training phase. High degree of transfer is equivalent to low specificity, and vice versa. In this section, we consider typical specificity indices that compare the performance at the beginning of the transfer phase to the performance during the initial training phase.
The index we used, based on Ahissar and Hochstein (1997), was
| (2) |
The values and are the contrast thresholds for the first and last blocks of the initial training phase for a given precision (i) and external noise (j) condition. The value is the contrast threshold for the first block in the transfer task. This measure, which estimates the portion of the initial learning that is transferred, is the measure most often used in the literature (Equation 2). An S of 0 indicates full transfer. This occurs when the threshold of the first block of the transfer phase is equal to the final block of the initial training phase. An S of 1 indicates full specificity, or no transfer. This occurs when the first block of the transfer phase starts at the same threshold as the first block of the initial training phase.
This specificity index requires that we compare like-precision tasks at initial training and transfer. This applies in experiments such as the current one where it is sensible to assume that the performances in the two tasks would have been the same without prior practice. We used the average of the two groups with the same precision and external noise level to estimate the first and last block thresholds for the initial training phase. It is necessary to use between-group definitions for the low-precision initial training to high-precision transfer task and for the high-precision initial training to low-precision transfer task. For consistency, we also used the average of the transfer point over the two same transfer conditions in all cases. This led to four specificity indices, one each for precision and noise conditions (Table 1). Within-observer comparisons are possible for the subset of groups who transferred from low-precision to low-precision or from high-precision to high-precision tasks; these values are quite close to those reported here. We examined several other closely related specificity indexes, but the results were substantially the same.
Table 1.
Specificity indices as a function of task precision and external noise for the average data.
| High precision
|
Low precision
|
||
|---|---|---|---|
| No noise | High noise | No noise | High noise |
| 0.38 | 0.65 | 0.21 | 0.15 |
Our results (Table 1) indicate low specificity (high transfer) to the low-precision transfer tasks of 15% and 21% specificity (85% and 79% transfer) in high and no external noise, respectively. The specificity scores, where within-individual comparisons are possible, were significantly below 1 (t-tests, p < 0.05 or p < 0.01) and not significantly above 0 (t-tests, p > 0.16). We characterize this as high transfer. In contrast, we observed higher specificity (lower transfer) to the high-precision transfer task of 65% and 38% specificity (35% and 62% transfer) in high and no external noise, respectively. For the conditions where within-individual comparisons are possible, the high-precision transfer conditions were all significantly above 0 (t-tests, p < 0.01) and significantly below 1 (t-tests, p < 0.05 or p < 0.01). We characterize this as partial specificity and partial transfer. Our transfer and specificity results are consistent with previous data for transfer between two low-precision (‘easy’) tasks and between two high-precision (‘difficult’) tasks (Ahissar & Hochstein, 1997; Liu & Weinshall, 2000) but go beyond them by examining cross-precision transfer conditions.
Although perceptual learning often exhibits relatively high observer variability, the consistency of this pattern of specificity/transfer can be seen in the individual observer data. Figures 3 and 4 show individual observer data in the initial training and initial transfer point for no-noise conditions (Figure 3, panels A–D) and high-noise conditions (Figure 4, panels E–H) for low- and high-precision tasks. The contrast thresholds for the low-precision and high-precision tasks are graphed in opposite directions (low precision to the left of midline, high precision to the right) to facilitate the comparison of like tasks, especially in the crossed-precision groups. These graphs show that the individual observer data were generally consistent with the aggregate data. They show initial learning (gray bars lower than white bars); and they show most transfer point thresholds for the high-precision task are between the two, indicating partial transfer and partial specificity, and closer to full transfer for the low-precision task.
Figure 3.
Individual subject data for no-noise conditions (panels A–D) for low- and high-precision tasks. Contrast threshold data for the first day of training (white bars), last day of training (gray bars), and the initial transfer point (black bars) are shown for all observers in each condition. Thresholds for low-precision tasks are graphed projecting to the left of the vertical midline; thresholds for high-precision tasks are graphed projecting to the right of the midline. Group means are presented in the middle panel, which provide direct comparisons for the transfer stages. Conditions are (A) low to low precision, (B) low to high precision, (C) high to low precision, and (D) high to high precision. The individual observer data were generally consistent with the aggregate data. Observers show learning in the initial training task (gray bars lower than white bars), while most transfer point thresholds are between the two, indicating partial transfer and partial specificity.
Figure 4.
Individual subject data for high-noise conditions (panels E–H) for low- and high-precision tasks. Contrast threshold data for the first day of training (white bars), last day of training (gray bars), and the initial transfer point (black bars) are shown for all observers in each condition. Thresholds for low-precision tasks are graphed projecting to the left of the vertical midline; thresholds for high-precision tasks are graphed projecting to the right of the midline. Group means are presented in the middle panel, which provide direct comparisons for the transfer stages. Conditions are (E) low to low precision, (F) low to high precision, (G) high to low precision, and (H) high to high precision. The individual observer data were generally consistent with the aggregate data. Observers show learning in the initial training task (gray bars lower than white bars), while most transfer point thresholds are between the two, indicating partial transfer and partial specificity.
Power function models of transfer
The classic approach to the measurement of specificity and transfer, reported in the previous section, focuses on proportional benefit in contrast threshold for the transfer task at the first block after the switch. In this section, we instead evaluate the data and aspects of transfer using a power function model of the learning process, of transfer, and of subsequent training on the transfer task. The value of the power function approach is that it characterizes the learning in the initial task, the learning in the transfer task following the task switch, as well as providing an alternative estimate of the extent of transfer. Importantly, it describes and offers an explanation for why learning in the transfer task (following the task switch) is visibly slower than learning in the initial training phase.
The power function is often cited as the primary ‘law of practice’ in speeded cognitive tasks (Anderson, 1982; Heathcote, Brown, & Mewhort, 2000) and has recently been shown to provide a good account of the functional form of contrast threshold learning curves averaged over observers (Dosher & Lu, 2007). Within the power function framework, transfer is measured by estimating the block-equivalent benefit of prior training on the transfer task. This also allows the estimation and characterization of learning rates and of the perceptual learning that occurs with practice following the switch to the transfer task.
An elaborated power function model was used to fit the perceptual learning contrast threshold data:
| (3a) |
The model fits a learning function with a lower minimum threshold α achieved after extended practice, an initial incremental threshold λ, and a learning rate ρ as a function of practice blocks, t, with τ indicating prior experience (Equation 3a).
The model we fit included an estimated τ for the transfer conditions but equated all the other parameters with the corresponding initial training conditions. The value of τ is set to zero for the initial training, which simplifies to
| (3b) |
A lattice of models with different numbers of parameters was explored. In the most complex (“fuller”) model, each curve in each phase was fit by an independent set of parameters (Equation 3a). In the end, a remarkably simple power function model with few parameters provided an excellent account of the entire pattern of contrast threshold data (Figure 2). This model estimated λ, α, and ρ values for the low-precision task and for the high-precision task within each external noise level; these are identical for the two groups in each pair. The initial training thresholds and the transfer thresholds were fit with identical parameter values, differing only in the experience factor τ estimated for the transfer task data. The value of τ was set to 0 for the initial training phase data (Equation 3b).
Parameter estimates for this model are shown in Table 2. The best-fitting model in no noise had an r2 of 0.874 and used 8 parameters (2λ, 2α, 2ρ, 2τ, one of each for low and high precision). The best-fitting model in high noise had an r2 of 0.963 and also used 8 parameters. For no external noise data, the 8-parameter model fit was similar to that of a 24-parameter full model with an r2 = 0.925 (F(16, 40) = 1.739, p = 0.08). The 24-parameter model (8λ, 8α, 8ρ) has separate parameters for low and high precision in the training and in the transfer stage, corresponding to independent descriptions of each curve. For high external noise data, the 8-parameter model again was similar to the 24-parameter full model (F(16, 40) = 1.688, p = 0.09), which has an r2 = 0.978. (Note that, because τ = 0 in the fuller model, the reduced model is not strictly nested within the full model. We cite these F-tests to assist in the comparison.)
Table 2.
Estimated parameters for the elaborated power function fits to average data. The initial level (λ), estimated asymptote (α), and rate (ρ) account for the learning curves, while the experience factors τ estimate the extent of transfer in each condition. The no noise r2 = 0.874 and the high noise r2 = 0.963. The * indicates that these values were set, not estimated. The identity of λ, α, and ρ’s for like-precision conditions were assumed by the model but are duplicated in the table.
| Noise | Parameter | Low-precision training task | Low-precision transfer task | High-precision training task | High-precision transfer task |
|---|---|---|---|---|---|
| No noise | λ | 0.191 | 0.191 | 0.508 | 0.508 |
| ρ | 0.429 | 0.429 | 0.505 | 0.505 | |
| α | 0.000 | 0.000 | 0.016 | 0.016 | |
| τ | 0* | 3.510 | 0* | 1.608 | |
| High noise | λ | 0.579 | 0.579 | 0.818 | 0.818 |
| ρ | 0.425 | 0.425 | 0.219 | 0.219 | |
| α | 0.000 | 0.000 | 0.000 | 0.000 | |
| τ | 0* | 4.73 | 0* | 0.770 |
The simple model (Table 2) differs in quality of fit from the full model primarily by fitting the two statistically equivalent low noise initial training curves by a single averaged curve as opposed to fitting two independent curves, which is the primary cause for the reduction in r2.
The parameter α could have been set without loss to 0. However, the contrast threshold even at extensive practice should still exceed zero; more practice measurements would be needed to more accurately estimate the asymptotic performance levels.
One property of the power function is that the relative learning rate is fastest in the early stages and diminishes throughout practice (Heathcote et al., 2000), corresponding to the appearance of most rapid improvements early in practice. The elaborated power function with transfer, τ > 0, provides a direct account of the apparently slower rate of learning through practice in the transfer phase—the new switched task inherits the reduced relative learning rate along with improved thresholds via transfer. It is as though the transfer task starts up at some point τ along the initial learning curve.
Of the 8 practice blocks in the initial training, the model estimated that about 3.5–4.5 practice blocks worth of benefit transferred to low-precision tasks, while about 1–2 practice blocks worth of benefit transferred to high-precision tasks. These estimates are analogous to the standard specificity indices—with more transfer and less specificity for low-precision transfer tasks (regardless of training task) and small estimated transfer and higher specificity for high-precision transfer tasks. The relative proportions of specificity and transfer are different because the power function estimates of transfer are in units of practice blocks and so scale differently due to the power form. A specificity score S of 0.5 will correspond to transfer of less than 50% of the number of initial training blocks because of the rapid improvements early in practice.
The elaborated power function offers a good, and relatively complete, account of the data, including converging estimates of the extent of transfer after the task switch. The results are straightforward: The specificity depends on the precision of the transfer task, not the training task. This leads to quite a different theoretical understanding.
Discussion
Previous studies concluded that “…This [perceptual] learning is specific to the stimuli used for training… the degree of specificity depends on the difficulty of the training conditions” (Ahissar & Hochstein, 1997, p. 401). Our new experiment and results require a reformulation of these claims and lead to a new precision-dependent framework for understanding the specificity of perceptual learning.
Task precision versus task difficulty
Task difficulty is usually associated with the accuracy of performance achievable in a task: a task leading to 70% accuracy is more difficult than one leading to 90% accuracy. As discussed earlier, many factors could in principle affect performance accuracy and so the corresponding task difficulty. The first major goal of this study was to document effects of precision on transfer, isolated from changes in performance accuracy. Staircases estimated contrast thresholds that on average tracked 75% correct.
We showed that task precision affected specificity and transfer even when task difficulty (accuracy) was held constant both between tasks and across the course of learning. The current results imply that precision is a major, and perhaps the major, controlling factor in transfer and specificity. Previous studies (Ahissar & Hochstein, 1997; Karni & Sagi, 1993; Liu & Weinshall, 2000) estimated thresholds through constant stimulus methods with changing sets of values, which probably yielded at best an approximate equivalency of accuracy during and across training and transfer conditions. Still, we interpret their results in support of the precision-dependent transfer framework.
By controlling for performance accuracy throughout learning, our results clearly identify precision as a core determinant of performance in a transfer task, following the task switch. Precision must account for the current results because difficulty is controlled. We believe that it accounts for almost all of the related prior results as well. The current procedure does not, however, experimentally evaluate the effects of difficulty during training on transfer. While the difficulty during training clearly will have implications for the speed of learning, we suspect that the independent effects of task difficulty on proportional transfer to the new task would be negligible. We believe that these too would be dominated by task precision. A full experimental evaluation of these intuitions would require an extensive new set of experimentation and theoretical development and testing.
Specificity depends on precision of the transfer task, not the training task
The second major goal of this study was to investigate the role of task precision during initial training and during transfer separately as controlling determinants of the transfer or specificity of perceptual learning. The experiment employed a fully crossed design in which the required precision of the initial training and subsequent transfer tasks was varied.
Our results show that the extent to which perceptual learning in one task transfers—or fails to transfer—to another similar task depends on the precision of the task to which learning is transferred. Although initial training in some form is critical, the transfer does not appear to depend strongly on the precision of the initial training task. The task precision of both the initial training task and the subsequent transfer task had a large and systematic effect on contrast threshold and on the absolute magnitude of learning. Yet the extent of transfer following training on high- and low-precision discriminations is essentially the same. It is likely that the remarkable equivalence of the effects of the low- and high-precision initial training on both immediate transfer and subsequent transfer training performance as learning continues is preconditioned upon equating both the amount of training and the achieved accuracy during that initial phase.
These conclusions hold for both no and high external noise conditions. The no-noise and high-noise conditions use very different stimulus contrasts and have been shown in related work to represent at least partially dissociable mechanisms of training (Dosher & Lu, 1998, 1999, 2005; Lu et al., 2006).
Our new framework for transfer makes new theoretical claims but subsumes previous empirical observations of transfer from the literature (Ahissar & Hochstein, 1997; Liu & Weinshall, 2000). Prior claims focused on the role of difficulty of the training task in controlling specificity at transfer. The vast majority of previous observations in support of the prior claims are consistent both with our observed data and with the changed theoretical emphasis on the transfer task in specificity of perceptual learning.
In the Ahissar and Hochstein (1997) experiments, for example, a target was an odd-orientation element in a 7 × 7 array of otherwise like-oriented elements. In their ‘easy’ (low-precision) condition, observers learned to detect a target element in two known locations that differed by 30°from the background elements and showed high transfer to a swap of locations and orientations. The orientations differed by 16° in their ‘difficult’ (high-precision) condition and showed specificity with partial transfer. These are analogs to our observations for our low-precision to low-precision group with ±12° (24° difference) and our high-precision to high-precision group with ±5° (10°difference). Our other two crossed conditions show, however, that the critical factor is the precision of the transfer task, not the precision of initial training.
Other conditions in the Ahissar and Hochstein data pose a further question, however. The orientation difference leading to full transfer was 90° and the orientation difference leading to partial specificity was 30° if the target could be at any location in the display. This observation—that the precision yielding transfer or specificity seems to depend on other factors in the task—highlights an obvious but previously unasked question: What level of precision causes learning to be specific? We offer several speculations below.
Channel segregation and transfer
What makes one orientation difference sufficiently low precision that perceptual learning transfers, and another difference sufficiently high precision that it does not? Previous researchers did not address this question. We speculate that the task-precision transfer boundary may be related to the channel segregation of the discrimination. Specificity is more likely when the precision demands of the task fall within the estimated bandwidth of a particular population of units relevant to the discrimination, here orientation difference.
Low-precision tasks discriminate stimuli separated by about the bandwidth (or more) of units tuned to the dimension of discrimination with relatively good channel segregation. High-precision tasks discriminate stimuli that both fall within a single channel bandwidth. In this case, orientation bandwidths are cited at about 25–30° (De Valois et al., 1982). We suggest that the orientation bandwidth sets important limitations in the mode of optimal learning, and hence opportunities to transfer. Ahissar and Hochstein’s (1997) unremarked observation that larger orientation differences are required for transfer if the target may occur in any one of a large number of locations suggests to us that either decision uncertainty (Graham, 1989; Shaw, 1980) or pooling (Parkes, Lund, Angelucci, Solomon, & Morgan, 2001) operations create an aggregate response whose bandwidth is wider, representing aggregation noise, and so requires higher orientation differences to yield segregated representations.
In computational neural networks, diagnostic neural input representations are selected by learning the weight structure that statistically optimizes discrimination performance through perceptual practice (Dosher & Lu, 1998, 1999; Petrov et al., 2005, 2006, see also Mollon & Danilova, 1996). The stimulus, including any external noise, is processed through early visual filters to produce a distributed set of activities over representational units, for example, units tuned to spatial frequency and orientation. The weights from these inputs to decision form the perceptual template(s) for the task. Initial training in the low- and high-precision tasks is similar insofar as they share locations, spatial frequencies, and generally similar orientations around the implied reference angles (i.e., the 55° of the 55° ± 5° or 55° ± 12°). This first-order similarity of weights after low- and high-precision training, together with the equalized accuracy of response, may help explain why initial training in either precision leads to a similar level of initial transfer to the new task.
Learning the high-precision task requires further optimization of weights beyond the learning for the low-precision tasks. We suggest that this further optimization may be analogous to common mode rejection in engineering or noise cancellation in electro-sensory models (Montgomery, 1984), which consider the effects of correlated noise in two pathways. The closer the to-be-discriminated targets are, the more likely the templates are to respond in a similar way, including the response to external noise. Common mode rejection refines weights so as to effectively balance (or subtract) the correlated response to shared noise.
So, learning the high-precision tasks requires more extensive optimization of weights than learning low-precision tasks. We speculate that only the relatively coarse weights common to low- and high-precision tasks transfer. This coarse information is insufficient to optimize the high-precision transfer task. This capacity or precision limitation in transfer accounts for why specificity of learning is predominantly controlled by the demand of the transfer task. Other cases of full transfer, e.g., between eyes, may reflect a common level of coding and/or learning or may reflect the relatively coarse demands of the tasks.
Conclusions
This new experiment leads to several new conclusions and several claims. First, task precision rather than task difficulty per se limits transfer of perceptual learning. Second, transfer of perceptual learning depends primarily on the precision of the transfer task not the precision of the initial training task. More transfer and less specificity is observed when switching to a low-precision task, while less transfer and more specificity is observed when switching to a high-precision task. These conclusions follow directly from the critical tests in the experiment. Our results suggest a task-precision framework to account for transfer and specificity. We propose that the task-precision boundary between transfer and specificity may correspond to the bandwidth of the relevant feature being discriminated. Finally, only the relatively coarse weights common to low- and high-precision tasks transfer, insufficient to optimize the high-precision transfer task. We conclude that transfer may in general be capacity or precision limited. Further experimentation will be useful in testing the boundaries of this framework and other possible factors.
Acknowledgments
This research was supported by the National Eye Institute and the National Institutes of Mental Health.
Footnotes
Commercial relationships: none.
Contributor Information
Pamela E. Jeter, Memory Attention Perception (MAP) Laboratory, Department of Cognitive Sciences, UC Irvine, Irvine, CA, USA
Barbara Anne Dosher, Memory Attention Perception (MAP) Laboratory, Department of Cognitive Sciences, Institute for Mathematical Behavioral Sciences, UC Irvine, Irvine, CA, USA.
Alexander Petrov, Department of Psychology, Ohio State University, Columbus, OH, USA.
Zhong-Lin Lu, Laboratory of Brain Processes (LOBES), Department of Psychology, University of Southern California, Los Angeles, CA, USA.
References
- Ahissar M, Hochstein S. Learning pop-out detection: Specificities to stimulus characteristics. Vision Research. 1996;36:3487–3500. doi: 10.1016/0042-6989(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;378:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Acquisition of cognitive skills. Psychological Review. 1982;89:369–406. [Google Scholar]
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Research. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Crist RE, Kapadia MK, Westheimer G, Gilbert CD. Perceptual learning of spatial localization: Specificity for orientation, position, and context. Journal of Neurophysiology. 1997;78:2889–2894. doi: 10.1152/jn.1997.78.6.2889. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Yund EW, Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Research. 1982;22:531–544. doi: 10.1016/0042-6989(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Doane SM, Sohn YW, Alderton DL, Pelligrino JW. Acquisition and transfer of skilled performance: Are visual discrimination skills stimulus specific? Journal of Experimental Psychology: Human Perception and Performance. 1996;22:1218–1248. [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning in clear displays optimizes perceptual expertise: Learning the limiting process. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5286–5290. doi: 10.1073/pnas.0500492102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. The functional form of performance improvements in perceptual learning: Learning rates and transfer. Psychological Science. 2007;18:531–539. doi: 10.1111/j.1467-9280.2007.01934.x. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio T, editors. Perceptual learning. Cambridge, MA: The MIT Press; 2002. [Google Scholar]
- Fine I, Jacobs RA. Perceptual learning for a pattern discrimination task. Vision Research. 2000;40:3209–3230. doi: 10.1016/s0042-6989(00)00163-2. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Learning in grating waveform discrimination: Specificity for orientation and spatial frequency. Vision Research. 1981;21:1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Graham N. Visual pattern analyzers. Oxford, UK: Oxford University Press; 1989. [Google Scholar]
- Heathcote A, Brown S, Mewhort DJ. The power law repealed: The case for an exponential law of practice. Psychonomic Bulletin & Review. 2000;7:185–207. doi: 10.3758/bf03212979. [DOI] [PubMed] [Google Scholar]
- Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in visual system of adults with anisometropic amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up–down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49:467–477. [PubMed] [Google Scholar]
- Liu ZL, Weinshall D. Mechanisms of generalization in perceptual learning. Vision Research. 2000;40:97–109. doi: 10.1016/s0042-6989(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Chu W, Dosher BA. Perceptual learning of motion direction discrimination in fovea: Separate mechanisms. Vision Research. 2006;46:2315–2327. doi: 10.1016/j.visres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- McKee SP, Westheimer G. Improvement in vernier acuity with practice. Perception & Psychophysics. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- Mollon JD, Danilova MV. Three remarks on perceptual learning. Spatial Vision. 1996;10:51–58. doi: 10.1163/156856896x00051. [DOI] [PubMed] [Google Scholar]
- Montgomery JC. Noise cancellation in the electrosensory system of the thornback ray; common mode rejection of input produced by the animal’s own ventilatory movement. Journal of Comparative and Physiological. 1984;55:103–111. [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience. 2001;4:739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Petrov AA, Dosher BA, Lu ZL. The dynamics of perceptual learning: An incremental reweighting model. Psychological Review. 2005;112:715–743. doi: 10.1037/0033-295X.112.4.715. [DOI] [PubMed] [Google Scholar]
- Petrov AA, Dosher BA, Lu ZL. Perceptual learning without feedback in non-stationary contexts: Data and model. Vision Research. 2006;46:3177–3197. doi: 10.1016/j.visres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Rubin N, Nakayama K, Shapley R. Abrupt learning and retinal size specificity in illusory-contour perception. Current Biology. 1997;7:461–467. doi: 10.1016/s0960-9822(06)00217-x. [DOI] [PubMed] [Google Scholar]
- Saarinen J, Levi DM. Perceptual learning in vernier acuity: What is learned? Vision Research. 1995;35:519–527. doi: 10.1016/0042-6989(94)00141-8. [DOI] [PubMed] [Google Scholar]
- Schoups AA, Vogels R, Orban GA. Human perceptual learning in identifying the oblique orientation: Retinotopy, orientation specificity and monocularity. The Journal of Physiology. 1995;483:797–810. doi: 10.1113/jphysiol.1995.sp020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Shaw ML. Pooling independent decisions about multiple sources of information. Bulletin of the Psychonomic Society. 1980;16:159–159. [Google Scholar]
- Watanabe T, Náñez JE, Sr, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than in higher-level visual motion processing in a passive perceptual learning task. Nature Neuroscience. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- Yu C, Klein SA, Levi DM. Perceptual learning in contrast discrimination and the (minimal) role of context. Journal of Vision. 2004;4(34):169–182. doi: 10.1167/4.3.4. http://journalofvision.org/4/3/4/ [DOI] [PubMed] [Google Scholar]