Abstract
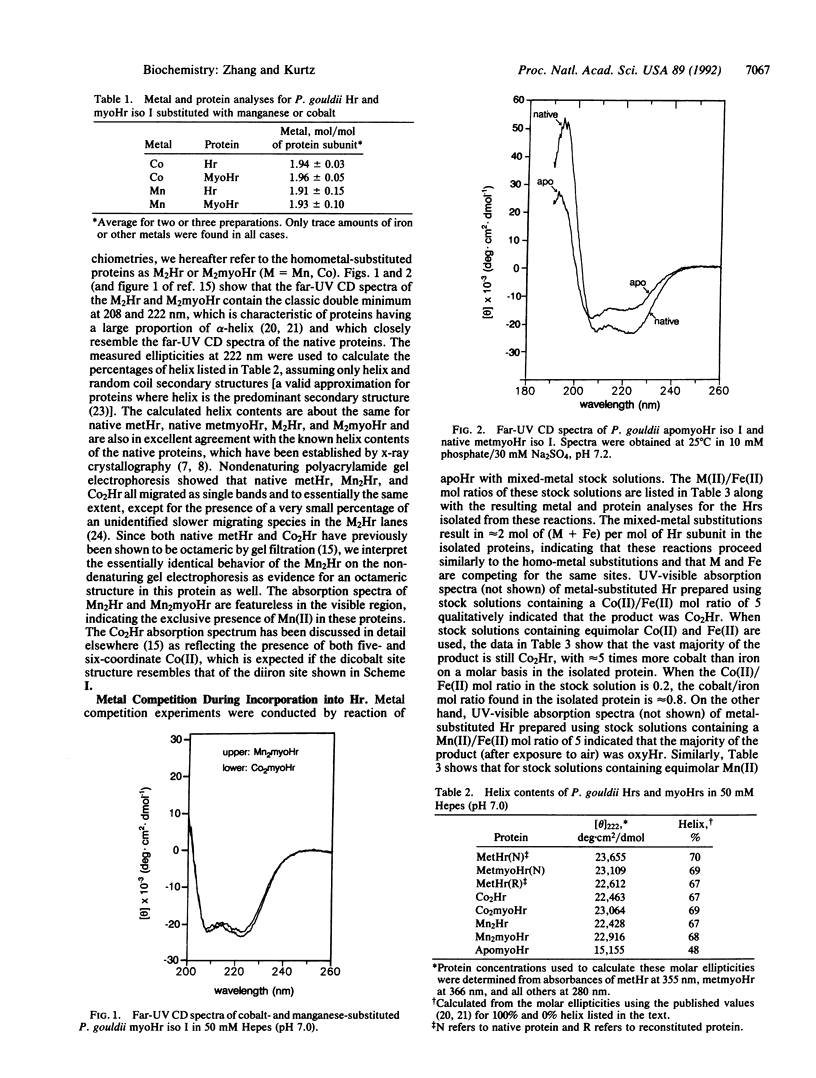
A general method is described for substitution of Mn(II) and Co(II) into the diiron sites of hemerythrin and myohemerythrin. Characterizations of these metal-substituted proteins show that their structures closely resemble those of the native proteins. In particular, the four-helix bundle structure appears to be maintained. The apomyohemerythrin retains most of the native helix content but is considerably less stable to denaturation than are the metal-containing proteins. The relative affinities of M(II) for apohemerythrin--namely, Co greater than Fe greater than Mn--parallel the stabilities of the M2myohemerythrins to denaturation by guanidinium chloride. These results indicate that for myohemerythrin (i) the majority of the helical structure found in the native protein does not require incorporation of M(II) and (ii) stabilization of the native structure relative to the fully unfolded structure appears to be due predominantly to M(II)-protein interactions, at least for M = Fe and Co. Incorporation of M(II) also generates unfolding cooperativity in myohemerythrin. This cooperativity can be attributed to interhelical interactions, which are prevented in the apoprotein by solvation of the seven metal ligand residues. The results are consistent with a minimal model for folding/unfolding of myohemerythrin and hemerythrin subunits consisting of the sequential equilibria, N in equilibrium with I in equilibrium with D, between native, intermediate, and fully unfolded states, respectively. The properties of apomyohemerythrin make it a candidate for the intermediate state, I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Bigelow C. C. Estimation of the free energy of stabilization of ribonuclease A, lysozyme, alpha-lactalbumin, and myoglobin. J Biol Chem. 1982 Nov 10;257(21):12935–12938. [PubMed] [Google Scholar]
- Bonomi F., Long R. C., Kurtz D. M., Jr Purification and properties of a membrane-bound NADH-cytochrome-b5 reductase from erythrocytes of the sipunculid worm, Phascolopsis gouldii. Biochim Biophys Acta. 1989 Nov 30;999(2):147–156. doi: 10.1016/0167-4838(89)90211-2. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Garbett K., Klotz I. M., Aktipis S., Keresztes-Nagy S. Optical rotatory properties of hemerythrin in the ultraviolet range. Arch Biochem Biophys. 1969 Aug;133(1):103–107. doi: 10.1016/0003-9861(69)90492-5. [DOI] [PubMed] [Google Scholar]
- Feng Y. Q., Sligar S. G. Effect of heme binding on the structure and stability of Escherichia coli apocytochrome b562. Biochemistry. 1991 Oct 22;30(42):10150–10155. doi: 10.1021/bi00106a011. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Garbett K., Darnall D. W., Klotz I. M., Williams R. J. Spectroscopy and structure of hemerythrin. Arch Biochem Biophys. 1969 Dec;135(1):419–434. doi: 10.1016/0003-9861(69)90559-1. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Mechanisms of metal ion incorporation into metalloproteins. Biofactors. 1990 Jul;2(3):179–184. [PubMed] [Google Scholar]
- Hecht M. H., Richardson J. S., Richardson D. C., Ogden R. C. De novo design, expression, and characterization of Felix: a four-helix bundle protein of native-like sequence. Science. 1990 Aug 24;249(4971):884–891. doi: 10.1126/science.2392678. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Le Trong I., Turley S., Sieker L. C., Stenkamp R. E. Structures of deoxy and oxy hemerythrin at 2.0 A resolution. J Mol Biol. 1991 Apr 5;218(3):583–593. doi: 10.1016/0022-2836(91)90703-9. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Stenkamp R. E. Structures of met and azidomet hemerythrin at 1.66 A resolution. J Mol Biol. 1991 Aug 5;220(3):723–737. doi: 10.1016/0022-2836(91)90113-k. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Pace C. N. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Stenkamp R. E., Sieker L. C., Jensen L. H. Influence of solvent accessibility and intermolecular contacts on atomic mobilities in hemerythrins. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1104–1107. doi: 10.1073/pnas.82.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H., Kurtz D. M., Jr, Xia Y. M., Debrunner P. G. Reconstitution of the diiron sites in hemerythrin and myohemerythrin. Biochemistry. 1991 Jan 15;30(2):583–589. doi: 10.1021/bi00216a037. [DOI] [PubMed] [Google Scholar]



