Abstract
Objective
Psychostimulants are partially effective in reducing cognitive dysfunction associated with attention-deficit/hyperactivity disorder (ADHD). Cognitive effects of guanfacine, an alternative treatment, are poorly understood. Given its distinct action on α2A receptors, guanfacine may have different or complementary effects relative to stimulants. This study tested stimulant and guanfacine monotherapies relative to combined treatment on cognitive functions important in ADHD.
Method
Children with ADHD (n = 182; age 7–14 years) completed an eight-week double blind randomized controlled trial with three arms: d-methylphenidate (DMPH), guanfacine (GUAN), or combination treatment with DMPH and GUAN (COMB). A non-clinical comparison group (n = 93) had baseline testing, and a subset was re-tested 8 weeks later (n = 38). Analyses examined treatment effects in four cognitive domains (working memory, response inhibition, reaction time, and reaction time variability) constructed from 20 variables.
Results
The ADHD group showed impaired working memory relative to the non-clinical comparison group (effect size = −0.53 SD units). The treatments differed in effects on working memory but not other cognitive domains. Combination treatment improved working memory more than GUAN, but was not significantly better than DMPH alone. Treatment did not fully normalize the initial deficit in ADHD relative to the comparison group.
Conclusion
Combined treatment with DMPH and GUAN yielded greater improvements in working memory than placebo or GUAN alone, but the combined treatment was not superior to DMPH alone, and did not extend to other cognitive domains. Although GUAN may be a useful add-on treatment to psychostimulants, additional strategies appear necessary to achieve normalization of cognitive function in ADHD.
Clinical trial registration information
Single Versus Combination Medication Treatment for Children With Attention Deficit Hyperactivity Disorder; http://clinicaltrials.gov/; NCT00429273
Keywords: cognition, attention-deficit/hyperactivity disorder, stimulant, guanfacine, working memory
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is associated with educational and social dysfunction in childhood and adolescence1 and disability in adulthood that are likely secondary to cognitive deficits2. There is consensus that ADHD is characterized by enormous heterogeneity, without a unitary cognitive pathology, but there is less agreement about the specific cognitive functions that are implicated due to both theoretical distinctions in the definitions of functions, and methodological differences in how functions are measured. At the group level, investigators agree that individuals with ADHD have impairments in broad domains comprising: response inhibition; working memory and/or executive functions (e.g., goal maintenance, interference control); processing speed; and reaction time variability3–7, but individual differences are large across these domains. Some investigators also identify impairments in arousal/activation or vigilance, temporal information processing, memory span, and organization/planning functions4,6.
Psychostimulants remain the mainstay of prescribed drug treatment for ADHD, and yield cognitive benefits on some measures, but fall short of normalizing cognitive performance deficits in ADHD8,9. Given both the plurality and heterogeneity of cognitive deficits, diverse cognitive responses could be expected to follow distinct mechanisms of action associated with different treatments. Intervention research so far has focused almost exclusively on psychostimulants, hypothesized to exert their effects via dopaminergic (DA) and noradrenergic (NE) reuptake blockade, and it has been suggested these agents may have effects primarily on vigilance functions with less impact on response inhibition, interference control, or other executive functions10.
Guanfacine, via its mechanism of action involving selective α2A receptor agonism, has been shown to enhance prefrontal cortical function in rodents and non-human primates11–13, and improve working memory in normal adults after single dose administration14. These observations have led to hypotheses about optimizing the balance of DA and α2A receptor agonism to facilitate cognitive function, with the role of guanfacine particularly in the normalization of prefrontal activity as manifest in working memory, response inhibition, and other cognitive control functions15.
Clinical trials have examined the impact of guanfacine on clinician and parent/teacher ratings of attention16,17, but so far there are sparse data on the cognitive consequences of guanfacine treatment on cognitive functions in youth with ADHD. One study found no significant effects of guanfacine on choice reaction time, digit symbol substitution, or spatial working memory tests18. Functional magnetic resonance imaging (fMRI) and behavioral data in healthy adults suggest possible effects of guanfacine on emotional regulation of attention19, and a study in schizotypal personality disorder reported improvement on context processing on a continuous performance test20. We are not aware of any studies yet examining neurocognitive responses to guanfacine compared to stimulants or guanfacine in combination with stimulants in children and adolescents with ADHD. This study aimed to provide these data.
METHOD
This report is one of three companion papers. The other two papers describe the overall clinical trial outcome (McCracken et al., under review) and the neurophysiological response to treatment as assessed using electroencephalography (EEG; Loo et al., in press).
Participants
This paper focuses on the 182 participants who completed 8 weeks of treatment (given 212 who were randomized to treatment, and 207 who received at least one dose of study medication; see Consolidated Standards of Reporting Trials [CONSORT] diagram, Figure 1, McCracken et al., under review). Parents and participants provided written informed permission and assent. All study procedures were approved by the University of California, Los Angeles (UCLA) Institutional Review Board and overseen by a data safety and monitoring board.
Inclusion criteria for patients were: 1) male or female aged 7–14 years; 2) DSM-IV ADHD (any subtype) diagnosed by semi-structured diagnostic interview (Kiddie-Schedule for Affective Disorders and Schizophrenia-PL41; KSADS-PL) and clinical interview; and 3) Clinical Global Impression—Severity (CGI-S) score ≥ 4 for ADHD.
Exclusion criteria for patients were 1) lifetime history of autistic disorder, chronic tic disorder, psychosis, bipolar disorder, or structural heart defects; 2) current major depression or panic disorder; 3) systolic or diastolic blood pressure > 95th or <5th percentile for age and body mass index (BMI); 4) medical condition contraindicating stimulants or alpha agonists; 5) need for chronic use of other central nervous system medications.
A non-clinical comparison group (n = 93) comprised boys and girls aged 7–14 who had no lifetime history of DSM-IV-TR Axis I mental disorders as determined by KSADS-PL and clinical interview, who did not satisfy any of the exclusion criteria noted for patients above, and who completed both baseline and follow-up assessments.
Further details are provided in McCracken et al (under review).
Procedures
One hundred eighty-two (182) children and adolescents with ADHD completed an eight-week double-blind randomized controlled trial with three arms: DMPH: d-methylphenidate extended-release (5 – 20 mg/day; treated from baseline to 4 weeks with placebo (PBO), and from week 4 to week 8 with DMPH); GUAN: guanfacine (1 – 3 mg/day for 8 weeks); COMB: treated from baseline to week 4 with guanfacine, and then from week 4 to week 8 with the combination of guanfacine and DMPH. Treatments were masked by the use of matching placebo capsules, and similar twice-daily study material administration. The study design is further detailed in McCracken et al (under review). Mean final (week 8) daily doses of DMPH were 16.0 (3.9) mg for DMPH and 15.1 (4.8) mg for COMB. Mean final total daily doses of guanfacine were 2.2 (0.7) mg for GUAN and 2.4 (0.6) mg for COMB, given twice daily. Mean mg/kg daily doses of guanfacine were 0.06 (0.03) mg/kg/d for both guanfacine groups. We consider here the cognitive assessment results obtained at baseline (week 0), midpoint (week 4), and endpoint (week 8). The main experimental design and analysis strategy are based on the assumption that for all medications after 4 weeks the participant has been titrated to their optimal dose(s), and that there are no further changes expected beyond the effects achieved after 4 weeks on a given treatment (see also McCracken et al). Participants were generally tested in the late morning or early afternoon (after initial clinical ratings); testing was executed by trained examiners blind to clinical history, clinical ratings, and treatment group assignment.
In the non-clinical comparison group, additional analyses focused on 38 individuals who had both baseline testing and re-testing 8 weeks later, to permit direct estimation of test-retest changes independently from treatment.
We constructed four cognitive domain scores (working memory, response inhibition, reaction time, and reaction time variability) using 20 test variables derived from 10 tests, based on a combination of a priori assignments of test variables to functional domains, then exploratory and finally confirmatory factor analysis (CFA) to assess the validity of the assignments. The specific test variables assigned to each cognitive domain are listed in Table 1. Each domain score was constructed as the mean of standardized scores on the contributing variables.
Table 1.
Listing of Cognitive Task Variables in Domains
| WMa | Spatial Working Memory – mean accuracy |
| WM | WISC-IV Digit Span Forward – total raw score |
| WM | WISC-IV Spatial Span Forward– total raw score |
| WM | WISC-IV Digit Span Backward – total raw score |
| WM | WISC-IV Spatial Span Backward – total raw score |
| WM | WISC-IV Letter Number Sequencing – total raw score |
| Inhibitiona | Stop Signal Test – mean Stop Signal Reaction Time |
| Inhibition | D-KEFS CWIT, Inhibition raw score |
| Inhibition | D-KEFS CWIT Inhibition/Switching raw score (controlling for Color Naming and Word Reading scores) |
| Inhibition | D-KEFS Trail Making Test Number-Letter Switching raw score (controlling for Visual Scanning and Number Sequencing scores) |
| Inhibitiona | Attention Networks Test, total errors (reversed sign) |
| SoPa | Time Discrimination Test, median correct RT |
| SoPa | Attention Networks Test, mean RT |
| SoPa | Go-NoGo Test, mean RT for valid hits |
| SoPa | Spatial Working Memory, mean RT correct trials |
| RTVa | Time Discrimination Test, SD of valid RT |
| RTVa | Attention Networks Test, SD of valid RT |
| RTVa | Stop Signal Test, SD of “Go” RT |
| RTVa | Go-NoGo Test, SD of valid RT |
| RTVa | Spatial Working Memory, SD of RT correct trials |
Note. Further details and key references for each test are provided in Supplement 1, available online. CWIT = Color-Word Interference Test; D-KEFS = Delis-Kaplan Executive Function System; RTV = Response Time Variability; SoP = Speed of Processing; WISC-IV = Wechsler Intelligence Scale for Children–Fourth Edition; WM = Working Memory.
Indicates computerized tests.
Further descriptions of each of the neurocognitive tests, the specific variables used, and references for these measures, are provided in Supplement 1: Neurocognitive Test Battery, available online.
We analyzed treatment effects using generalized mixed models, with each of the cognitive domain scores serving as the outcome in 4 separate analyses, and within-subject variables including the time of the visit (baseline, week 4, or week 8), and medication at the time of the visit (DMPH, GUAN, COMB, or PBO), and age as a covariate. To facilitate interpretation of results, we computed each cognitive domain score as a standard score relative to the mean and standard deviations of the non-clinical comparison group at baseline. The reported cognitive domain scores are thus interpretable generally as reflecting the difference (similar to Cohen’s d) between the actively treated patients and the non-clinical comparison group.
RESULTS
We conducted preliminary exploratory factor analyses on 409 participants with suspected ADHD, chronic tic disorder, or Tourette disorder who underwent research assessments, and 93 non-clinical comparison participants who completed identical testing protocols. All participants were 7 – 14 years of age. These analyses prompted slight revision of variables to be included in domain scales, primarily to reduce cross-loadings on the a priori domains. For confirmatory factor analysis, the number of cases with complete data across all variables (n=209) was suboptimal (i.e., the structural equation model has 65 free parameters), so we imputed missing data using the EM algorithm. The results revealed that most factors had relatively good fit, but that these factors do not explain all the variability in the data (Chi square =2757, CFI = .79, RMSEA=0.13). We examined modification indices to determine what cross loadings might improve the model fit, but none of these led to substantially improved solutions. The correlation matrix including all variables used to construct the domain scores, table of CFA weights, and structural equation model diagram are shown in Tables S1 and S2 and Figure S1, available online.
Mixed Models Analysis
Results of the mixed models analyses are shown in Tables 2 and 3. Table 2 shows the statistical effects, which include significant effects of age on each of the 4 cognitive domain scores (serving as a validity check), and significant effects of visit on both reaction time (RT) and reaction time variability (RTV), suggesting that the RT became faster and RTV less variable over time across groups, independent of treatment. We also provide summary statistics for the original variables (prior to standardization) for both the patients and non-clinical comparison group at baseline in Table S3, available online.
Table 2.
Mixed Models Analysis of Medication Effects on Cognitive Factors
| Domain | Effect | Num df | Den df | F | p |
|---|---|---|---|---|---|
| WM | Intercept | 1 | 511.7 | 311.1 | .001 |
| Visit | 1 | 558.8 | 0.1 | .78 | |
| Age | 1 | 398.9 | 260.8 | .001 | |
| Medication | 4 | 563.1 | 7.3 | .001 | |
| INH | Intercept | 1 | 567.2 | 69.8 | .001 |
| Visit | 1 | 696.9 | 2.6 | .11 | |
| Age | 1 | 390.3 | 82.6 | .001 | |
| Medication | 4 | 721.7 | 0.5 | .74 | |
| RTV | Intercept | 1 | 558.4 | 189.5 | .001 |
| Visit | 1 | 602.7 | 11.6 | .001 | |
| Age | 1 | 422.1 | 187.9 | .001 | |
| Medication | 4 | 663.7 | 1.2 | .30 | |
| RT | Intercept | 1 | 571.0 | 184.9 | .001 |
| Visit | 1 | 663.9 | 5.4 | .02 | |
| Age | 1 | 428.5 | 211.4 | .001 | |
| Medication | 4 | 711.9 | 0.6 | .64 |
Note: INH = inhibition; RT = reaction time; RTV = reaction time variability; WM = working memory.
Table 3.
Estimated Marginal (EM) and Observed Means of Standardized Neurocognitive Domain Scores for Groups With Attention-Deficit/Hyperactivity Disorder (ADHD) Relative to the Non-Clinical Comparison Group’s Baseline Scores for Each Treatment Condition
| Working Memory | Inhibition | RTV | RT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base- line |
Week 4 | Week 8 | Base- line |
Week 4 | Week 8 |
Base- line |
Week 4 |
Week 8 | Base- line |
Week 4 | Week 8 |
|
| GUAN | ||||||||||||
| Med | NONE | GUAN | GUAN | NONE | GUAN | GUAN | NONE | GUAN | GUAN | NONE | GUAN | GUAN |
| EM | −.53 (.04) |
−.53 (.05) |
−.60 (.06) |
−.22 (.04) |
−.03 (.06) |
.11 (.07) |
−.32 (.05) |
−.11 (.07) |
.04 (.08) |
−.14 (.05) |
.06 (.06) |
.22 (.07) |
| Observed | −.48 (.08) |
−.52 (.13) |
−.36 (.10) |
−.13 (.06) |
.08 (.16) |
.08 (.07) |
−.31 (.11) |
.03 (.16) |
−.15 (.10) |
−.09 (.08) |
.09 (.12) |
.14 (.10) |
| COMB | ||||||||||||
| Med | NONE | GUAN | COMB | NONE | GUAN | COMB | NONE | GUAN | COMB | NONE | GUAN | COMB |
| EM | −.53 (.04) |
−.53 (.05) |
−.25 (.07) |
−.22 (.04) |
−.03 (.06) |
.15 (.09) |
−.32 (.05) |
−.11 (.07) |
.24 (.09) |
−.14 (.05) |
.06 (.06) |
.13 (.09) |
| Observed | −.48 (.08) |
−.58 (.13) |
−.25 (.10) |
−.28 (.08) |
−.07 (.15) |
.13 (.07) |
−.50 (.10) |
−.06 (.15) |
.17 (.11) |
−.23 (.08) |
.16 (.14) |
.13 (.11) |
| DMPH | ||||||||||||
| Med | NONE | NONE | DMPH | NONE | NONE | DMPH | NONE | NONE | DMPH | NONE | NONE | DMPH |
| EM | −.53 (.04) |
−.60 (.06) |
−.30 (.07) |
−.22 (.04) |
−.09 (.07) |
.08 (.07) |
−.32 (.05) |
−.17 (.08) |
.18 (.09) |
−.14 (.05) |
.01 (.07) |
.26 (.09) |
| Observed | −.57 (.07) |
−.79 (.10) |
−.38 (.07) |
−.33 (.08) |
.01 (.14) |
.07 (.05) |
−.31 (.08) |
.13 (.14) |
.25 (.08) |
−.22 (.10) |
.13 (.13) |
.28 (.09) |
Note: Scores are coded so that higher values reflect better performance (negative values represent impairments relative to the comparison group; for response time (RT) and RT variability (RTV), lower scores were considered better). The shading in each cell indicates the treatments received at each time-point (white = no treatment or placebo, 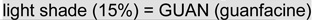 ,
,  d-methylphenidate),
d-methylphenidate),  ): at baseline none of the groups had yet received treatment; at week 4 both GUAN and combination (COMB) groups were receiving guanfacine, while the DMPH group remained on placebo; and at week 8 the GUAN group was receiving guanfacine, the COMB group was receiving the combined treatment, and the DMPH group was receiving only DMPH. Note that the placebo effect is estimated based on performance observed across the 3 groups at baseline and in the DMPH group at week 4.
): at baseline none of the groups had yet received treatment; at week 4 both GUAN and combination (COMB) groups were receiving guanfacine, while the DMPH group remained on placebo; and at week 8 the GUAN group was receiving guanfacine, the COMB group was receiving the combined treatment, and the DMPH group was receiving only DMPH. Note that the placebo effect is estimated based on performance observed across the 3 groups at baseline and in the DMPH group at week 4.
The only significant effect of treatment was on the working memory (WM) cognitive domain. Estimated marginal means were highest for COMB and DMPH, which did not differ from each other; both COMB and DMPH scores were higher than the means for GUAN and PBO (see Table 3). Table 3 shows the estimated marginal means for each of the neurocognitive domain scores within each of the treatment conditions over time.
Table 3 shows that the estimated magnitude of WM impairment in the ADHD group relative to the non-clinical comparison group was −.53 standard deviation (SD) units relative to our non-clinical comparison group (see estimated marginal means at baseline), and that the deficit was reduced under COMB (to −.25 SD) and DMPH (to −.30 SD), but there was no reduction in the GUAN group (in fact, the WM deficit remained at −.53 SD by Week 4, and was non-significantly worse at −.60 SD by Week 8). Table 3 also shows that Inhibition (INH), RT Variability, and Reaction Time domains all tended to have small to moderate impairments relative to our non-clinical comparison group at baseline, and to improve over time in treatment, regardless of the treatment received.
Given that the cognitive domain scores were computed relative to our non-clinical comparison group, we tested for differences between the subset of non-clinical comparison group participants who completed a follow-up exam at 8 weeks, and those who did not. We found that mean cognitive domain scores of the completers were within 1/10th SD unit of the whole group, and scores of the completers did not differ significantly from non-completers for any cognitive domain score. We found small changes from baseline to follow-up for those who completed both sessions, consistent with prior exposure or practice effects. The difference was significant for the RT Variability domain score, which improved by .27 SD units (F[1,36]=10.8, p<.01). The other cognitive domain scores showed smaller changes (in SD units: WM = .11, INH = .03, RT = .19), none of which was statistically significant (all p>.40).
DISCUSSION
Combined treatment with methylphenidate and guanfacine led to greater improvements in working memory than guanfacine alone, but the combined treatment was not significantly superior to methylphenidate alone, and benefits were not observed on other cognitive domain scores for any of the treatments. These findings go beyond the one prior study that examined cognitive effects of guanfacine alone on cognitive parameters in ADHD, and found no significant effects on three cognitive tests despite improvement on clinical ratings18, highlighting a fundamental distinction between stimulant and α2 agonist treatments for ADHD. The current study corroborates the lack of efficacy of guanfacine on cognitive outcomes as a monotherapy, and further suggests it does not benefit cognition as an adjunct to stimulant treatment by direct comparison.
The lack of an advantage for the COMB treatment relative to DMPH alone, and the absence of a cognitive benefit for guanfacine monotherapy, both suggest that guanfacine did not provide a significant cognitive benefit for patients with ADHD. Our clinical findings showing an advantage for the COMB treatment on ADHD clinical outcomes suggest that behavioral gains associated with the addition of guanfacine are not reflected by changes in cognition as measured in this study (see McCracken et al., under review). It is possible that this discrepancy reflects guanfacine’s impact on cognitive functions not measured by our test battery, such as emotional regulation of attention. It is also possible that the addition of guanfacine yields more complex effects, for example a mixture of cognitive benefit but also increased sedation, that ultimately cancel out benefits on our cognitive domain score, but that yield reductions in clinical ratings of impulsive and/or inattentive behavior. A related hypothesis is suggested by a recent study showing benefits of guanfacine relative to placebo on clinically observed symptoms, but not cognitive indicators of inhibition; in that study clinical improvements were associated with reduced fMRI signal in the right midcingulate cortex/supplementary motor area and the left posterior cingulate cortex, but not changes in the inferior prefrontal cortex regions often associated with enhanced response inhibition21. One interpretation of these results is that the clinical benefits of guanfacine were achieved through dampening of ascending noradrenergic “arousal” from the locus coeruleus (LC), more than an enhancement of the systems involved in inhibitory cognitive control, such as the ventrolateral frontal cortex22. Future research might specifically focus on differentiating the actions of guanfacine on post-synaptic α2A receptors in local prefrontal cortical microcircuits (putatively enhancing cognition via increased synaptic efficacy) relative to its presynaptic actions in the LC, where it reduces firing rates23,24. Regardless of possible mechanisms that may explain the distinctions between our cognitive outcomes and the clinical outcomes reported in the companion paper (McCracken et al., under review), it should be appreciated that the clinical outcomes are in part dependent on parent ratings, which may be subject to biases that are less likely manifest in objective cognitive test results.
Our observation of significant treatment effects on working memory but not other cognitive domains contributes to a growing literature on the cognitive effects of pharmacological treatments for ADHD. These findings differ from earlier reports suggesting that stimulant monotherapy has its greatest impact on vigilance functions25, or impact on inhibitory functions often associated with the right inferior frontal gyrus26, but are generally consistent with another meta-analysis that showed the largest effect of stimulants on “executive memory” tests that include what are more often referred to as working memory measures27. At the same time, the lack of a significant effect on response inhibition indicates that this gain is not likely part of a generalized beneficial effect on cognitive control functions, which encompass both working memory and response inhibition functions. Unfortunately, heterogeneity in the methods used to assess cognitive functions hinders cross-study comparisons. Future focus on developing standardized instrumentation for cognitive assessment in clinical trials of ADHD may be necessary to answer these questions more definitively. It is also possible that drugs with other mechanisms may be necessary to ameliorate ADHD-related cognitive deficits other than those that are apparent in working memory.
It is noteworthy that, while not yielding a significant cognitive benefit, guanfacine monotherapy or addition of guanfacine to stimulant treatment did not cause deterioration of cognitive function. For example, the study of Kollins et al. had the primary goal of testing for adverse effects of guanfacine on cognitive measures, based on findings from the Phase III clinical trials that guanfacine was associated with adverse sedative effects28,29. Our results may be seen as corroborating their finding that guanfacine, even as a monotherapy, did not lead to significant declines on any cognitive measures despite our findings of increased sedation, fatigue, and somnolence (see McCracken et al., under review).
It is difficult to reconcile the absence of any apparent cognitive benefit of guanfacine, alone or in combination, with the preclinical and non-clinical control evidence showing cognitive enhancement after acute administration. Dosing differences do not appear to explain this discrepancy, as final daily doses of guanfacine in our study averaged 0.03 mg/kg twice daily, almost identical to the 0.029 mg/kg single dose in the Jakala et al report,14 which found improved working memory and planning performance. Important differences may include acute administration in the prior studies versus the extended, 8-week administration of guanfacine in our protocol. It is possible that cognitive benefits of acute administration fade with repeated exposure, or that developmental differences exist in either dose-response or in α2A receptor regulation of working memory and other cognitive processes. It is also possible that individual titration approaches, distinct from our titration method based on clinical response, are necessary to optimize effects on cognitive functioning. Finally, given that the biology of ADHD and its attendant cognitive deficits remains incompletely understood, disorder-related differences in α2A receptor function, if present, may not be fully ameliorated by straightforward α2A receptor agonist administration, and instead require a different mechanism. If guanfacine can be thought of as increasing “tonic” noradrenergic function, conceivably an approach augmenting “phasic” cognitive control circuit responses, such as more potent noradrenergic reuptake blockade, might demonstrate greater cognitive benefits in ADHD.
Our cognitive assessment approach had strengths in the relatively comprehensive nature of assessments, which enabled us to specify a model with multiple test measures (indicators) for each of the major domains we intended to measure, presumably a more stable assessment of function in each domain. At the same time, our factor analyses suggested that the overall “fit” of our data to the theoretical model was adequate but not excellent. Further work will likely be needed to better specify the ideal measurement methods to detect cognitive impairments and pharmacological enhancement in ADHD.
Our inclusion of a non-clinical comparison group enables us to make approximate statements about the degree of cognitive impairment in our ADHD sample and the extent to which the treatments helped to normalize these deficits. In contrast, the one prior report18 lacked a non-clinical comparison group, making any inferences about baseline performance characteristics or degree of normalization of cognitive function challenging. In the present study patients with ADHD had an initial working memory (WM) deficit of .53 SD units relative to the non-clinical comparison group, and by the end of treatment the WM deficit was only .25 SD units for COMB treatment and .30 SD units for DMPH monotherapy. This reflects an improvement from baseline of .28 (COMB) or .23 (DMPH). This improvement is modest, and similar to the improvement observed on re-testing of our untreated non-clinical comparison group after 8 weeks (.27 SD units). But it should be noted that (a) our patients did not show any improvement in WM while on placebo from baseline to week 4; (b) the members of the COMB group treated with guanfacine did not improve from baseline to week 4; and (c) the members of the GUAN group showed no improvement at either week 4 or week 8 (indeed, they showed a non-significant decline of .10 SD units by week 8). These findings suggest that the improvement of WM functioning was a result of treatment with DMPH or the combination treatment, not just a prior exposure effect. To better characterize the true placebo rates of repeated test exposure in a patient with ADHD, future research might consider designs with a non-treatment but clinically affected comparison group (i.e., with placebo extended for 8 weeks), although that design may be challenging to execute, and careful consideration would need to be paid to whether the potential benefits of the knowledge gained would outweigh keeping children off treatment for the longer duration.
Are these gains clinically significant? Gains of .23 to .28 SD units in cognitive function may be difficult to discern clinically, yet the endpoint WM performance of our patients with ADHD was only ¼ SD below the non-clinical baseline mean, suggesting that the patient group’s performance was substantially closer to being “normalized.” It is possible that the benefits seen in working memory performance, though modest, are indeed clinically significant, but additional investigations of real-world outcomes will be necessary to answer this question definitively. More importantly, these findings reinforce the conclusion that while our treatment of both the clinical symptoms and cognitive deficits of ADHD have improved with modern medication treatments, there is still a major need for new treatments—both pharmacological and non-pharmacological— that may be capable of yielding more robust normalization of cognition and behavior.
Supplementary Material
Acknowledgments
This work is supported by the National Institute of Mental Health (NIMH) Research Center grant P50MH077248, “Translational Research to Enhance Cognitive Control” (J.T.M.).
Ms. Whelan and Drs. Hellemann and Sugar served as the statistical experts for this research.
The authors would like to thank the children and parents for their participation.
Dr. Bilder has received consulting income or honoraria from EnVivo Pharmaceuticals, Forum Pharmaceuticals, Lumos Labs, Maven Research, Neurocog Trials Inc., OMDUSA, LLC, Snapchat, Takeda-Lundbeck, and ThinkNow Inc. He has received research support from the National Institute of Mental Health, the John Templeton Foundation, and Johnson and Johnson. Dr. McGough has received consultant honoraria from Neurovance; research support from Purdue, material research support for investigator initiated studies from NeuroSigma and Shire; book royalties from Oxford University Press; and DSMB honoraria from Sunovion. He has provided expert testimony for Shire. Dr. McCracken has received consultant honoraria from Dart Neuroscience and Think Now, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Loo, Hellemann, Sugar, Del’Homme, Cowen, Ms. Whelan, Ms. Sturm, and Mr. Hanada report no biomedical financial interests or potential conflicts of interest.
References
- 1.Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. Journal of clinical psychiatry. 2002;63:10–15. [PubMed] [Google Scholar]
- 2.Fayyad J, De Graaf R, Kessler R, et al. Cross–national prevalence and correlates of adult attention–deficit hyperactivity disorder. The British Journal of Psychiatry. 2007;190(5):402–409. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 3.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Sciences. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinussen R, Tannock R. Working memory impairments in children with attention-deficit hyperactivity disorder with and without comorbid language learning disorders. J Clin Experimental Neuropsychology. 2006;28(7):1073–1094. doi: 10.1080/13803390500205700. [DOI] [PubMed] [Google Scholar]
- 6.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 7.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. [06/01/2005];Biological psychiatry. 57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Tucha O, Prell S, Mecklinger L, et al. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2006 Apr;185(3):315–326. doi: 10.1007/s00213-006-0318-2. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes SM, Coghill DR, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology (Berl) 2004 Sep;175(3):319–330. doi: 10.1007/s00213-004-1833-7. [DOI] [PubMed] [Google Scholar]
- 10.Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007 Mar;113(3):523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000 Sep;23(3):240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten AF, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J Neurosci. 1988 Nov;8(11):4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakala P, Riekkinen M, Sirvio J, Koivisto E, Riekkinen P., Jr Clonidine, but not guanfacine, impairs choice reaction time performance in young healthy volunteers. Neuropsychopharmacology. 1999 Oct;21(4):495–502. doi: 10.1016/S0893-133X(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 15.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS drugs. 2009;23(1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Newcorn JH, Stein MA, Childress AC, et al. Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry. 2013;52:921–930. doi: 10.1016/j.jaac.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Wilens TE, Bukstein O, Brams M, et al. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012 Jan;51(1):74–85. e72. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Kollins SH, Lopez FA, Vince BD, et al. Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011 Apr;21(2):111–120. doi: 10.1089/cap.2010.0064. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KP, Clerkin SM, Fan J, Halperin JM, Newcorn JH. Guanfacine modulates the influence of emotional cues on prefrontal cortex activation for cognitive control. Psychopharmacology. 2013;226(2):261–271. doi: 10.1007/s00213-012-2893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure MM, Barch DM, Romero MJ, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol psychiatry. 2007;61:1157–1160. doi: 10.1016/j.biopsych.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Bedard AC, Schulz KP, Krone B, et al. Neural mechanisms underlying the therapeutic actions of guanfacine treatment in youth with ADHD: a pilot fMRI study. Psychiatry Res. 2015 Mar 30;231(3):353–356. doi: 10.1016/j.pscychresns.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Congdon E, Altshuler LL, Mumford JA, et al. Neural activation during response inhibition in adult attention-deficit/hyperactivity disorder: Preliminary findings on the effects of medication and symptom severity. Psychiatry Research: Neuroimaging. 2014;222(1):17–28. doi: 10.1016/j.pscychresns.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnsten AF, Jin LE. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. The Yale journal of biology and medicine. 2012 Mar;85(1):45–58. [PMC free article] [PubMed] [Google Scholar]
- 24.Berridge CW, Arnsten AF. Catecholamine mechanisms in the prefrontal cortex: proven strategies for enhancing higher cognitive function. Current Opinion in Behavioral Sciences. 2015;4:33–40. [Google Scholar]
- 25.Sander C, Arns M, Olbrich S, Hegerl U. EEG-vigilance and response to stimulants in paediatric patients with attention deficit/hyperactivity disorder. Clin Neurophysiol. 2010 Sep;121(9):1511–1518. doi: 10.1016/j.clinph.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biological psychiatry. 2014 Oct 15;76(8):616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biological psychiatry. 2014 Oct 15;76(8):603–615. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Sallee FR, McGough J, Wigal T, et al. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009 Feb;48(2):155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- 29.Biederman J, Melmed RD, Patel A, McBurnett K, Donahue J, Lyne A. Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS spectrums. 2008 Dec;13(12):1047–1055. doi: 10.1017/s1092852900017107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


