Abstract
The relationship between mercury (Hg) and selenium (Se) toxicity is complex, with coexposure reported to reduce, increase, and have no effect on toxicity. Different interactions may be related to chemical compound, but this has not been systematically examined. Our goal was to assess the interactive effects between the two elements on growth in the nematode Caenorhabditis elegans, focusing on inorganic and organic Hg (HgCl2 and MeHgCl) and Se (selenomethionine, sodium selenite, and sodium selenate) compounds. We utilized aqueous Hg/Se dosing molar ratios that were either above, below, or equal to 1 and measured the internal nematode total Hg and Se concentrations for the highest concentrations of each Se compound. Observed interactions were complicated, differed between Se and Hg compounds, and included greater-than-additive, additive, and less-than-additive growth impacts. Biologically significant interactions were only observed when the dosing Se solution concentration was 100–25 000 times greater than the dosing Hg concentration. Mitigation of growth impacts was not predictable on the basis of internal Hg/Se molar ratio; improved growth was observed at some internal Hg/Se molar ratios both above and below 1. These findings suggest that future assessments of the Hg and Se relationship should incorporate chemical compound into the evaluation.
Graphical abstract
INTRODUCTION
Mercury (Hg) is an environmental contaminant of great concern owing to its persistence in the environment; human and wildlife exposures are common, with recognized toxic impacts, making it important to understand factors that can mediate Hg toxicity. Hg exists naturally in the environment but also has considerable anthropogenic mobilization, with industrial inputs expected to increase in the future.1 In the environment, Hg exists in three main chemical forms: elemental Hg(0), inorganic divalent Hg(II), and organic forms such as monomethylmercury (MeHg).2 The majority of human exposures are to inorganic and organic Hg through occupational and dietary routes, respectively. Chemical speciation is important to toxicity; for example, harmful impacts to the nervous and reproductive systems are associated with MeHg exposure, and negative renal system impacts are associated with inorganic Hg exposure.3–6 Hg toxicity can also be altered by a variety of other factors, including selenium coexposure.
Selenium (Se), like Hg, is a naturally occurring element in the environment, but unlike Hg, Se is a necessary micronutrient. It is necessary for the proper function of selenoenzymes, which have important roles including antioxidant functions. Due to its biological importance, organic Se (e.g., selenomethionine) and inorganic Se (e.g., selenate, selenite) have commonly been used in human and animal supplements. However, although Se is required at low levels, it has a very narrow therapeutic index and is toxic at higher concentrations. The interaction between Hg and Se is complex and has commonly been described as antagonistic, with Se coexposures having the ability to mitigate Hg toxicity.7, 8 Conversely, synergistic effects have also been observed.9–11 The difference between antagonistic and synergistic impacts may result from the chemical species and dosing concentrations used and may depend on the organism and biological outcome examined. However, the mechanisms that underlie either interaction are not well-understood.
Proposed mechanisms for antagonism include: (1) reductions in bioavailable Hg due to Hg–Se complex formation (i.e., reduced uptake); (2) decreased distribution to target tissues or increased excretion of Hg due to Hg–Se complex formation; and (3) improved antioxidant function, as some antioxidants are Se-dependent enzymes (ex. glutathione peroxidase, GPX; thioredoxin reductase, TrxR), and supplemental Se could reduce the Hg-induced depletion of selenoenzymes.12, 13 Related to the first two hypotheses, it has been specifically postulated that the protective effects of Se occur when the molar Se/Hg molar ratio is ≥1 because Se may reduce the biological availability of Hg through physical sequestration due to the high affinity between Hg and Se.14–17 Potential mechanisms for synergism are less clear, but may include altering antioxidant capabilities, thereby promoting a prooxidative environment.8, 9, 18
We sought further knowledge of Hg and Se interactions by systematically varying multiple chemical species and concentrations in the model organism Caenorhabditis elegans. C. elegans growth was used as the toxic end-point because it represents an integrated measure of multiple developmental processes. Growth impairments from exposure to some species of Hg or Se individually have also been previously described in C. elegans, making it a logical model to investigate coexposures.19–21 Additionally, many antioxidants in C. elegans act similarly to mammalian homologues in terms of reducing oxidative stress (GPX, TrxR, and superoxide dismutase SOD).22–25 We note that C. elegans may be a particularly useful model for addressing the antagonism theories described in the preceding paragraph because the likelihood of the third hypothesis is especially low in C. elegans. C. elegans has only one selenoenzyme (TrxR-1) compared to the 25 in humans,26–28 deletion of this gene has not been observed to alter Se toxicity in C. elegans,29 and low levels of Se stimulate nematode growth21, 30 and are protective against oxidative stress in C. elegans.31–34 Our objective was to measure growth impacts from multiple combinations of inorganic and organic forms of Hg and Se and, in doing so, examine the importance of (1) Se compound and concentration, (2) Se/Hg molar ratio of the exposure medium, and (3) internal Se/Hg molar ratio to toxicity recovery.
MATERIALS AND METHODS
C. elegans Maintenance
N2 (Bristol) nematodes were maintained at 20 °C on K agar plates seeded with Escherichia coli (OP50 strain). Synchronized L1 larvae were obtained by treating gravid adults with a 5% sodium hypochlorite solution and hatching eggs in the absence of food (K medium plus MgSO4, CaSO4, and cholesterol).35
Individual Dose–Response Curves for Selecting Hg and Se Concentrations for the Coexposure Growth Assay
L1 nematodes were distributed into 24 well plates (100–300 nematodes per well in 1 mL of dosing solution) containing a range of concentrations of Hg or Se compounds in EPA reconstituted moderately hard water plus UVC-killed E. coli (UVRA strain) to eliminate the potentially confounding effect of bacterial metabolism on exposures, as previously described.36 Dose–response curves were obtained for HgCl2 (0–10 µM), MeHgCl (0–10 µM), selenomethionine (0–1 mM, SeMeth), sodium selenite (0–5 mM, Se(IV)), and sodium selenate (0–50 mM, Se(VI)). Nematodes were exposed at 20 °C for 48 h, with additional UVC-killed E. coli added after 24 h (5% of solution volume). Following 48 h, nematode size was measured using a COPAS Biosort (Union Biometrica, Holliston MA), using extinction, the optical density of a nematode, as a growth end-point (72–1479 nematodes measured per exposure).37
Compound concentrations for the coexposure growth assay were chosen on the basis of growth reductions. We selected concentrations of 2 and 5 µM MeHgCl and HgCl2 because of their similar impacts on growth (both species caused ~15 and ~35% reduction at these doses) and because these exposure levels alter neuromuscular function (locomotion) in C. elegans.20 We chose Se concentrations that included a high concentration that reduced growth 10–20% (500 µM SeMeth, 1500 µM Se(IV), and 50 mM Se(VI)) along with lower concentrations to assess the impacts of dosing solutions with molar ratios of Hg/Se that were either above, below, or equal to 1. Although we did not observe a growth response at the lower Se concentrations (0.25, 2, and 5 µM), which could suggest that Se may not be entering the nematodes, these doses were considered appropriate for the coexposure growth assay, as toxicities to other end-points have been observed in this concentration range in other invertebrate studies. We note that although some Se concentrations are high compared to those employed in some other studies, they are sublethal and consistent with previous reports of the same chemicals in studies with C. elegans.19, 29, 38, 39
Hg and Se Coexposure Growth Assay
L1 nematodes were exposed to HgCl2, MeHgCl, SeMeth, Se(IV), or Se(VI) using the same methods that were used to obtain the individual dose–response curves. The following conditions were assayed individually and in Hg–Se combinations: control; 2 and 5 µM HgCl2; 2 and 5 µM MeHgCl; 0.25, 2, 5, and 500 µM SeMeth; 0.25, 2, 5, and 1500 µM Se(IV); and 0.25, 2, 5, 1000, 10000, and 50000 µM Se(VI). Following the 48 h exposure, nematode size was determined (163–409 nematodes per exposure).
Internal Hg and Se Exposure Determination
L1 nematodes were distributed into 250 mL tissue culture flasks (49 000–85 000 nematodes per flask in 30 mL of dosing solution) and exposed to a range of concentrations of HgCl2, MeHgCl, SeMeth, Se(IV), or Se(VI). Internal concentrations were only determined for the highest Se concentrations because interactive impacts on growth in combination with Hg exposure were most clearly present at these exposure levels. Following the 48 h of exposure, nematodes were washed three times with K medium and briefly damaged with a Bullet Blender (Next Advance, Averill Park, NY; four repetitions of 15 s pulses) to break the cuticle. The solution containing damaged nematodes was then centrifuged (3 min at 300g) to remove the cuticle from the solution. The remaining solution was considered to be representative of the internal nematode environment.
Total Hg concentrations from the internal worm solution were measured using direct thermal decomposition, amalgamation, and atomic absorption spectrometry (Milestone-DMA-80).40 Analysis of standard reference material (DORM-4 Fish Protein) resulted in Hg measurements that were 95 ± 4.2% of the certified value. The limit of detection (LOD) for Hg by this method was 0.001 mg/kg. For Se determination, 0.5 mL of sample was microwave digested in 2.5 mL of 90% nitric acid solution (CEM Discover SP-D Microwave Digestion System). Total Se was determined by inductively coupled plasma–mass spectrometry (ICP–MS; Agilent 7700X equipped with Octopole Reaction System). Analysis of a standard reference material (DORM-4 fish protein) resulted in recoveries of 101 ± 2.1% (n = 7) of the certified Se value. The LOD for Se by this method was 3.8 µg/L. For internal nematode Hg and Se determination, the LOD value was used for samples with concentrations below the LOD for each method. Hg and Se content were normalized to total protein content to account for nematode size differences between treatments. Protein content was determined following manufacturer instructions in the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific Pierce BCA Protein Assay Kit). Average protein concentration per sample was approximately 80 µg/mL, ranging from 39 to 133 µg/mL.
Statistical Analysis
Individual dose–response curves were analyzed using ANOVA, followed by Dunnett’s post-hoc test for pairwise comparisons to the control. Interactions between Hg and Se were assessed by using contrast matrices to measure differences in nematode growth from the coexposure assay. Using these matrices, null hypotheses were defined as additive, with equal weight given to both Hg and Se individual exposures. Significant deviations from the null would indicate interactions that were either greater or less than additive, with less than additive responses signifying an antagonistic interaction. Matrices were constructed, for each Hg and Se compound pair, in a stepwise manner, and significance was determined using an F-statistic (see Supporting Information). For cases in which the expected nematode size was smaller than the size before dosing, the coexposure treatment was compared to the size before dosing so that the calculated change in growth would not be overinflated. Changes in expected growth were expressed as percentage relative to control nematode growth. A multiplicative model was also assessed, and instances in which multiplicative interactions are significant and differ from additive are described in the text. Additive representations are presented graphically because they are more straightforward to interpret. Additive and multiplicative models were compared using an F-test, Akaike information criterion (AIC), and Bayesian information criterion (BIC) scores. An amelioration of growth inhibition (i.e., toxicity reduction, sometimes described as “rescue” in the pharmacological or genetic literature), is referred to as a reduction or mitigation of toxicity in this manuscript. Internal Hg and Se analyses were performed twice, separated in time. The impact of the highest Se exposures on the internal Hg and Se concentrations and the Hg/Se molar ratio was determined using a two-way ANOVA. Statistics were calculated using R version 3.2.2 (Vienna, Austria), and significance was accepted at a level of p < 0.05
Equilibrium Speciation Calculations
The speciation of Hg(II) and MeHg in the Hg–Se(IV) and Hg–Se(VI) mixtures was calculated using Visual MINTEQ (v. 3.1). Input parameters included the recipe for the EPA moderately hard water matrix (96 mg of NaHCO3, 60 mg of MgSO4, 60 mg of CaSO4, and 4 mg of KCl in 1 L of H2O), pH 7.5, and 2 µM total Hg (as Hg(II) or MeHg). The concentration of total Se as Se(VI) or Se(IV) was varied using the same range as the coexposure experiments (from 0.1 µM to 50 000 µM). The calculations utilized the thermodynamic stability constants in the Visual MINTEQ database, including constants for Hg2+ complexes with OH−, Cl−, and SeO32−. Constants for CH3Hg+ complexes with OH−, Cl−, SeO32−, and SeO42− were obtained from the reference literature (NIST) and manually entered into the program database (Table SI-2).41 The calculations assumed that other components of the exposure matrix (e.g., UV-killed bacteria, C. elegans) did not alter the solution-phase speciation of Hg and Se.
RESULTS
Individual Dose–Response Curves for Selecting Hg and Se Concentrations for the Coexposure Growth Assay
Nematodes were exposed to a range of individual Hg and Se compound concentrations to evaluate exposure concentrations that impact growth and could be used in the coexposure assay. Data for the individual dose–response curves represents one to nine biological experiments separated in time. All tested Hg and Se compounds exhibited dose–responses with respect to growth with the following observed toxicity order: MeHg ≈ HgCl2 > SeMeth > Se(IV) > Se(VI) (Figure SI-1 top panel). For individual compounds, the lowest concentrations at which consistent and significant growth reductions were observed were: 1 µM MeHg, 2 µM HgCl2, 200 µM SeMeth, 700 µM Se(IV), and 50 mM Se(VI) (Figure SI-1 bottom panel).
Hg and Se Coexposure Growth Assay
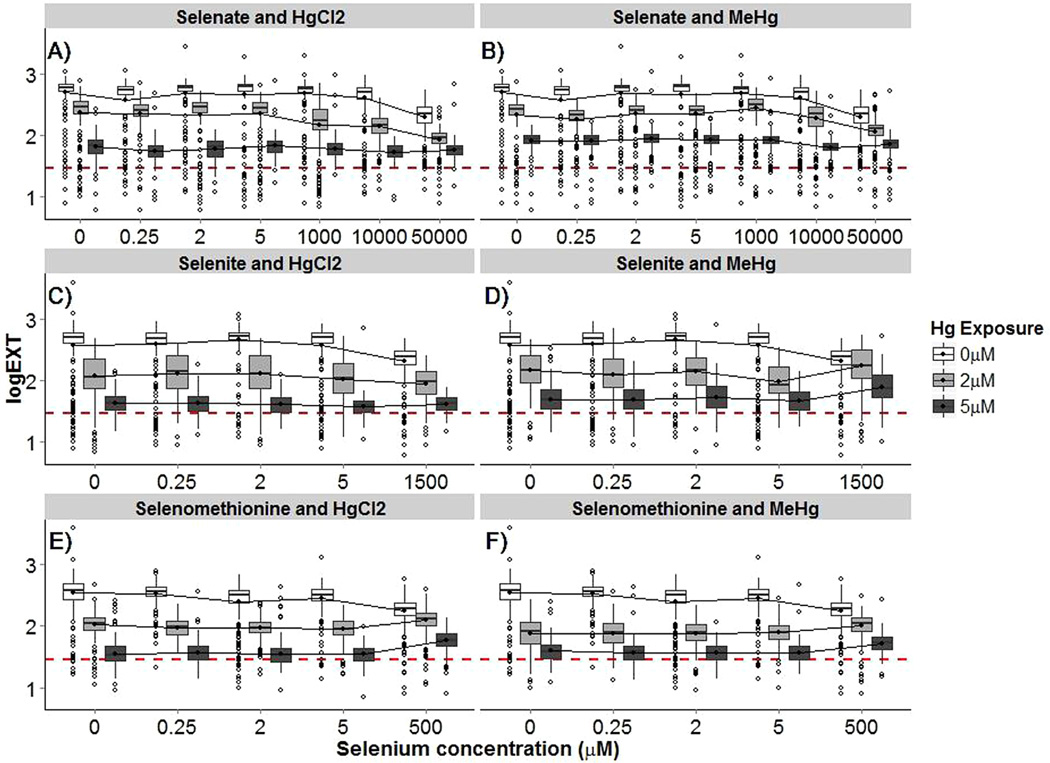
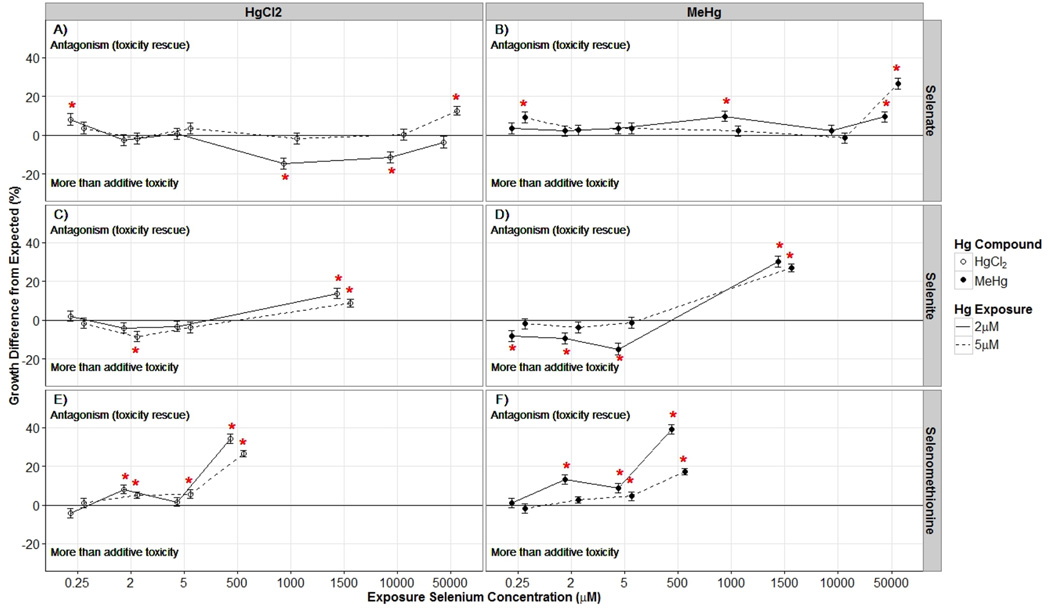
Next, to assess the interaction between Hg and Se, F-statistics were computed to determine if there were significant interactions and whether these interactions were less than additive (antagonistic) or greater than additive. Nematode size for each treatment is displayed in Figure 1. Data for coexposure experiments represents three to four biological experiments separated in time. Initial matrices for all Hg–Se comparisons rejected the null hypotheses that Hg exposure in general (HAa) and at each dosing concentration (HAb1 and HAb2) had no impact on nematode growth compared to that in Se-only exposures. Additive interaction directionality (antagonistic or more than additive) for each Hg–Se pairing is indicated in Figure 2. Multiplicative interactions were significant and different from the additive model at all instances in which growth differed from additive conditions, with the exception of no significant multiplicative interaction at 0.25 µM Se(VI) in combination with 2 µM HgCl2. In most instances, differences between additive and multiplicative models was modest (AIC and BIC score difference <20). However, for coexposures that resulted in more than 15% growth differences additive and multiplicative models differed greatly (AIC and BIC score difference >70), with the multiplicative models having the lower AIC and BIC scores. The better model fit with the multiplicative models in these instances is likely due to the defined multiplicative interaction being a more intermediate response compared to the additive model.
Figure 1.
Nematode size (logEXT) following combination mercury (HgCl2 and MeHg) and selenium (sodium selenate, sodium selenite, and selenomethionine) exposures. Coexposures include: (A) selenate and HgCl2, (B) selenate and MeHg, (C) selenite and HgCl2, (D) selenite and MeHg, (E) selenomethionine and HgCl2, and (F) selenomethionine and MeHg. Selenium exposure is represented on the x-axis against nematode growth (logEXT), with mercury exposure represented by the grouped lines and box-plots. White box-plots indicate 0 µM Hg exposure, light gray box-plots indicate 2 µM Hg exposure, and dark gray box-plots indicate 5 µM Hg exposure. Data for each group represents three to four biological experiments separated in time with between 163 and 409 individual nematodes. Interaction lines and box-plots are dodged for better visual representation. Lines between box-plots connect the means for each treatment. For each selenium–mercury pairing, mercury altered nematode growth compared to selenium-only exposures (p < 0.05). The dashed red line indicates the average nematode size before dosing (L1).
Figure 2.
Nematode growth difference (average % of control ± SE) from expected growth under additive conditions for all selenium and mercury coexposures. Coexposures include: (A) selenate and HgCl2, (B) selenate and MeHg, (C) selenite and HgCl2, (D) selenite and MeHg, (E) selenomethionine and HgCl2, and (F) selenomethionine and MeHg. The x-axis has categorically increasing selenium exposures (µM). HgCl2 and MeHg exposures are plotted separately and are indicated by open and closed circles, respectively. Exposure concentration is represented by a solid line for 2 µM exposures and a dashed line for 5 µM exposures. Positive percentages indicate an antagonistic interaction (more growth than expected) and negative percentages greater than additive interactions (less growth than expected). Asterisks indicate significance compared to the expected additive relationship (p < 0.05).
Following Se(VI) exposures, antagonistic interactions were mainly observed at the highest Se(VI) dosing concentration (50 000 µM). Smaller (<15%) antagonistic and greater than additive interactions also occurred at lower Se(VI) doses (Figure 2). Following Se(IV) exposures, antagonistic interactions only occurred at the highest Se(IV) dosing concentration (1500 µM), with significant growth improvements (>20%) observed in combination with both MeHg concentrations. Greater-than-additive interactions with Se(IV) were also observed at one coexposure with HgCl2 and multiple coexposures with 2 µM MeHg (Figure 2). Following SeMeth exposures, significant antagonistic interactions (>20%) were observed at the highest Se dosing concentrations for all Hg compounds and doses. Smaller antagonistic interactions were also observed at lower SeMeth doses in combination with both low and high concentrations of HgCl2 and MeHg (Figure 2). Antagonistic relationships identified for some very high (5 µM) Hg exposures (Figure 2, Table SI-3) should be taken with caution because growth was highly impaired at this Hg concentration. There were seven cases in which the expected nematode size was smaller than the size before dosing. These cases are indicated in Table SI-3.
Internal Hg and Se Exposure Determination
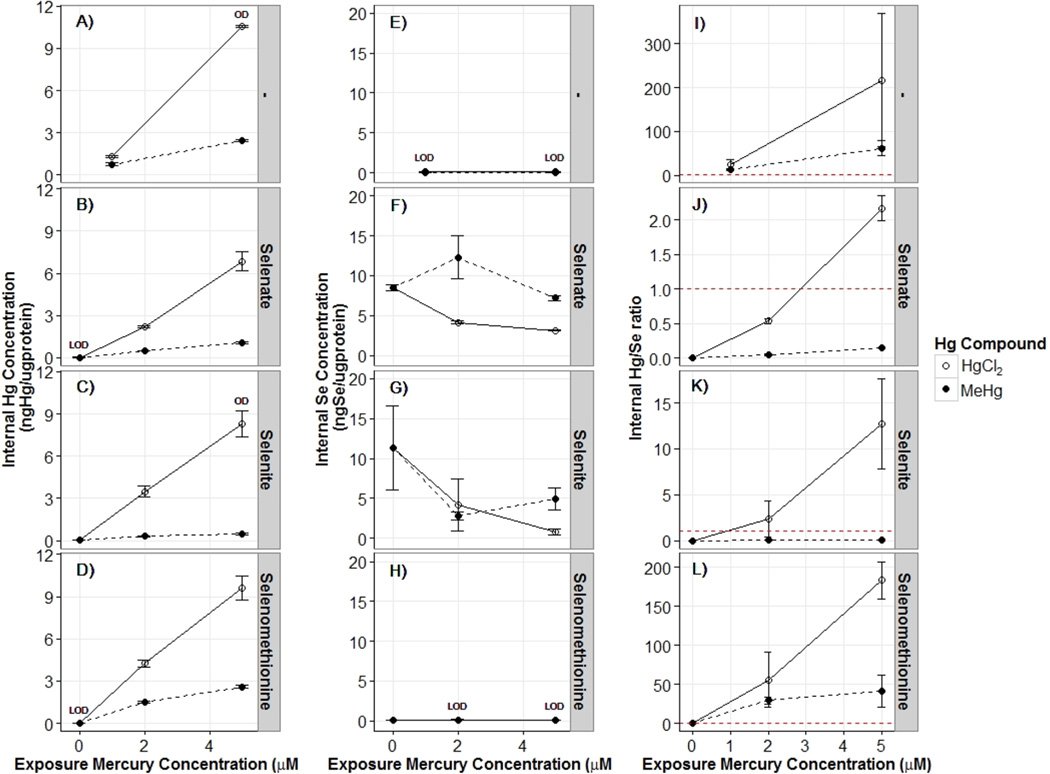
We next determined if the interactions observed at the highest Se dosing concentrations, which were uniformly antagonistic, were related to the intraorganismal Se/Hg molar ratio. We measured internal Hg and Se concentrations for individual Hg exposures and for the coexposures that occurred at the highest Se dosing concentration for each Se compound. As expected, Hg concentrations increased with increasing exposure concentration, but unexpectedly, higher Hg concentrations were detected in HgCl2-exposed rather than MeHg-exposed nematodes (p < 0.05 for Hg compound effect). In Se(IV) and Se(VI) coexposed nematodes, internal HgCl2 concentrations were reduced but not significantly (p = 0.074). In SeMeth and MeHg coexposed nematodes, the internal Hg concentration was similar to the internal concentration of the treatment exposed only to MeHg, and the internal Hg concentration was significantly reduced in Se(IV) and Se(VI) coexposures (p < 0.05). Nematodes exposed to Se(IV) had significantly reduced internal Hg compared to the Se(VI) treatment (Figure 3).
Figure 3.
Interaction plots for internal mercury and selenium concentrations (average ng of compound/µg of protein ± SE) and internal Hg/Se ratios (average ± SE). The figure is grouped into three main sections with internal mercury concentrations are represented in the left panels (A–D), internal selenium concentrations are represented in the middle panels (E–H), and internal Hg/Se ratios in the right panels (I–L). Each of these sections contains faceted plots to represent exposure to no selenium compounds (none; A, E, I), selenate (50 mM; B, F, J), selenite (1500 µM; C, G, K), or selenomethionine (500 µM; D, H, L). The x-axis has categorically increasing mercury exposures (µM) to either HgCl2 (open circle, solid line) or MeHg (closed circle, dotted line). In the internal Hg/Se ratio section, note that the y-axis scale is different for each plot, and the red dashed line indicates a 1:1 Hg/Se ratio. In the internal mercury and selenium sections, points with at least one value above or below detection limits are indicated by OD and LOD, respectively; for a full list of sample values, see Table SI-1. For the internal mercury plots, main effects for mercury compound and concentration and their interaction were significant (p < 0.05) for each selenium exposure. For the internal selenium plots, the main effect of mercury compound was significant (p < 0.05) in the selenate plot but not (p = 0.08) in the selenite or selenomethionine plots.
Internal Se concentrations were not statistically significantly impacted by either Hg compound in SeMeth and Se(IV) coexposed nematodes (Figure 3). In nematodes exposed to Se(VI), the internal Se(VI) concentration was higher in MeHg coexposed nematodes (Figure 3).
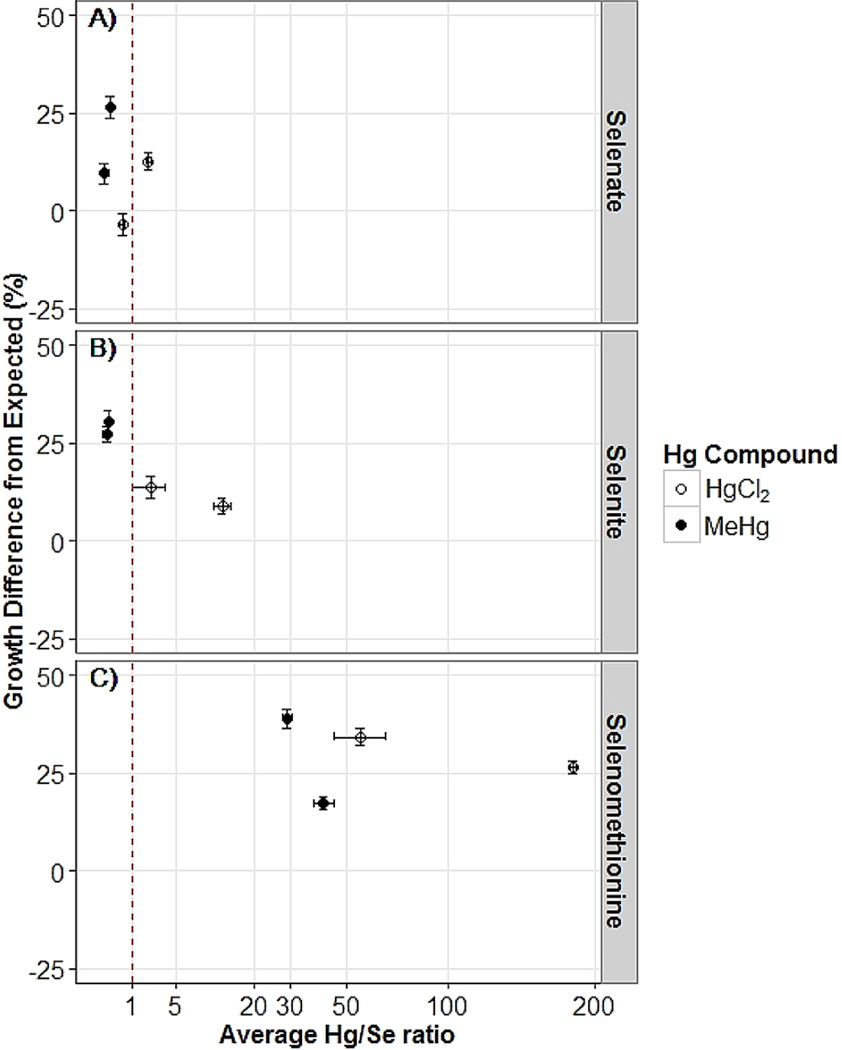
Finally, we assessed the effects of coexposures on Hg/Se molar ratios (Figure 3), and the relationship between those ratios and growth effects (Figure 4). We observed significant main effects for Hg concentration and compound and their interaction on Hg/Se molar ratios for Se(VI) and SeMeth (Figure 3). For Se(IV), the main effect of Hg compound was significant, but the main effect for Hg concentration (p = 0.053) and the interaction between compound and concentration (p = 0.055) were not. As Se concentrations were held constant at the highest exposure level, increasing the Hg exposures increased the Hg/Se molar ratio, and the Hg/Se molar ratio was higher in HgCl2 coexposed than in MeHg coexposed nematodes. Antagonistic interactions resulting in greater than expected growth were observed at Hg/Se molar ratios both above and below 1 for all Se compounds at the highest Se exposure levels (Figure 4). We also observed one instance (Se(VI) treatment) in which a Se/Hg molar ratio of >1 did not result in the reduction of growth inhibition (Figure 4).
Figure 4.
Hg/Se ratio (average ± SE) plotted against nematode growth difference (average % of control ± SE) from expected growth under additive conditions for selenium–mercury coexposures at the highest selenium exposure. Faceted plots represent exposure to (A) selenate (50 mM), (B) selenite (1500 µM), or (C) selenomethionine (500 µM). HgCl2 and MeHg exposures are indicated by open and closed circles, respectively. Red dashed line indicates a 1:1 Hg/Se ratio.
DISCUSSION
We observed growth impairments from single exposures to either Hg or Se that were consistent with other C. elegans studies. Reduced growth was comparable for MeHg and HgCl2 and occurred in the low-µM range, as observed by McElwee and Freedman.20 At first glance, this finding is unexpected because MeHg is frequently identified as the more toxic compound for most end points, but in both of our studies, growth was reduced similarly on the basis of external dosing solution concentrations. However, our measurements of internal Hg demonstrated that MeHg was more toxic than HgCl2 because internal nematode MeHg concentrations were lower than HgCl2 concentrations for identical external exposure concentrations.
The tested Se compounds were at least 2 orders of magnitude less toxic than the Hg compounds. Biological impairments resulting from Se(IV) occurred in the same range (1–5 mM) reported by Li et al.19 for growth impairment and by Morgan et al.34 for impaired motility. Growth sensitivity to Se varied greatly between compounds, with toxicity observed in following order: SeMeth > Se(IV) > Se(VI); this order has also been observed in other invertebrates.29, 38, 39 Coexposure outcomes were not as straightforward.
In our experiments, the instances in which Hg and Se did interact were complex and depended greatly on the chemical compound and the concentration administered. For reference, most dosing Se concentrations that we utilized were in the range of total Se concentrations found in child (2–32 µM) and adult (3–12 000 µM) blood and plasma in populations exposed to Hg through diet.42–47 We observed growth impacts from coexposures that ranged from antagonistic to greater-than-additive. Antagonism has been the most frequently reported Hg and Se interaction and was observed in this study primarily with Se(IV) and SeMeth exposures. Growth improvements, compared to the individual Hg exposure, were observed with Se(IV) and organic Hg, while growth improvements were noted for SeMeth in combination with both inorganic and organic Hg (Figure 2). Some antagonistic interactions occurred at some lower Se exposures and resulted in small growth improvements (≤13%), and biologically meaningful antagonism, which we defined here as that which altered growth by 20% or more, only occurred when the Se exposure was much greater than the Hg exposure (100–25 000 times). Toxicity reduction at the highest Se exposures also varied between compounds, with SeMeth causing the greatest reduction in combination with both Hg compounds (34–47%), followed by Se(IV) with MeHg (30–42%). Se(IV) in combination with HgCl2 and Se(VI) with both Hg compounds provided 9–28% toxicity reduction. Antagonism between Hg and Se has been observed previously, with other end-points with many different selenocompounds in a number of ameliorated or partially ameliorated end points including enzyme activity, enzyme expression, and DNA damage.8, 18, 48
In addition to the antagonistic interactions described above, we observed additive and greater than additive interactions. Additive impacts were most common, especially at the lower Se exposures (Figure 2). Various interactions between the two elements have also been noted in other studies. Impacts ranging from little impact of Se supplementation to reduction of toxicity were also highlighted in a rodent dietary study in which Se delayed and reduced MeHg’s impact for some end points but not others.49 Though only observed to a small degree in this study, greater than additive toxicity has been noted in other aquatic toxicology studies, resulting in reduced survival and development in aquatic insect larvae,50 lower hatching success and survival in ducklings,51 and reduced reproduction in fish.10 This synergism in younger life-stages appears to have been noted most frequently in studies that have assessed end-points at young and adult life-stages. However, the same group that reported synergistic effects of reduced reproduction also observed partial mitigation of embryonic selenoprotein gene mRNA levels, GPX activity, and larval locomotion.18 The mechanisms that underlie greater than additive toxicity are not well-understood.
One theory that seeks to explain some of these interactions is that antagonism and toxicity mitigation occur when the Se/Hg molar ratio is ≥1. This hypothesis is based on the premise that when the two elements are present at equimolar ratios, Se binds to and sequesters Hg.14, 17 However, the majority of the studies addressing Se/Hg molar ratios have been ecological in nature, focusing on natural ratios present in fish.52–54 In laboratory studies with rodents, both beneficial and nonadvantageous health outcomes of Se coexposure have been noted.14, 55 Furthermore, although Se has a beneficial impact on some health outcomes, there has not been strong evidence for interactions between Hg and Se in epidemiological studies.42, 43, 46, 47 The reasons for which different interactions have been observed are not entirely clear but could be attributable to study design differences including: different studies employing different species of Hg and Se, typically a relatively small number of concentrations, different species of test organism, and different toxic end-points. The results of our study, in which we directly tested outcomes in experiments in which Se/Hg molar ratio were deliberately manipulated over a wide range of ratios do not support the Se/Hg molar ratio hypothesis. Antagonism was not consistently observed when either the external or internal Se/Hg molar ratio was ≥1, suggesting that the simple model of Se-mediated sequestration of Hg is an insufficient explanation for antagonism. Another possibility, less dependent on the molar ratio per se, is that Se supplementation replenishes Se sequestered by Hg, thereby rescuing toxicity by providing ample Se for selenoprotein production and activity.14, 17 This hypothesis, however, presumes that all Hg toxicity can be explained by selenoprotein depletion or inhibition, which seems unlikely given that Hg also has a well-documented interaction with sulfhydryl groups in proteins. Of note, this possibility is especially unlikely in our model organism because C. elegans has only one selenoprotein. However, it is important to note that this study did not assess tissue-specific impacts or effects beyond growth. While this means that we capture many potentially important biological targets, it also means that we could not distinguish specific impacts at the cellular or tissue level. To address the potential interactions between the elements in the dosing solution, we calculated that changes in Se and Hg speciation and the binding of the two in solution are unlikely to explain the biological impacts observed.
The speciation of Se and Hg in exposure experiments was likely dependent on the Se/Hg molar ratio and the type of Se and Hg tested. For example, the calculated equilibrium speciation for the exposure media in our study suggested that Se(VI) was not binding substantial amounts of MeHg+ in the experimental solutions (Figure SI-2), even though we observed antagonism at the highest Se(IV)/MeHg molar ratio. Se(IV) also was not influencing inorganic Hg2+ and MeHg+ speciation at most Se/Hg molar ratios. The exception was the mixture with the highest Se(IV)/MeHg molar ratio (1500 µM Se(IV) and 2 µM MeHg), where the CH3HgSeO3− concentration was predicted to be 1.3 µM (or 67% of the total MeHg). We also note that at this highest Se(IV)/MeHg molar ratio, antagonistic effects were observed for the growth of the nematodes (Figure 2). Stability constants for Hg2+ complexes with Se(VI) and Hg2+ as well as MeHg+ complexes with SeMeth were not found in the literature, so speciation calculations were not performed for these mixtures. Therefore, the effects of these ligands, particularly SeMeth, on Hg speciation in the exposure mixtures could not be determined. Overall, the changes in Hg speciation in the exposure medium coincided with antagonisms in one case (Se(IV)–MeHg mixtures) but not in other cases (Se(VI)–MeHg and Se(IV)–Hg mixtures).
Understanding factors that may mitigate Hg toxicity is important to environmental and human health. Our experiments focused on Se and utilized multiple Hg and Se compounds and concentrations to assess interaction impacts on toxicity. Our results indicate that even in a simple system, the relationship between the Hg and Se is complex and that straight antagonism is not the only interaction. Our most noteworthy findings emphasize the importance of including Se compound as a part of the analysis when assessing Hg and Se interaction and de-emphasizing the weight given to the Se/Hg molar ratio.
Supplementary Material
Acknowledgments
We thank Elena Turner for assistance with the COPAS Biosort. This research was supported in part by the U.S. Department of Energy (DE-SC0006938) and the National Institute of Environmental Health Sciences (R01ES024344). S.E.D. was supported by a doctoral scholarship from the Duke Global Health Institute.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Table SI-1: internal mercury and selenium concentrations (average ng of compound/µg of protein) for nematodes exposed to selenium (50 mM selenite, 1500 µM selenite, and 500 µM selenomethionine) and mercury (HgCl2, MeHg). Table SI-2: stability constants for the mercury–selenium species considered in the equilibrium calculations. Table SI-3: expected growth (logEXT) following coexposure assuming an additive interaction between mercury and selenium compounds. Figure SI-1: nematode size (logEXT) dose–responses for individual mercury and selenium compounds. Figure SI-2: the calculated equilibrium speciation of MeHg species and selenite species for the MeHg+selenite test mixtures. Figure SI-3: equilibrium speciation of mercury in the Hg(II)-selenite mixtures and the MeHg-selenite mixtures. (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.UNEP Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. Geneva, Switzerland: UNEP Chemicals Branch; 2013. [Google Scholar]
- 2.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 3.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19(6):417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 4.Lebel J, Mergler D, Lucotte M, Amorim M, Dolbec J, Miranda D, Arantes G, Rheault I, Pichet P. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methylmercury. Neurotoxicology. 1996;17(1):157–167. [PubMed] [Google Scholar]
- 5.Drake PL, Rojas M, Reh CM, Mueller CA, Jenkins FM. Occupational exposure to airborne mercury during gold mining operations near El Callao, Venezuela. Int. Arch. Occup. Environ. Health. 2001;74(3):206–212. doi: 10.1007/s004200000206. [DOI] [PubMed] [Google Scholar]
- 6.Jarosinska D, Horvat M, Sallsten G, Mazzolai B, Dabkowska B, Prokopowicz A, Biesiada M, Barregard L. Urinary mercury and biomarkers of early renal dysfunction in environmentally and occupationally exposed adults: A three-country study. Environ. Res. 2008;108(2):224–232. doi: 10.1016/j.envres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Deng DF, Teh FC, Teh SJ. Effect of dietary methylmercury and seleno-methionine on Sacramento splittail larvae. Sci. Total Environ. 2008;407(1):197–203. doi: 10.1016/j.scitotenv.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 8.El-Demerdash FM. Effects of selenium and mercury on the enzymatic activities and lipid peroxidation in brain, liver, and blood of rats. J. Environ. Sci. Health, Part B. 2001;36(4):489–499. doi: 10.1081/PFC-100104191. [DOI] [PubMed] [Google Scholar]
- 9.Brandao R, Lara FS, Pagliosa LB, Soares FA, Rocha JBT, Nogueira CW, Farina M. Hemolytic effects of sodium selenite and mercuric chloride in human blood. Drug Chem. Toxicol. 2005;28(4):397–407. doi: 10.1080/01480540500262763. [DOI] [PubMed] [Google Scholar]
- 10.Penglase S, Hamre K, Ellingsen S. Selenium and Mercury have a synergistic negative effect on fish reproduction. Aquat. Toxicol. 2014;149:16–24. doi: 10.1016/j.aquatox.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Weber DN, Connaughton VP, Dellinger JA, Klemer D, Udvadia A, Carvan MJ. Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol. Behav. 2008;93(1–2):250–260. doi: 10.1016/j.physbeh.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luque-Garcia JL, Cabezas-Sanchez P, Anunciacao DS, Camara C. Analytical and bioanalytical approaches to unravel the selenium-mercury antagonism: A review. Anal. Chim. Acta. 2013;801:1–13. doi: 10.1016/j.aca.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Cuvinaralar MLA, Furness RW. Mercury and Selenium Interaction - A Review. Ecotoxicol. Environ. Saf. 1991;21(3):348–364. doi: 10.1016/0147-6513(91)90074-y. [DOI] [PubMed] [Google Scholar]
- 14.Ralston NVC, Blackwell JL, Raymond LJ. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007;119(3):255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- 15.Sormo EG, Ciesielski TM, Overjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM. Selenium Moderates Mercury Toxicity in Free-Ranging Freshwater Fish. Environ. Sci. Technol. 2011;45(15):6561–6566. doi: 10.1021/es200478b. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura Y, Tamai Y, Tanaka H. Selenium Protection Against Mercury Toxicity - High Binding Affinity of Methylmercury by Selenium-Containing Ligands in Comparison with Sulfur-Containing Ligands. Bioinorg. Chem. 1978;9(2):167–180. doi: 10.1016/s0006-3061(00)80288-4. [DOI] [PubMed] [Google Scholar]
- 17.Ralston NVC, Azenkeng A, Raymond LJ. Mercury-Dependent Inhibition of Selenoenzymes and Mercury Toxicity. In: Ceccatelli S, Aschner M, editors. Methylmercury and Neurotoxicity, Current Topics in Neurotoxicity 2. 2012. pp. 91–99. [Google Scholar]
- 18.Penglase S, Hamre K, Ellingsen S. Selenium prevents downregulation of antioxidant selenoprotein genes by methylmercury. Free Radical Biol. Med. 2014;75:95–104. doi: 10.1016/j.freeradbiomed.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Li W-H, Ju Y-R, Liao C-M, Liao VH-C. Assessment of selenium toxicity on the life cycle of Caenorhabditis elegans. Ecotoxicology. 2014;23(7):1245–1253. doi: 10.1007/s10646-014-1267-x. [DOI] [PubMed] [Google Scholar]
- 20.McElwee MK, Freedman JH. Comparative toxicology of mercurials in Caenorhabditis elegans. Environ. Toxicol. Chem. 2011;30(9):2135–2141. doi: 10.1002/etc.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner EA, Kroeger GL, Arnold MC, Thornton BL, Di Giulio RT, Meyer JN. Assessing Different Mechanisms of Toxicity in Mountaintop Removal/Valley Fill Coal Mining-Affected Watershed Samples Using Caenorhabditis elegans. PLoS One. 2013;8(9):e75329. doi: 10.1371/journal.pone.0075329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda Y, Honda S. Life span extensions associated with upregulation of gene expression of antioxidant enzymes in Caenorhabditis elegans, studies of mutation in the age-1, PI3 kinase homologue and short-term exposure to hyperoxia. Journal of the American Aging Association. 2002;25(1):21–28. doi: 10.1007/s11357-002-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jee C, Vanoaica L, Lee J, Park BJ, Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes to Cells. 2005;10(12):1203–1210. doi: 10.1111/j.1365-2443.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson WM, Yao C, Siedlak SL, Wang WZ, Zhu XW, Caldwell GA, Wilson-Delfosse AL, Mieyal JJ, Chen SG. Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson’s disease. Hum. Mol. Genet. 2015;24(5):1322–1335. doi: 10.1093/hmg/ddu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto T, Maebayashi K, Nakagawa Y, Imai H. Deletion of the four phospholipid hydroperoxide glutathione peroxidase genes accelerates aging in Caenorhabditis elegans. Genes to Cells. 2014;19(10):778–792. doi: 10.1111/gtc.12175. [DOI] [PubMed] [Google Scholar]
- 26.Buettner C, Harney JW, Berry MJ. The Caenorhabditis elegans homologue of thioredoxin reductase contains a selenocysteine insertion sequence (SECIS) element that differs from mammalian SECIS elements but directs selenocysteine incorporation. J. Biol. Chem. 1999;274(31):21598–21602. doi: 10.1074/jbc.274.31.21598. [DOI] [PubMed] [Google Scholar]
- 27.Gladyshev VN, Krause M, Xu XM, Korotkov KV, Kryukov GV, Sun QA, Lee BJ, Wootton JC, Hatfield DL. Selenocysteine-containing thioredoxin reductase in C-elegans. Biochem. Biophys. Res. Commun. 1999;259(2):244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- 28.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 29.Boehler CJ, Raines AM, Sunde RA. Deletion of Thioredoxin Reductase and Effects of Selenite and Selenate Toxicity in Caenorhabditis elegans. PLoS One. 2013;8(8):1–8. doi: 10.1371/journal.pone.0071525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li WH, Hsu FL, Liu JT, Liao VHC. The ameliorative and toxic effects of selenite on Caenorhabditis elegans. Food Chem. Toxicol. 2011;49(4):812–819. doi: 10.1016/j.fct.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Stefanello ST, Gubert P, Puntel B, Mizdal CR, de Campos MM, Salman SM, Dornelles L, Avila DS, Aschner M, Soares FA. Protective effects of novel organic selenium compounds against oxidative stress in the nematode. Toxicology reports. 2015;2:961–967. doi: 10.1016/j.toxrep.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W-H, Shi Y-C, Chang C-H, Huang C-W, Liao VH-C. Selenite protects Caenorhabditis elegans from oxidative stress via DAF-16 and TRXR-1. Mol. Nutr. Food Res. 2014;58(4):863–874. doi: 10.1002/mnfr.201300404. [DOI] [PubMed] [Google Scholar]
- 33.Li WH, Shi YC, Tseng IL, Liao VH. Protective efficacy of selenite against lead-induced neurotoxicity in Caenorhabditis elegans. PLoS One. 2013;8(4):e62387. doi: 10.1371/journal.pone.0062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan KL, Estevez AO, Mueller CL, Cacho-Valadez B, Miranda-Vizuete A, Szewczyk NJ, Estevez M. The Glutaredoxin GLRX-21 Functions to Prevent Selenium-Induced Oxidative Stress in Caenorhabditis elegans. Toxicol. Sci. 2010;118(2):530–543. doi: 10.1093/toxsci/kfq273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis JA, Fleming JT. Basic culture methods. Methods Cell Biol. 1995;48(48):3–29. [PubMed] [Google Scholar]
- 36.Meyer JN, Lord CA, Yang XYY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR, Auffan M. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat. Toxicol. 2010;100(2):140–150. doi: 10.1016/j.aquatox.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Yang XY, Gondikas AP, Marinakos SM, Auffan M, Liu J, Hsu-Kim H, Meyer JN. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012;46(2):1119–1127. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- 38.Hyne RV, Hogan AC, Pablo F, Roach AC. Toxicity of selenomethionine- and seleno-contaminated sediment to the amphipod Corophium sp. Ecotoxicol. Environ. Saf. 2002;52(1):30–37. doi: 10.1006/eesa.2002.2157. [DOI] [PubMed] [Google Scholar]
- 39.Maier KJ, Foe CG, Knight AW. Comparative Toxicity of Selenate, Selenite, Seleno-DL-Methionine and Seleno-DL-Cystine to Daphnia-Magna. Environ. Toxicol. Chem. 1993;12(4):755–763. [Google Scholar]
- 40.EPA. Method 7473: Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation and Atomic Absorption Spectrophotometry. Washington, DC: EPA; 1998. [Google Scholar]
- 41.Smith RD, Martell AE. NIST Critical Stability Constants of Metal Complexes Database v. 2.0. Gaithersburg, MD: NIST; 1993. [Google Scholar]
- 42.Ayotte P, Carrier A, Ouellet N, Boiteau V, Abdous B, Sidi EAL, Chateau-Degat ML, Dewailly E. Relation between Methylmercury Exposure and Plasma Paraoxonase Activity in Inuit Adults from Nunavik. Environ. Health Perspect. 2011;119(8):1077–1083. doi: 10.1289/ehp.1003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillion M, Lemire M, Philibert A, Frenette B, Weiler HA, Deguire JR, Guimarães JRD, Larribe F, Barbosa F, Mergler D. Visual acuity in fish consumers of the Brazilian Amazon: risks and benefits from local diet. Public Health Nutrition. 2011;14(12):2236–2244. doi: 10.1017/S1368980011001765. [DOI] [PubMed] [Google Scholar]
- 44.Lemire M, Fillion M, Barbosa F, Jr, Guimarães JRD, Mergler D. Elevated levels of selenium in the typical diet of Amazonian riverside populations. Sci. Total Environ. 2010;408(19):4076–4084. doi: 10.1016/j.scitotenv.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Lemire M, Fillion M, Frenette B, Passos CJS, Guimarães JRD, Barbosa F, Mergler D. Selenium from dietary sources and motor functions in the Brazilian Amazon. NeuroToxicology. 2011;32(6):944–953. doi: 10.1016/j.neuro.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Saint-Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Despres C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. NeuroToxicology. 2006;27(4):567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Steuerwald U, Weihe P, Jorgensen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jorgensen E, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J. Pediatr. 2000;136(5):599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 48.Grotto D, Barcelos GRM, Valentini J, Antunes LMG, Angeli JPF, Garcia SC, Barbosa F. Low levels of methylmercury induce DNA damage in rats: protective effects of selenium. Arch. Toxicol. 2009;83(3):249–254. doi: 10.1007/s00204-008-0353-3. [DOI] [PubMed] [Google Scholar]
- 49.Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, Newland MC. Dietary selenium protects against selected signs of aging and methylmercury exposure. NeuroToxicology. 2010;31(2):169–179. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen PD, Sorensen MA, Walton WE, Trumble JT. Lethal and sublethal responses of an aquatic insect Culex quinquefasciatus (Diptera: Culicidae) challenged with individual and joint exposure to dissolved sodium selenate and methylmercury chloride. Environ. Toxicol. 2007;22(3):287–294. doi: 10.1002/tox.20254. [DOI] [PubMed] [Google Scholar]
- 51.Heinz GH, Hoffman DJ. Methylmercury chloride and selenomethionine interactions on health and reproduction in mallards. Environ. Toxicol. Chem. 1998;17(2):139–145. [Google Scholar]
- 52.Squadrone S, Benedetto A, Brizio P, Prearo M, Abete MC. Mercury and selenium in European catfish (Silurus glanis) from Northern Italian Rivers: Can molar ratio be a predictive factor for mercury toxicity in a top predator? Chemosphere. 2015;119:24–30. doi: 10.1016/j.chemosphere.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 53.Arribere MA, Ribeiro Guevara SR, Bubach DF, Arcagni M, Vigliano PH. Selenium and mercury in native and introduced fish species of patagonian lakes, Argentina. Biol. Trace Elem. Res. 2008;122(1):42–63. doi: 10.1007/s12011-007-8059-6. [DOI] [PubMed] [Google Scholar]
- 54.Peterson SA, Ralston NVC, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS. How Might Selenium Moderate the Toxic Effects of Mercury in Stream Fish of the Western US? Environ. Sci. Technol. 2009;43(10):3919–3925. doi: 10.1021/es803203g. [DOI] [PubMed] [Google Scholar]
- 55.Ralston NVC, Ralston CR, Blackwell JL, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. NeuroToxicology. 2008;29(5):802–811. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.