Abstract
Introduction
Infection accounts for over 40% of preterm premature rupture of the fetal membranes (PPROM), a major cause of preterm birth. Toll-like receptors (TLR) play key roles in pathogen surveillance but their expression and function in amnion mesenchymal cells (AMC) is unclear. The aims of this study were to determine the expression of all TLR isoforms and the effect of macrophage-activating lipoprotein-2 (MALP-2), derived from a common pathogen involved in PPROM, on human AMC.
Methods
AMC were isolated from normal, term, amnion from repeat caesarean section. Semi-quantitative RT-PCR, immunocytochemistry, immunohistochemistry and western blotting were used to detect TLR isoform expression. Immunocytochemistry of NF-κB p65, pro-inflammatory cytokine secretion (ELISA), MTT assay, LDH assay, immunoblotting of cytosolic cytochrome c and cleaved caspase-3, and expression of 84 microRNAs by Qiagen miRNA PCR array were used to determine the functional effect of MALP-2 on AMC.
Results
TLR1-10 was detected in AMC, and protein expression of TLR2, 4, and 6 were confirmed. MALP-2 induced nuclear translocation of p65, reaching significance after 45 minutes (ANOVA, P < 0.05). MALP-2 did not cause apoptosis but did lead to significant secretion of IL-4, IL-6, and IL-8 (P < 0.05, 0.01, 0.001, respectively) and significant changes in miRNA-320a and miRNA-18a (P < 0.05).
Discussion
These results suggest that AMC elicit a pro-inflammatory response following stimulation with the known TLR2/6 ligand MALP-2. This data supports the idea that AMC express the innate immune system receptors that could help with immune surveillance during infection and contribute to inflammatory responses that lead to PPROM.
Keywords: toll-like receptor (TLR), macrophage-activating lipoprotein-2 (MALP-2), pro-infllammatory cytokine, nuclear factor kappa B (NF-κB), microRNA, amnion mesenchymal cells
1. Introduction
Preterm birth (PTB) is a major obstetric problem in the United States, constituting nearly 12% of births annually [1]. Despite significant medical advances and a recent slowly declining rate, the U.S. still has a higher rate of PTB than other developed countries [2], such as Canada, Germany, and the United Kingdom. It is also the leading cause of perinatal morbidity and mortality [3], creating extensive financial and emotional burdens on the families of preterm infants as complications arising from prematurity can extend into adulthood [2]. Preterm premature rupture of the membranes (PPROM) is one of the known causes of PTB, accounting for more than one-third of pregnancies ending before 37 weeks gestation [4,5]. Currently, there is no prevention or treatment for PPROM to interrupt the delivery of the fetus. While the precise biological and pathophysiological mechanisms of PPROM are still unknown, nearly 40% of these pregnancies present with evidence of intrauterine infection and inflammation [6–8]. Amongst bacterial-associated intrauterine infections, mycoplasmas are one of the common pathogens shown to cause severe inflammation that contribute to infection-driven PPROM [9,10].
The Toll-like receptor (TLR) family play a key role in the innate immune system by recognizing pathogens via conserved pathogen-associated molecular patterns (PAMPs) as well as endogenous ligands expressing danger-associated molecular patterns (DAMPs) [11,12]. Currently, there are 10 known functional isoforms expressed in humans that are able to detect both bacterial (1, 2, 4, 5, 6, 9) and viral (3, 7, 8, 9) components to elicit immune responses. Thus, each receptor isoform has been shown to detect specific ligands, which in turn trigger intracellular signaling cascades either through MyD88-dependent or - independent pathways, but ultimately lead to the activation of NF-κB and subsequent cytokine production [11]. One major difference between these signaling cascades is that MyD88-independent pathways activated by TLR3 and TLR4 also lead to the phosphorylation of IRF-3 and the production of type I interferons; responses typical of virus detection [13].
The importance of TLRs in pregnancy has been demonstrated in various reproductive tissues, such as the uterus, placenta, and the fetal membranes [14–16]. One such study has demonstrated that primary epithelial cells from the human amnion (AEC) express all of the ten TLR transcripts and that several of the detected isoforms were functional [17]. Upon treatment with known TLR ligands, the AEC mounted different immune responses, suggesting that the activation of TLR signaling pathways may contribute to PTB through inflammation and apoptosis. This was one of the first studies that proposed a role for inflammation stemming from the amnion in PPROM. However, the role of TLR in amnion mesenchymal cells (AMC) has not been fully investigated. One of the few studies of TLR, focused mainly on TLR4 from amnion mesenchymal cells demonstrating its expression and functionality in response to fetal fibronectin and therefore suggested that TLR signaling activation could play a role in the pathogenesis of PTB and PPROM [18]. This data also suggested that the mesenchymal layer of the amnion may contain other innate immune receptors; however, a functional screen of TLR isoform expression in this layer has not been performed. Therefore, the extent of AMC contribution to the stimulation of inflammatory responses in the presence of invading pathogens is not understood.
The purpose of this study was to (1) examine if primary AMC could play a role in microbial defense within the amnion and (2) provide evidence that pathogen-mediated inflammatory responses within these cells have the potential to contribute to the weakening and rupture of the fetal membranes associated with PPROM. Thus, the aims of this work were to characterize the expression profile of the members of the TLR family in primary human AMC and to determine if specific TLR isoforms (TLR2 and 6) are functional within these mesenchymal cells, capable of causing apoptosis and/or inflammation, using a known TLR2/6 agonist, macrophage-activating lipoprotein-2 (MALP-2) originally isolated from Mycoplasma fermentans.
2. Materials and Methods
2.1. Tissue collection and primary amnion cell culture
Fetal membranes were collected immediately following singleton, repeat Cesarean section, before labor (≥ 38 weeks gestation) at Kapi‛olani Medical Center for Women and Children (Honolulu, HI, USA) with approval from the Institutional Review Board (Hawaii Pacific Health). The reflected fetal membranes were removed 1 inch from the placenta and transported back to the laboratory in sterile phosphate buffered saline (PBS) within 30 minutes. Primary AMC and AEC were isolated as previously described [19,20]. Briefly, the amnion was stripped from the underlying choriodecidua and the epithelial cells were isolated by four consecutive trypsin (0.2%) digestions (Roche, Indianapolis, IN). The tissue was incubated further with collagenase A (3.3 mg/mL) and DNase I (3.3 mg/mL) (Roche, Indianapolis, IN) for 60 minutes at 37°C to release the remaining mesenchymal cells. Cells were cultured in DMEM/F-12 media containing 10% fetal bovine serum (FBS) (Corning Life Sciences, Tewksbury, MA), penicillin (100 U/mL), streptomycin (100 µg/mL) and incubated at 37°C in 95% air/5% CO2. The media was replaced every 2 – 3 days. Epithelial cells were utilized without passage upon reaching confluence, while mesenchymal cells were passaged up to 3 – 4 times before being utilized.
2.2. Semi-quantitative real-time RT-PCR
Transcript gene expressions of TLR1-10 were determined in cultured AMC and AEC. Total RNA was isolated using the Qiagen RNeasy Mini Kit and RNAse-free DNAse (Qiagen, Valencia, CA) according to manufacturer’s instructions. Reverse transcription was performed with the High Capacity RNA-to-cDNA kit and RT-PCR reactions were executed with TaqMan Gene Expression Assays for human TLR1-10 (Applied Biosystems, Grand Island, NY). The ABI standard protocol was used as follows: one cycle of 50°C for 2 minute s and 95°C for 10 minutes and 40 cycles of 95°C for 15 sec and 60°C for 1 min utes. Each 96-well plate contained a reverse transcriptase blank, water blank, and no cDNA template control. Real-time PCR was carried out on an Applied Biosystems StepOne Real-Time PCR System. Relative standard curves were generated using serial-diluted cDNA from placental tissue. Each patient sample (n = 4) was run in triplicate and the results were normalized to the expression of 18S in each sample.
2.3. Immunohistochemistry
Fetal membrane tissue sections (5 µm) were fixed in formaldehyde, embedded in paraffin and mounted on +EPIC+ charged microscope slides (Epic Scientific, Tualatin, OR), then deparaffinized and hydrated with deionized water. Antigen unmasking was performed in a steamer (>80°C) with 1 0 mM citric acid buffer, pH 6.0 for 20 minutes. Sections were treated with 0.3% hydrogen peroxide in methanol for 30 minutes to block endogenous peroxidase activity, followed by blocking with normal horse or goat serum (1.5%) from the Vectastain ABC kits (Vector Laboratories, Burlingham, CA) for 30 minutes, then incubated with TLR2 (mouse, 1:100, Millipore, Billerica, MA), TLR4 or TLR6 (mouse and goat, respectively, 1:100, Santa Cruz Biotechnology, Dallas, TX) antibodies for 30 minutes at room temperature. Adjacent tissue sections served as IgG (mouse or goat, same concentration as primary antibody) and buffer-only controls. Sections were rinsed three times in 1× phosphate buffered saline (PBS) (9 minutes total), incubated with a biotinylated secondary antibody for 30 minutes, rinsed three times in 1× PBS (9 minutes total), and treated with avidin-biotin-peroxide complex (ABC) for 30 minutes and diaminobenzidine. Sections were counterstained with hematoxylin, dehydrated and coverslips mounted with Permount (Fisher Chemicals, Pittsburgh, PA).
2.4. Immunocytochemistry
Primary isolated AMC (6.25 × 104 cells/well) and AEC (1.25 × 105 cells/well) were seeded into 4-well chamber slides pre-coated with poly-L-lysine, grown to 60% confluency before fixation with 4% paraformaldehyde in 1× PBS for 15 minutes, followed by two washes of 1× PBS. Non-specific binding was blocked with 10% normal goat serum or 5% normal rabbit serum (Jackson ImmunoResearch, West Grove, PA) for 30 minutes. Cells were incubated with TLR2 (1:25, Abcam, Cambridge, MA), TLR4 (1:25, R&D Systems, Minneapolis, MN), or TLR6 (1:25, Santa Cruz Biotechnology, Dallas, TX) for 60 minutes at room temperature, washed three times with 1× PBS (15 minutes total), then incubated with secondary antibody (Alexa Fluor, Grand Island, NY) for 60 minutes. Cells were rinsed three times with 1× PBS (15 minutes total) and DAPI (1:5000, Calbiochem, Billerica, MA) was used as a nuclear indicator. Slides were mounted with ClearMount with Tris buffer (Electron Microscopy Sciences, Hatfield, PA). Expression was visualized by fluorescence microscopy (Nikon C1 Plus Ti Eclipse epi-fluorescence).
Functionality studies of TLR2 and TLR6 were performed by treating AMC with macrophage-activating lipoprotein-2 (MALP-2, Santa Cruz Biotechnology, Dallas, TX) at different intervals up to 2 hours and the nuclear translocation of NF-κB p65 was measured. Isolated AMC were seeded into chamber slides and allowed to adhere overnight, then media was changed to DMEM/F-12 media with 0.5% FBS. The next day, AMC were treated with MALP-2 (1, 10, 100 ng/mL) at intervals up to 2 hours. After treatment, the cells were fixed with 4% paraformaldehyde and immunocytochemistry for NF-κB p65 (1:100, Upstate, Billerica, MA) was performed in the same manner, except prior to blocking with 1% bovine serum albumin, the fixed cells were permeabilized with 0.25% Triton X-100 (EMD Chemicals, Gibbstown, NJ) for 10 minutes, followed by three 1× PBS washes (15 minutes total).
2.5. Western blotting
Isolated AMC and AEC (n = 4) were allowed to reach ~70% confluency in culture before harvest with modified RIPA buffer (50 mM Tris, pH 7.4, 1% NP-40, 0.2% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM sodium orthovanadate, 1 mM NaF and Roche complete mini EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN)). Amnion tissue was prepared into lysates with 1:3 (weight: volume) modified RIPA buffer. For MALP-2 treated AMC, cells were seeded at 100,000 cells/6 cm dish (5 mL) and allowed to grow for 72 hours before being switched to DMEM/F-12 media with 0.5% FBS overnight. The following day, cells were treated with 100 ng/mL MALP-2 at intervals up to 2 hours. Lysates for cytochrome c western blots were spun down at 20,000 × g for cytosol samples. The Pierce BCA Protein Assay Kit (Thermo Scientific, Grand Island, NY) was used to determine protein concentrations, as per manufacturer’s instructions.
Cell lysates were denatured at 95°C for 5 minutes in 5× SDS Laemmli buffer with 5% β-mercaptoethanol and resolved on 10% (5 µg, TLR) or 18% (5 µg, cytochrome c and caspase-3) SDS-PAGE gel electrophoresis, transferred to nitrocellulose or PDVF (for caspase-3) membranes, and blocked overnight at 4°C in 5% milk, 2% fish skin gelatin, or 2% bovine serum albumin in 1× PBS with 0.1% Tween-20 (PBS-T). Membranes were incubated with primary antibody in blocking buffer for 2 hours. Antibodies used were TLR2 (1:250, Santa Cruz Biotechnology, Dallas, TX), TLR4 (1:500, Santa Cruz Biotechnology, Dallas, TX) and TLR6 (1:1000, Abcam, Cambridge, MA), cytochrome c (1:1000, R&D Systems, Minneapolis, MN) and caspase-3 (1:400, R&D Systems, Minneapolis, MN). After washing with PBS-T, membranes were incubated with secondary antibody conjugated to horseradish-peroxidase for 60 minutes before being washed with PBS-T, developed with enhanced chemiluminescence (Amersham, Piscataway, NJ) and exposed to hyperfilm-enhanced chemiluminescence (Amersham, Piscataway, NJ). Rat basophilic leukemia (RBL) cells (ATCC, Manassas, VA) were used as a positive control for TLR expression in Western blots [21,22].
Membranes were stripped (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) for 30 minutes at 50°C with gentle agitation, washed twice with 1× PBS (20 minutes total), and blocked overnight. Membranes were reprobed with loading control β-actin (1:500,000, Abcam, Cambridge, MA). Films were scanned, densitometry performed using ImageJ software (National Institute of Health, Bethesda, MD) and bands normalized to loading controls.
2.6. MTT Assay
Cell viability of MALP-2 treated (100 ng/mL) AMC was assessed by MTT Assay [23]. Briefly, cells were seeded at 500,000 cells/well in 24-well plates and allowed to grow for 72 hours before being switched to DMEM/F-12 media with 0.5% FBS overnight. The following day, cells were treated with 100 ng/mL MALP-2 at intervals up to 72 hours. At the appropriate time point, conditioned media was removed and incubated with 500 µg/mL MTT reagent (Millipore, Billerica, MA) dissolved in DMEM/F-12 media with 0.5% FBS for 4 hours at 37°C, followed by addition of 0.04 N HCl in isopropanol. Absorbance was read within 30 minutes at a test wavelength of 570 nm and reference wavelength of 630 nm. Cell viability was calculated as a percent of untreated AMC cells for each experiment (n = 4).
2.7. Lactate Dehydrogenase (LDH) Assay
Viability of AMC was also evaluated by LDH assay as described by Zewe and Fromm [24]. Conditioned media from MALP-2 treated AMC were added to 96-well plates, in addition to 0.2 M Tris-HCl, pH 7.3, 6.6 mM NADH, and 30 mM sodium pyruvate. The change in absorbance was monitored at 340 nm every 15 seconds for 10 minutes. Rates were transformed to OD/minutes/mg protein.
2.8. Pro-inflammatory cytokine levels
The levels of pro-inflammatory cytokines (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17α, IFN-γ, TNF-α, GM-CSF) were assessed in conditioned media from AMC treated with MALP-2 (100 ng/mL) for 24 hours using the Human Inflammatory Cytokines Multi-Analyte ELISArray (Qiagen, Valencia, CA), as per manufacturer’s instructions. The absorbance reading from the negative control for each cytokine was subtracted from sample absorbance readings to remove background. The resulting data was analyzed as percent fold-change between paired untreated and treated AMC.
2.9. Human miFinder Microarray
The expression of 84 common microRNAs (miRNA) was determined in untreated and MALP-2-treated AMC (100 ng/mL, 24 hours) with the miScript Human miFinder miRNA PCR Array (Qiagen, Valencia, CA). Total RNA, containing miRNAs, was isolated using the miRNeasy Micro Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. Reverse transcription was performed with the miScript II RT kit, and cDNA was immediately used for RT-PCR with miScript SYBR Green PCR kit used with miScript miRNA PCR Array (Qiagen, Valencia, CA). The real-time PCR program was used as per the manufacturer’s instruction: initial activation step of 95°C for 15 minutes and 40 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 70°C for 30 seconds was performed on an Applied Biosystems StepOne Real-Time PCR System. Analysis was also performed as recommended by manufacturer’s manual. Briefly, changes in Ct values (ΔCt) for each miRNA was generated by subtracting the average of five internal controls for each group within each plate from the raw Ct value. SNORD96A was excluded as an internal control due to low Ct value in one patient group. Log2 expression changes were calculated as ΔΔCt (DDCt) = delta Ct (MALP-2) − ΔCt (untreated) for each miRNA. Fold-change for each miRNA was calculated as 2(−DDCt). For statistical analysis, Ct values for undetected sample values (but were detected in their paired sample) were given the maximum cycle number value of Ct = 40, which is a conservative estimate of their expression level. Five independent experiments were run for five patient sample sets (MALP-2 treated and untreated).
2.10. Statistical Analysis
Statistical analysis was performed using Prism 5.0 (GraphPad Software, Inc., San Diego, CA) with statistical significance set at α = 0.05. Data groups for RT-PCR were compared by multiple unpaired t-tests. Statistics for the densitometry of NF-κB translocation was performed using the one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison post-hoc test. Ratio paired t-tests were used for statistical analysis of pro-inflammatory cytokines. Microarray data was calculated as mentioned and paired t-tests were performed to determine statistical differences.
3. Results
3.1. TLR1-10 isoforms are present in AMC and AEC
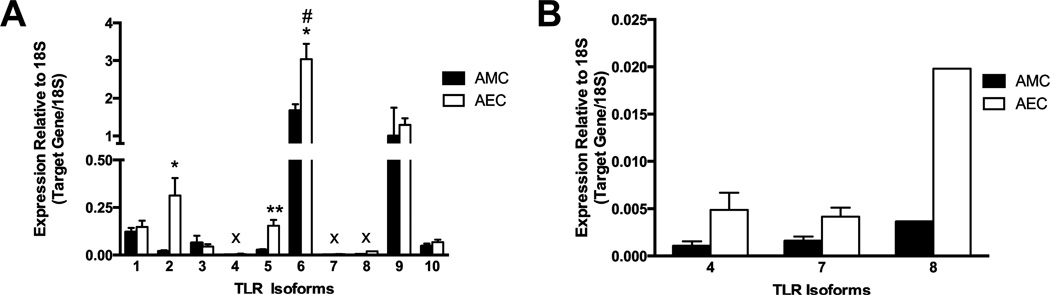
As a comprehensive characterization of the different TLRs expressed in AMC has not been performed previously, the gene expressions of TLR1-10 were assessed. Relative expression levels were investigated in AMC isolated from human amnion and compared to that seen in AEC. All of the ten TLR isoforms were detected in AMC (Figure 1). Overall, AEC had higher expression levels of most of the TLR isoforms compared to AMC, with the expressions of TLR2 (0.31 ± 0.09 vs. 0.02 ± 0.01, P < 0.05), TLR5 (0.15 ± 0.03 vs. 0.03 ± 0.01, P < 0.01), and TLR6 (3.04 ± 0.41 vs. 1.67 ± 0.67, P < 0.05) significantly greater in AEC. However, TLR3 expression was greater in AMC than in AEC (0.06 ± 0.04 vs. 0.04 ± 0.01, respectively). Interestingly, TLR6 and TLR9 were expressed at the highest levels in both amnion cell types (Figure 1A). Within AMC, TLR6 was expressed at significantly higher levels than all other TLR isoforms (P < 0.01). The expressions of TLR4, 7, and 8 were very low (Figure 1B), with TLR8 expression detected at very low levels in only one out of four patient cell samples.
Figure 1. TLR1-10 are expressed in cultured human AMC and AEC.
Expression of all TLR isoforms was detected in AMC and AEC (A), with low expression of TLR4, 7 and 8, as displayed larger in (B) and denoted with the x. Data shown represents data from n = 4 different patients’ AMC and AEC presented as mean ± SEM and normalized to 18S. # signifies the significant expression in TLR6 compared to all other TLR isoforms in AMC (P <0.05). * P < 0.05, ** P < 0.01 signifies the significance of individual TLR isoform expression level in AEC compared to the same isoform in AMC.
3.2. TLR2, 4, and 6 proteins are present in human amnion, AMC, and AEC
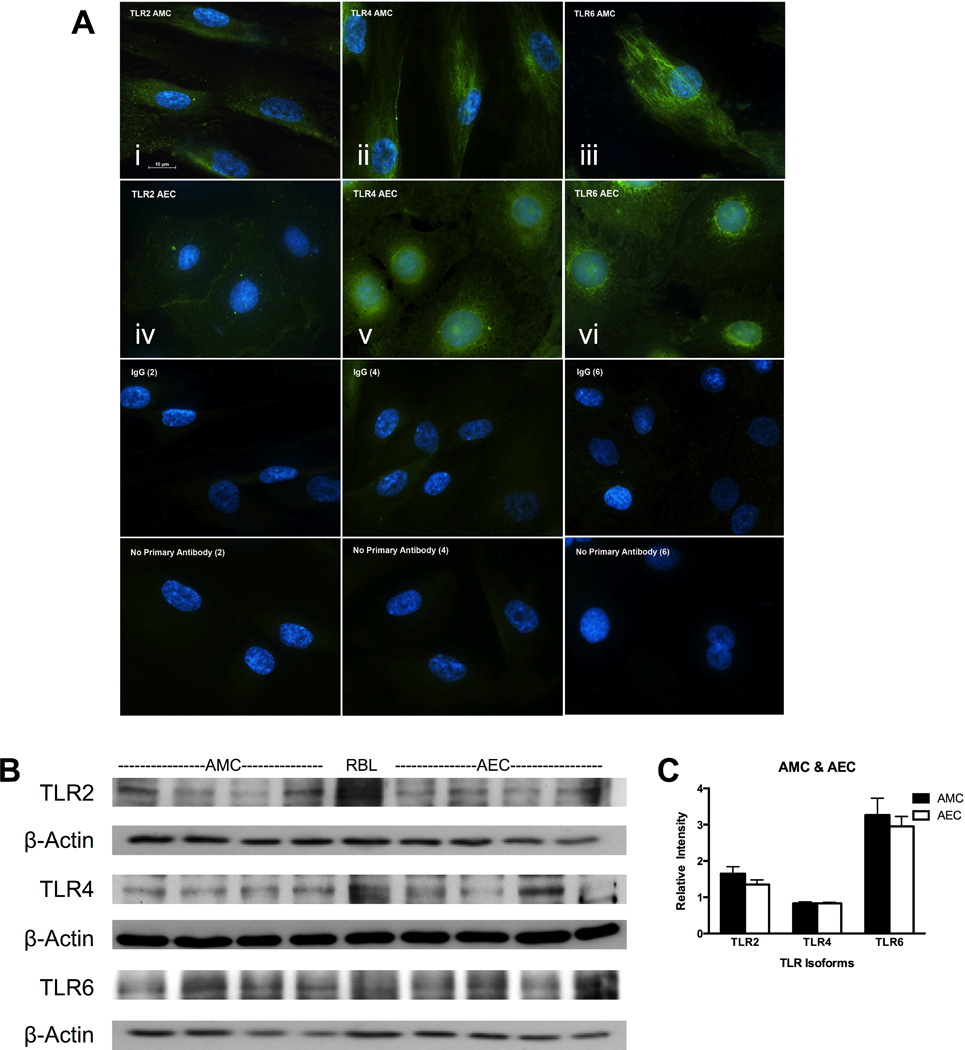
In order to investigate bacterial TLR signaling in AMC, TLR2, 4, and 6 were chosen for further study. This was determined by detection of the high expression level of TLR6 measured in AMC (Figure 1), the well-known effects of TLR4 in other cells, and TLR2’s function as the heterodimer partner of TLR6 [11]. Thus the ex vivo expression of TLR2, 4, and 6 was demonstrated in fetal membrane sections by immunohistochemistry (Figure 2A). TLR2 expression was localized to the apical surface of AEC (2Ai) but was not easily detectable in AMC, which reflects the low mRNA expression observed in Figure 1. TLR4 (2Aii) displayed apical expression on AEC while only faint labeling was seen in AMC. The expression of TLR6 (2Aiii) was strongly seen at the apical surface of AEC and dispersed throughout AMC. As expression by IHC was qualitative, TLR expression for the three isoforms within amnion cell lysates, comprised of both AEC and AMC, was confirmed by Western blotting (Figure 2B).
Figure 2. The ex vivo expression of TLR2, 4, and 6 was detected in human fetal membranes.
(A) Labeling for TLR 2 (i), 4 (ii), and 6 (iii) was observed along the apical surface of AEC with minor staining of AMC. Arrows point to brown immunolabeling in AMC and AEC. IgG (iv – vi) and no primary antibody controls (vii – ix) are shown for each antibody. AMC = amnion mesenchymal cells, AEC = amnion epithelial cells, CD = choriodecidua. Images were taken at a magnification of 50× and are representative of results seen in n = 4 patients. Western blotting (B) for TLR2, 4, and 6 was performed from amnion layer lysate samples. Each well represents a sample from an individual patient (n = 4) with RBL cells as positive control. (C) Quantitation is shown by relative intensities of expression bands normalized to β-actin.
Clear expression of the TLR 2, 4 and 6 isoforms was detected by immunohistochemistry (Figure 2), therefore protein re-expression was confirmed when amnion cells were grown in culture. It was thought that the expression of these receptors may be altered when the cells were isolated from tissues, and subsequently moved from in vivo to in vitro conditions. However, re-expression of all three TLR isoforms (2, 4 and 6) was detected by immunocytochemistry on both cultured AMC (Figure 3A i –iii) and AEC (Figure 3A iv–vi). Western blotting (Figure 3B and 3C) further confirmed the expression of the TLR isoforms in cultured AMC and AEC patient samples.
Figure 3. In vitro expression of TLR2, 4, and 6 was observed in cultured human AMC and AEC.
(A) Immunocytochemistry of TLR2 (i, iv), TLR4 (ii, v) and TLR6 (iii, vi) on AMC (i – iii) and AEC (iv – vi) was observed on the plasma membrane surface. IgG (vii – ix) and no primary antibody (x – xii) controls are shown for each antibody. Cells were imaged at a magnification of 100× are representative of results seen in n = 4 patients. (B) Western blotting for TLR2, 4, and 6 was performed from AMC and AEC lysate samples. Each well represents a sample from an individual patient (n = 4) with RBL cells as positive control. (C) Quantitation is shown by relative intensities of expression bands normalized to β-actin.
3.3. MALP-2 causes activation of NF-κB in AMC
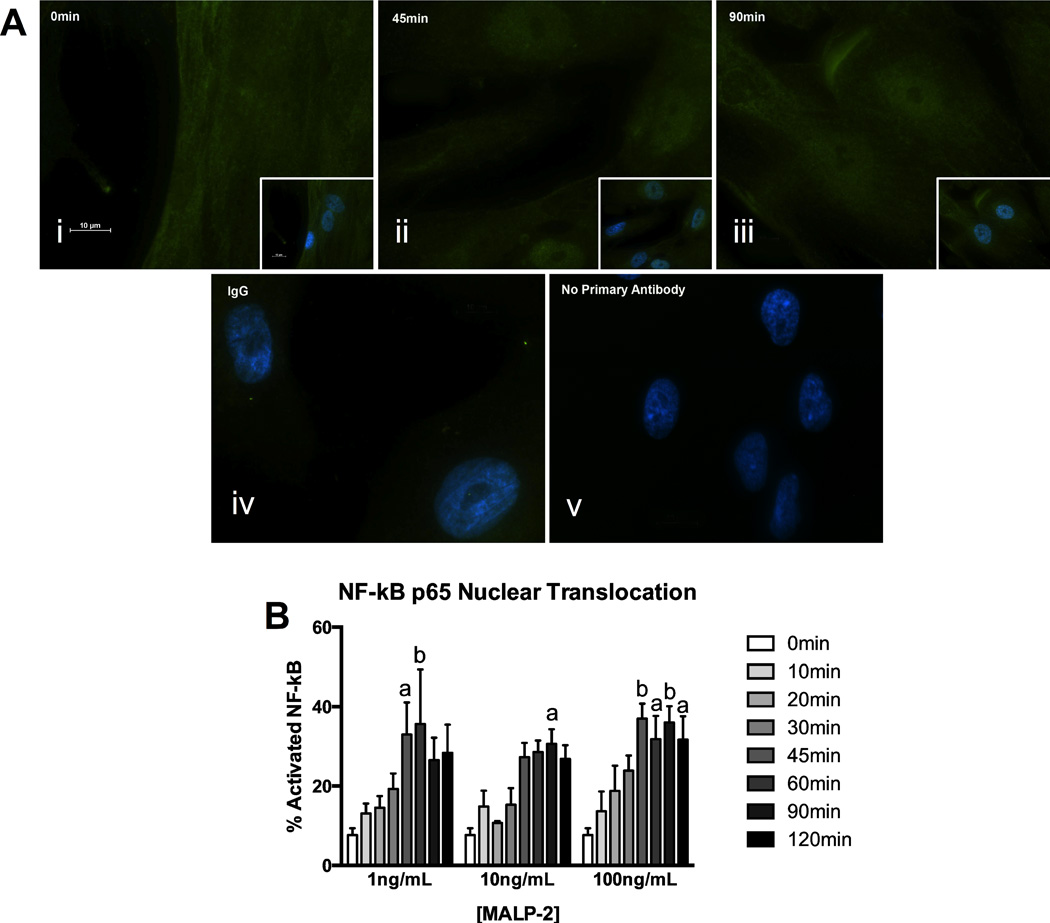
Given that the gene expression data showed that TLR6 was high in AMC (Figure 1) and it is known to have an important role in bacterial detection, functionality of this TLR isoform and its heterodimer partner, TLR2, were further investigated in isolated AMC. A known TLR2/6 agonist, macrophage-activating ligand-2 (MALP-2) was used to activate these receptors and determine if they were able to activate the pro-inflammatory transcription factor NF-κB. AMC were treated with MALP-2 (1, 10, 100 ng/mL) at intervals up 2 hours and nuclear translocation of NF-κB p65 subunit was measured by immunofluorescence (Figure 4). Some of the p65 subunits were seen to translocate to the nucleus of AMC after 10 minutes and this movement reached a peak after 45 minutes. Thus, the percentage of cells with translocation was significantly higher after 45 minutes compared to the untreated control (0 minute time point) after treatment with MALP-2 at either 1 ng/mL or 100 ng/mL (32.96% ± 8.10, P < 0.05; 36.95% ± 3.84, P < 0.01, respectively). After 45 minutes, the nuclear translocation of the p65 subunit was seen to remain high up to the 2 hours investigated. Activation of one of the upstream NF-κB-activating MAPK pathways was also investigated via Western blotting of both the total and phosphorylated forms of ERK1/2. However, no significant changes were detected (data not shown).
Figure 4. The TLR2/6 agonist, MALP-2, activates NF-κB in AMC.
(A) NF-κB p65 was localized to the cytoplasm at time 0 minute (i), p65 subunit nuclear translocation peaked at 45 minutes (ii) and could be seen in the nucleus up to 90 minutes (iii) in MALP-2 treated AMC (100 ng/mL shown). Inlays in panels i – iii show DAPI nuclear staining merged with NF-κB p65 fluorescence. IgG and no primary antibody controls are shown in panels iv and v. Images are representative of cells from n = 4 individual patients, and were taken at a magnification of 100×. (B) Quantification of activated NF-κB p65 was determined by the number of cells demonstrating p65 subunit nuclear translocation divided by total number of cells counted times 100%. The data is representative of n = 4 for each concentration of MALP-2 and presented as mean ± SEM. a: P < 0.05, b: P < 0.01 compared to time 0 minute control.
3.4. MALP-2 does not cause apoptosis in AMC
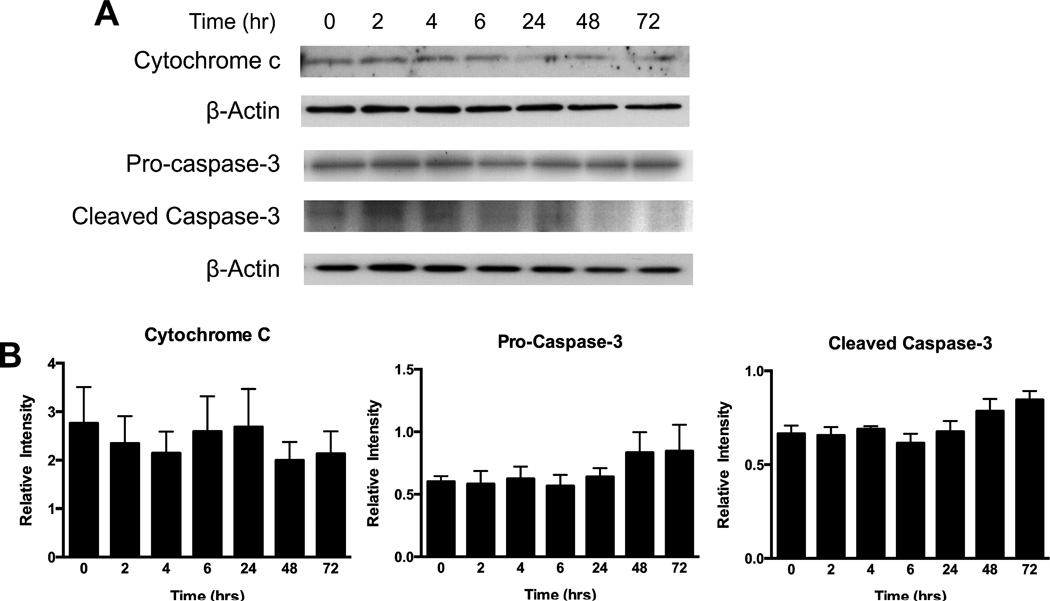
As the data demonstrated that TLR2 and 6 are present and functional in AMC (Figures 1–4), the biological role of these receptors in the cells after MALP-2 stimulation was investigated. TLRs have been shown to cause apoptosis in other cell types [11,15], therefore the protein expression of cytosolic cytochrome c and cleaved caspase-3 were measured by Western blotting but were found to be not significantly altered by MALP-2 (100 ng/mL) treatment up to 72 hours (Figure 5). This also did not lead to a reduction in AMC cell viability, as tested by MTT or LDH assays (data not shown). This data suggests that MALP-2 does not cause cell death in AMC, up to the 72 hours tested.
Figure 5. MALP-2 did not induce apoptosis in AMC.
(A) No significant cell death was measured by western blotting following MALP-2 (100ng/ml) up to 72 hours for cytochrome c, pro-caspase 3, and cleaved caspase-3 and (B) quantitation of the western blotting for cytochrome c and caspase-3 (pro- and cleaved) are shown (n = 4), performed on cell lysates from AMC and normalized to β-actin.
3.5. MALP-2 induces inflammatory cytokine production by AMC
The ability of MALP-2 to induce inflammation in AMC was also examined. Cells were treated with MALP-2 (100 ng/mL) for 24 hours and the conditioned media was collected and assessed for inflammatory cytokine secretion (Figure 6A). IL-6, IL-8 and GM-CSF exhibited greater secretion due to MALP-2 treatment compared to untreated AMC (fold-change compared to untreated control; 21.25 ± 7.79, 33.27 ± 9.48, 7.88 ± 6.24, respectively). This increase was significant for IL-6 and IL-8 (P = 0.01, 0.001, respectively). Interestingly, the level of IL-4 secretion was also significant in treated AMC compared to untreated, despite a modest fold-change (1.21 ± 0.05, P = 0.02).
Figure 6. MALP-2 treatment led to increased cytokine secretion and alterations in miRNA expression in AMC.
(A) MALP-2 treated AMC caused significant increases in secretion of IL-4, IL-6 and IL-8 compared to untreated AMC, with increases in GM-CSF that were not significant. IL = interleukin, IFN = interferon, GM-CSF = granulocyte-macrophage colony-stimulating factor. Fold-changes greater than 0 are viewed as an increase in secretion compared to untreated AMC. (B) Of the 84 miRNAs probed, miR-320a was found to be significantly up-regulated (fold-change (FC) > 2.0, P < 0.05) in MALP-2 treated AMC compared to untreated. MiR-18a-5p expression was significantly reduced by 30% in treated cells (P <0.05). Although not significant, 13 other miRNAs were differentially expressed following MALP-2 treatment. FC > 2.0 was viewed as an up-regulation and as a down-regulation if 0 < FC < 1.0. (C) FC of miRNA expression patterns for all 84 miRNA in response to MALP-2 treatment in AMC are presented in the heat map from n = 5 separate patient experiments. * P < 0.05, *** P < 0.001.
3.6. MALP-2 alters miRNA expression in AMC
To further understand if and how MALP-2 can affect TLR2/6 signaling and lead to increased inflammation in AMC, the expression of 84 common miRNAs were assessed following 24-hour treatment of MALP-2 (100 ng/mL). Of the miRNAs investigated, 68 miRNAs (81%) were detected in all treated and untreated AMC samples. A total of 14 miRNAs were differentially expressed in the MALP-2 treated group (Figure 6B), with fold-changes greater than two for 12 of the miRNAs (320a, let-7e, let-7d, 155, 423, let-7c, 30c, 124, 150, let-7b, 223, 32). MiR-320a was found to be significantly up-regulated and miR18a significantly reduced in MALP-2 treated cells (fold-change: 2.74 ± 0.68, 0.71 ± 0.08, respectively, P < 0.05). An 81% down-regulation of miR-22 was observed in treated AMC, although not significant (fold-change: 0.19, P = 0.07). Interestingly, miR-144 was expressed at very low levels (Ct > 33) or not detectable in AMC samples. Expression patterns of the 84 miRNAs demonstrating fold-changes between MALP-2 treated and untreated AMC from five independent patient sample sets are displayed in the heat map (Figure 6C).
4. Discussion
The ability of TLRs to activate pro-inflammatory signaling pathways in reproductive tissues has lead to the suggestion that they are involved in several pregnancy pathologies, such as preterm labor and PPROM [14]. However, this is the first study to demonstrate the expression of the ten known TLR isoforms in primary human AMC, two of which, TLR2 and TLR6, were able to produce pro-inflammatory responses in AMC when treated with the known TLR agonist MALP-2. AMC treatment with MALP-2 led to increased cytokine secretion (Figure 6A), as well as altered expression of miRNAs (Figure 6). This is important because changes in inflammatory signaling in AMC, may help to further understanding of how AMC contribute to the weakening and rupture of the fetal membranes in infection-driven PPROM. Thus taken together, this data supports the hypothesis that in vivo AMC have innate immune receptors that are capable of initiating inflammation when exposed to an infectious stimulus and can contribute to the resultant inflammatory environment in the fetal membranes.
The mesenchymal cells of the amnion have a central role in the maintenance of the integrity of the fetal membranes as they produce the majority of the collagens that configure the extracellular matrix [3]. Although the pathophysiology is known to differ between normal rupture of the fetal membranes (ROM) and infection-driven PPROM, the pathways that contribute to the remodeling of the amnion including: extracellular matrix breakdown, cellular apoptosis, inflammation and stretch-induced physical weakening, are similar. This study provides evidence that AMC may also play a role in microbial defense of the fetal membranes. According to the traditional paradigm of ascending infection, pathogens from the reproductive tract invade in the direction of the decidua, chorion, and amnion before infiltration into the amniotic cavity. Therefore, the invading pathogens must cross the amnion mesenchymal layer before breaching the epithelium, often resulting in membrane rupture. Thus a key strength of this work is the use of primary AMC isolated from human tissue as previous studies investigating infection-driven TLR signaling have been focused on fetal membrane explants of amnion, chorion, and decidua [16,25], making it difficult to assign specific cellular responses. While AMC are not secluded in vivo, this approach allowed the demonstration of their reaction to MALP-2 independent of other cell types.
The high level of TLR 6 and 9 (Figure 1) in AMC supports the notion that these cells can participate in bacterial surveillance, which is of particular interest as several studies support the premise that bacterial invasion is the predominant cause of chorioamnionitis in PPROM [8,26,27]. This was further validated by the utilization of MALP-2 as a bacterial stimulus. Indeed mycoplasma, from which this lipoprotein is derived, is one of the more common microorganisms found in the amniotic cavity [9] that can cause intense inflammatory responses leading to PPROM [10]. In this study MALP-2 activated the canonical NF-κB pathway (Figure 4A), similarly to that previously observed in AEC [17]. This is key to drive the known mechanisms of membrane weakening as MALP-2 activated NF-κB has also been shown to increase COX-2 expression and prostaglandin production in human placental trophoblasts [28] and AEC [17].
Cytokines play important roles in normal parturition (e.g. cervical ripening and rupture of membranes (ROM)) [29,30] and lead to tissue remodeling and apoptosis [31,32], but are also produced in large amounts by the fetal membranes in the presence of bacterial products. These cytokine-driven processes are thought to be central to the mechanisms leading to ROM and may also suggest a role for PAMP recognition by the TLR family within the amnion. In this study, activation of TLR2/6 by MALP-2 induced secretion of IL-6, IL-8 and GM-CSF (Figure 6A) in AMC. Although GM-CSF was not significant it has been shown to be involved in fetal membrane weakening [33]. This is similar to the production of cytokines documented from AEC [17], suggesting that both AMC and AEC have pro-inflammatory roles in bacterial infection.
Recently, several miRNAs have gained attention as potential immunomodulators of the TLR signaling pathway [34], involved in stress responses, apoptosis, and immune activation. Studies have also provided evidence for the potential role of miRNAs in human parturition, as seen by their differential expression patterns in the chorioamnion between preterm and term labor [35] or histologic chorioamnionitis [36]. In this study, miR-320a and miR-18a, had significantly altered expression in AMC after MALP-2 treatment compared to untreated controls (Figure 6B). MiR-320a has been shown to directly target MAPK1 in human lymphocytes, and inhibit ERK activity, while up-regulating JNK activity [37]. Thus this may explain the lack of phosphorylated ERK1/2 observed in AMC treated with MALP-2 (data not shown) despite the activation of NF-κB. The significant reduction in miR-18a is notable, as several studies have reported that its over-expression induces apoptosis in colon cancer cells [38] and reduces hepatocyte expression of PIAS3, a suppressor of NF-κB signaling [39,40]. Therefore, the reduction of miR-18a may explain why MALP-2 did not cause apoptosis despite NF-κB activation and increases in the levels of pro-inflammatory cytokines (Figures 5 and 6), suggesting that in AMC miR-18a may function as a negative regulator of MALP-2/NF-κB signaling after 24 hours.
It is known that small alterations in expression level of miRNAs have considerable biological effects as they act synergistically to target multiple mRNA leading to translational repression [36]. Interestingly, several of the differentially expressed (insignificant changes) miRNAs, specifically miR-223, miR-155, miR-150, and let-7e, have been shown to target molecules involved in TLR and/or NF-κB signaling [41]. MiR-223 up-regulation also correlates with the presence of chorioamnionitis [36]. A limitation of this work is the restricted number of miRNAs investigated to understand their role in AMC. In addition, miRNAs have been shown to have temporal expression patterns [34], rapidly fluctuating, and by studying changes after 24 hours, early response miRNA will have been missed. Despite these limitations, this data confirms the importance of miRNA in TLR signaling and suggests that they could have considerable impact on the control of specific immune responses.
This is the first study to characterize TLR expression within human AMC, highlighting these receptors as contributors in microbial surveillance during infection and also suggesting a role in inflammatory responses during normal parturition following endogenous ligand activation. This work also demonstrated the ability of AMC to elicit pro-inflammatory responses in the presence of an infectious agent that may help to drive fetal membrane weakening, leading to eventual ROM. While this work was focused on infection-driven TLR signaling, it also suggests that TLR activation in AMC may play a role in normal gestation through the activation of this system by PAMPs and DAMPs. Therefore further studies investigating the importance of TLR isoforms in the normal processes of parturition are also needed.
Highlights.
Expression of TLR1-10 isoforms was confirmed in human amnion mesenchymal cells.
MALP-2 induced TLR2/6-mediated NF-κB signaling.
MALP-2 was pro-inflammatory in AMC, but not apoptotic.
MicroRNA-320a and 18a expressions in AMC were significantly altered by MALP-2.
Acknowledgments
This work is funded by the National Institutes of Health, National Institute on Minority Health and Health Disparities (P20MD000064084) and National Center for Research Resources (5P20RR016467-11) awarded to CEKW. We would like to thank Drs. John Chen and Lu Wang from the Biostatistics and Data Management Core of the John A. Burns School of Medicine, University of Hawaii for their assistance with the biostatistical analysis. We would also like to especially thank the mothers, nurses and staff at the labor and delivery ward of Kapi‛olani Medical Center for Women and Children for their help with the tissue collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010;39:1–8. [PubMed] [Google Scholar]
- 2.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, Kinney M, Lawn J. Born Too Soon: The global epidemiology of 15 million preterm births. Reprod. Health. 2013;10:S2–S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry S, Strauss JF. Premature Rupture of the Fetal Membranes. N. Engl. J. Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 4.Mercer BM. Preterm Premature Rupture of the Membranes: Current Approaches to Evaluation and Management. Obstet. Gynecol. Clin. North Am. 2005;32:411–428. doi: 10.1016/j.ogc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Mazor M. Infection and preterm labor. Clin. Obstet. Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract. Res. Clin. Obstet. Gynaecol. 2007;21:467–478. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, Tosner J, Jacobsson B. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2012;25:2014–2019. doi: 10.3109/14767058.2012.671873. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The Role of Inflammation and Infection in Preterm Birth. Semin Reprod Med. 2007;25:021–039. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh KJ, Lee KA, Sohn Y-K, Park C-W, Hong J-S, Romero R, Yoon BH. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 2010;203:211.e1–211.e8. doi: 10.1016/j.ajog.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Kaisho T, Akira S. Toll-like Receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting Edge: A Novel Toll/IL-1 Receptor Domain-Containing Adapter That Preferentially Activates the IFN-β Promoter in the Toll-Like Receptor Signaling. J. Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 14.Koga K, Mor G. Toll-Like Receptors at the Maternal–Fetal Interface in Normal Pregnancy and Pregnancy Disorders. Am. J. Reprod. Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrahams VM. Pattern Recognition at the Maternal-Fetal Interface. Immunol. Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 16.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial Modulation of Human Fetal Membrane Toll-like Receptor Expression. Am. J. Reprod. Immunol. N. Y. N 1989. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillaux C, Méhats C, Vaiman D, Cabrol D, Breuiller-Fouché M. Functional Screening of TLRs in Human Amniotic Epithelial Cells. J. Immunol. 2011;187:2766–2774. doi: 10.4049/jimmunol.1100217. [DOI] [PubMed] [Google Scholar]
- 18.Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal Fibronectin Signaling Induces Matrix Metalloproteases and Cyclooxygenase-2 (COX-2) in Amnion Cells and Preterm Birth in Mice. J. Biol. Chem. 2013;288:1953–1966. doi: 10.1074/jbc.M112.424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol. Reprod. 1996;55:1253–1260. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 20.Kendal-Wright CE, Hubbard D, Gowin-Brown J, Bryant-Greenwood GD. Stretch and inflammation-induced Pre-B cell colony-enhancing factor (PBEF/Visfatin) and Interleukin-8 in amniotic epithelial cells. Placenta. 2010;31:665–674. doi: 10.1016/j.placenta.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maaetoft-Udsen K, Greineisen WE, Aldan J, Magoay H, Ligohr C, Shimoda LM, Sung C, Turner H. Comparative Analysis of Lipotoxicity Induced by Endocrine, Pharmacological, and Innate Immune Stimuli in Rat Basophilic Leukemia Cells. J. Immunotoxicol. 2015;12:385–394. doi: 10.3109/1547691X.2014.990655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passante E, Ehrhardt C, Sheridan H, Frankish N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm. Res. 2009;58:611–618. doi: 10.1007/s00011-009-0028-4. [DOI] [PubMed] [Google Scholar]
- 23.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 24.Zewe V, Fromm HJ. Kinetic studies of rabbit muscle lactate dehydrogenase II. Mechanism of the reaction. Biochem - US. 1965;4:782–792. doi: 10.1021/bi00880a024. [DOI] [PubMed] [Google Scholar]
- 25.Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM. Human Fetal Membranes Generate Distinct Cytokine Profiles in Response to Bacterial Toll-Like Receptor and Nod-Like Receptor Agonists. Biol. Reprod. 2014;90:39, 1–9. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 2014:1–16. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn PA, Butany J, Taylor J, Hannah W. Chorioamnionitis: its association with pregnancy outcome and microbial infection. Am. J. Obstet. Gynecol. 1987;156:379–387. doi: 10.1016/0002-9378(87)90288-2. [DOI] [PubMed] [Google Scholar]
- 28.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, Terakawa N. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E2 via toll-like receptor 2 in human placental trophoblast cells. J. Reprod. Immunol. 2006;72:46–5. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the Placenta and Extra-placental Membranes: Roles and Regulation During Human Pregnancy and Parturition. Placenta. 2002;23:257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 30.Keelan JA, Blumenstein M, Helliwell RJA, Sato TA, Marvin KW, Mitchell MD. Cytokines, Prostaglandins and Parturition—A Review. Placenta. 2003;24(Supplement A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 31.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG Int. J. Obstet. Gynaecol. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 32.Flores-Herrera H, García-López G, Díaz NF, Molina-Hernández A, Osorio-Caballero M, Soriano-Becerril D, Zaga-Clavellina V. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1β, TNFα) and proMMP-9 in human fetal membranes. Placenta. 2012;33:271–277. doi: 10.1016/j.placenta.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Kumar D, Moore RM, Nash A, Springel E, Mercer BM, Philipson E, Mansour JM, Moore JJ. Decidual GM-CSF is a critical common intermediate necessary for thrombin and TNF induced in-vitro fetal membrane weakening. Placenta. 2014;35:1049–1056. doi: 10.1016/j.placenta.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 35.Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, Kim JS, Lee DC, Erez O, Gotsch F, Hassan SS, Kim CJ. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J. Pathol. 2009;217:113–121. doi: 10.1002/path.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montenegro D, Romero R, Pineles BL, Tarca AL, Kim YM, Draghici S, Kusanovic JP, Kim J-S, Erez O, Mazaki-Tovi S, Hassan S, Espinoza J, Kim CJ. Differential expression of microRNAs with progression of gestation and inflammation in the human chorioamniotic membranes. Am. J. Obstet. Gynecol. 2007;197:289.e1–289.e6. doi: 10.1016/j.ajog.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Z, Qiu S, Jiang L, Zhang A, Bao W, Liu P, Liu J. MiR-320a is downregulated in patients with myasthenia gravis and modulates inflammatory cytokines production by targeting mitogen-activated protein kinase 1. J. Clin. Immunol. 2013;33:567–576. doi: 10.1007/s10875-012-9834-5. [DOI] [PubMed] [Google Scholar]
- 38.Fujiya M, Konishi H, Mohamed Kamel MK, Ueno N, Inaba Y, Moriichi K, Tanabe H, Ikuta K, Ohtake T, Kohgo Y. microRNA-18a induces apoptosis in colon cancer cells via the autophagolysosomal degradation of oncogenic heterogeneous nuclear ribonucleoprotein A1. Oncogene. 2014;33:4847–4856. doi: 10.1038/onc.2013.429. [DOI] [PubMed] [Google Scholar]
- 39.Brock M, Trenkmann M, Gay RE, Gay S, Speich R, Huber LC. MicroRNA-18a enhances the interleukin-6-mediated production of the acute-phase proteins fibrinogen and haptoglobin in human hepatocytes. J. Biol. Chem. 2011;286:40142–40150. doi: 10.1074/jbc.M111.251793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang HD, Yoon K, Shin YJ, Kim J, Lee SY. PIAS3 Suppresses NF-κB-mediated Transcription by Interacting with the p65/RelA Subunit. J. Biol. Chem. 2004;279:24873–24880. doi: 10.1074/jbc.M313018200. [DOI] [PubMed] [Google Scholar]
- 41.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell. Mol. Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]