Abstract
The bacterial protein Rho triggers transcription termination at the ends of many operons and when transcription and translation become uncoupled. In addition to these genome-wide activities, Rho implements regulation of specific genes by dictating whether RNA polymerase terminates transcription within the 5’ leader region or continues into the downstream coding region. Here, we report that the Mg2+ channel gene corA in Salmonella enterica serovar Typhimurium, which was previously thought to be constitutively expressed, is regulated by a Rho-dependent terminator located within its 5’ leader region. We demonstrate that the unusually long and highly conserved corA leader mRNA can adopt two mutually exclusive conformations that determine whether or not Rho interacts with a Rho utilization (rut) site on the nascent RNA and thereby prevents transcription of the corA coding region. The RNA conformation that promotes Rho-dependent termination is favored by efficient translation of corL, a short open reading frame located within the corA leader. Thus, corA transcription is inversely coupled to corL translation. This mechanism resembles those governing expression of Salmonella’s other two Mg2+ transport genes, suggesting that Rho links Mg2+ uptake to translational signals.
Keywords: gene regulation, RNA secondary structure, Rho utilization (rut) site, Salmonella, short open reading frame (ORF)
Graphical Abstract
INTRODUCTION
The transcription termination factor Rho is an ATP-dependent RNA helicase that performs critical roles in bacteria. For example, Rho defines the 3’ end of many operons, suppresses antisense transcription, and implements transcriptional polarity by triggering RNA release from RNA polymerase (RNAP) when the normal coupling between transcription and translation is disrupted1; 2; 3. Whereas Rho executes these homeostatic functions genome-wide, it can also regulate expression of specific genes by controlling whether RNAP terminates transcription within a 5’ leader region or continues into the associated coding region4. In such cases, the efficiency of Rho-dependent termination is modulated by particular physiological signals. Here, we establish that the mRNA leader of the Mg2+ channel gene corA in the bacterium Salmonella enterica serovar Typhimurium harbors a Rho-dependent terminator whose efficiency is inversely correlated with translation of a short open reading frame (ORF) in the corA leader.
Rho-dependent transcription termination is a multi-step process. First, a primary binding site (PBS) located on an exposed face of the Rho hexamer must recognize a Rho utilization (rut) site on a nascent RNA5; 6. The PBS contains six clefts (one from each monomer) that can each accommodate a YC (CC or UC) dinucleotide7; 8. Correspondingly, rut sites tend to be pyrimidine-rich, at least 70 nt in length and relatively unstructured9; 10; 11. Once the PBS binds an RNA substrate, transient opening of the ring-shaped protein allows a downstream RNA segment to thread into the central channel and contact a secondary binding site8; 12. This secondary interaction activates Rho’s ATPase and helicase activities to initiate translocation along the RNA in a 5’ to 3’ direction13; 14. When a translocating Rho molecule catches up to a paused RNAP, it induces dissociation of the elongation complex2; 15.
The accessibility of rut sites within 5’ mRNA leaders can be modulated by a variety of intracellular signals including ions16, small molecule metabolites16, amino acid availability17, sRNAs18 and proteins19. These disparate signals all act via one of two mechanisms. In one mechanism, sequences required for recognition by Rho are sequestered or exposed by selective RNA secondary structure formation. For example, binding of the protein CsrA to the pgaA leader or the small molecule flavin mononucleotide to the ribB leader in Escherichia coli both unfold RNA secondary structures that normally sequester a rut site16; 19. Because transcription and translation occur at the same time and location in bacteria, the presence or absence of translating ribosomes can also control rut site accessibility. For instance, excess tryptophan promotes ribosome stalling during translation of a short ORF in the leader region of the E. coli tnaA mRNA, thereby exposing a rut site that would normally be occluded by a ribosome17. Similarly, the Salmonella sRNA ChiX can base pair with the chiPQ transcript at the chiP translation start site to uncouple transcription and translation, thus allowing Rho to abort transcription of chiQ18.
The leader mRNA of the Salmonella Mg2+ transporter gene mgtA utilizes both of the above mechanisms to regulate Rho-dependent transcription termination. That is, the formation of RNA secondary structures controlling rut site availability is independently determined by direct binding of Mg2+ to the mgtA leader mRNA and by translation of mgtL, a short ORF in the mgtA leader20. High levels of cytoplasmic Mg2+ and efficient mgtL translation both promote an RNA conformation that facilitates Rho access to a rut site16; 20. By contrast, low cytoplasmic Mg2+ or a reduction in availability of proline-charged tRNAPro (which leads to ribosome stalling during translation of mgtL) favor an alternative RNA conformation that sequesters the rut site within a stem. Notably, the architecture of the mgtA leader region creates a situation opposite to canonical polarity; whereas Rho generally aborts transcription of incompletely translated messages, it instead attenuates transcription of mgtA when translation of mgtL is efficient20. A similar mechanism regulates another Salmonella Mg2+ transporter gene, mgtB; transcription of the mgtB coding region is inversely related to translation of a short ORF in its mRNA leader via a Rho-dependent mechanism21; 22.
Here we report that the corA gene, which specifies the third and primary Mg2+ transporter operating in Salmonella, harbors a Rho-dependent transcription terminator in its 5’ leader region. We determine that the corA leader mRNA is highly conserved and can adopt two mutually exclusive conformations. One conformation promotes premature transcription termination by exposing sequences required for recognition by Rho, whereas the alternative conformation sequesters these regions to allow read-through by RNAP. Whether the corA leader RNA adopts one conformation or the other is dictated by translation of the short ORF corL; efficient translation of corL favors the RNA conformation that presents an accessible rut site. Thus, expression of all three Mg2+ transporters in Salmonella is governed by Rho-mediated inverse coupling of transcription and translation, suggesting that this mechanism represents a general strategy for linking Mg2+ uptake to the translational status of the cell.
RESULTS
A long, highly conserved mRNA leader represses expression of the corA coding region
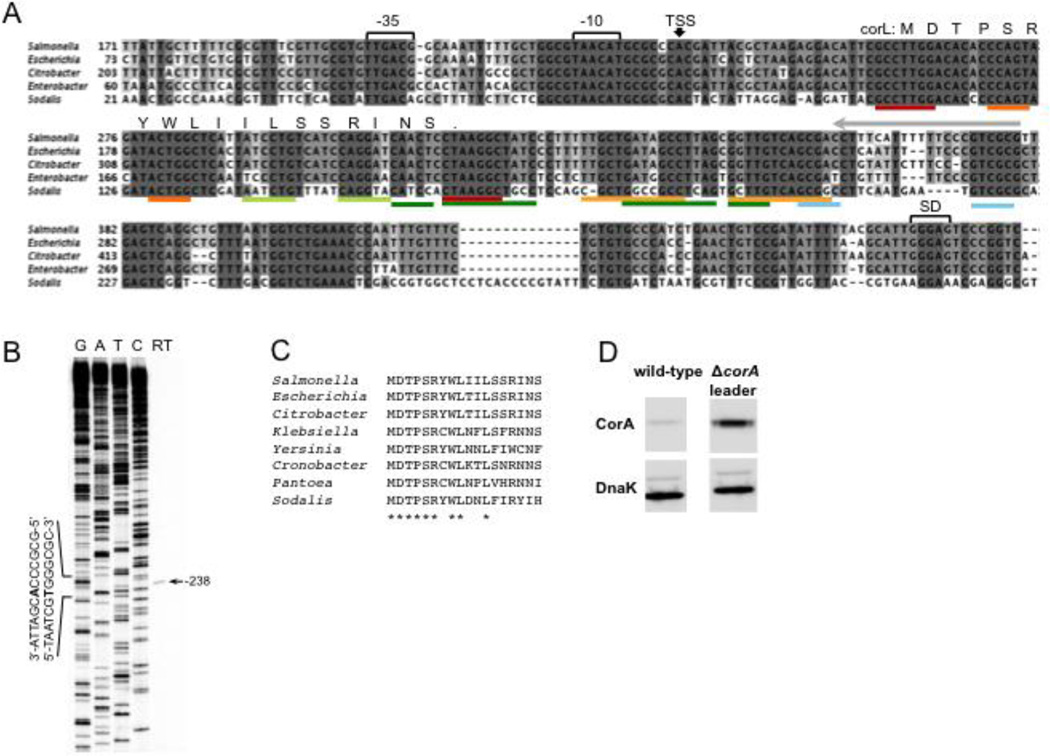
Given that 5’ leader mRNAs in Salmonella have an average length of 20–65 nucleotides (nt)23, the presence of a >400 nt intergenic region upstream of the corA start codon (Fig. 1A) raises the possibility that an unusually long mRNA leader precedes the corA coding region. To determine at which position corA transcription begins, we performed primer extension on total RNA harvested from Salmonella grown to mid-log phase in N-minimal medium. Reverse transcription yielded a single band corresponding to a position 238 nt upstream of the corA start codon (Fig. 1B). This transcription start site, hereafter denoted nucleotide +1, has near-consensus −10 and −35 promoter elements (Fig. 1A) and matches that previously identified via RNA-seq performed using a different Salmonella strain and growth condition23. Therefore, a 238 nt 5’ leader precedes the coding region in the corA transcript.
Fig. 1. The corA transcript includes a long, highly conserved 5’ leader region that functions as a repressor.
(A) Nucleotide alignment of the corA 5’ intergenic region from selected enteric bacterial genomes. The location of promoter elements, RNA secondary structures (colored bars, see Fig. 3A), the short ORF corL and the corA Shine-Dalgarno (SD) sequence are shown. The transcription start site (TSS) mapped in (B) is denoted with a black arrow.
(B) Primer extension of total RNA isolated from wild-type Salmonella (14028s) grown to mid-log phase in N-minimal medium (10 mM MgCl2) using a 5’ radiolabeled oligonucleotide that anneals to nucleotides −93 to −114 relative to the corA start codon (Fig. 1A, grey arrow). The band obtained from reverse transcription (RT) was mapped to position −238 relative to the corA start codon using a DNA sequencing ladder generated from plasmid pYS1040FL (Materials and Methods).
(C) Alignment of the deduced amino acid sequence of corL from selected enteric bacterial genomes. Asterisk indicates positions conserved in all eight listed species.
(D) Western blot of cell lysates prepared from wild-type (14028s) Salmonella or a mutant strain in which the corA leader was replaced by a scar sequence from plasmid pKD4 (MK112). Bacteria were grown in N-minimal medium (10mM MgCl2) for 2 h prior to switching to N-minimal media without MgCl2 for 2 h.
The nucleotide sequence of the corA promoter and leader region is highly conserved among members of the family Enterobacteriaceae (Fig. 1A). In addition, all examined homologs of the Salmonella corA leader contain a short ORF, termed corL, whose existence was first reported in a ribosome profiling study in E. coli24. corL’s position, length (18 sense codons) and use of a non-canonical UUG start codon are universally conserved, as is the deduced amino acid sequence of its first six codons (Fig. 1A,C).
If the corA leader mRNA is required for normal expression of the corA coding region, then deletion of the corresponding DNA sequence from the Salmonella chromosome should alter the abundance of the CorA protein. Indeed, replacement of the chromosomal corA leader with an unrelated 85 nt sequence increased CorA protein levels 10-fold compared to the wild-type (Fig. 1D). This result indicates that the corA leader represses expression of the corA coding region.
The corA leader mRNA contains a Rho-dependent transcription terminator
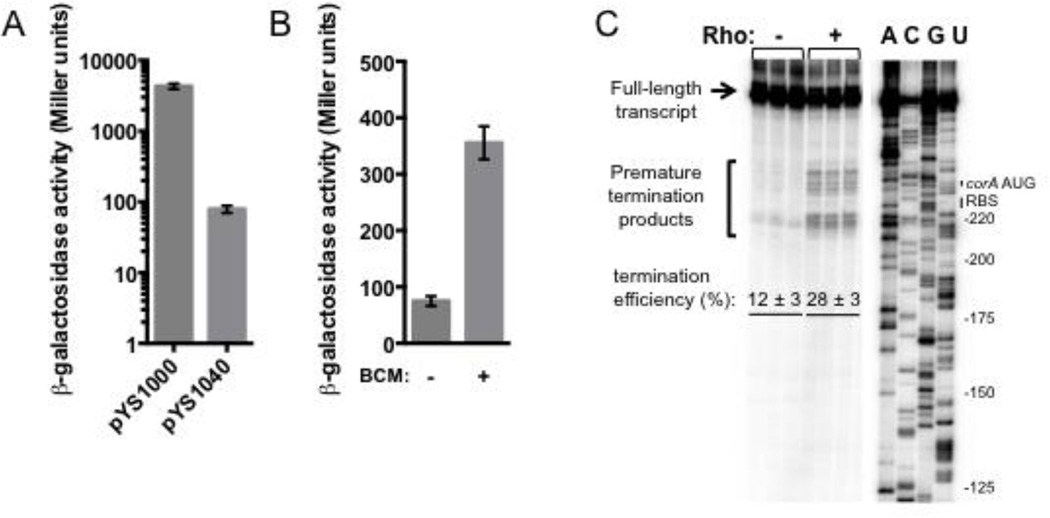
That the corA leader reduces CorA abundance raises the possibility that it attenuates transcription or translation of the coding region. To determine whether the corA leader affects transcription of the associated coding region, we compared the β-galactosidase activity of wild-type Salmonella harboring plasmid pYS1000, in which a constitutive promoter drives transcription of the E. coli lacZ gene, or its derivative pYS1040, in which the corA leader is inserted upstream of the lacZ Shine-Dalgarno (SD) sequence. The β -galactosidase activity originating from the pYS1040-containing strain was 54-fold lower than that of the isogenic strain harboring pYS1000, indicating that the corA leader represses transcription of its associated coding region (Fig. 2A).
Fig. 2. The corA leader mRNA contains a Rho-dependent transcription terminator.
(A) β-galactosidase activity (Miller units) from wild-type Salmonella (14028s) harboring plasmid pYS1000, in which the plac1-6 promoter drives expression of lacZ, or the derivative pYS1040, in which the Salmonella corA leader sequence is present between plac1-6 and the lacZ SD sequence. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(B) β-galactosidase activity (Miller units) from wild-type Salmonella (14028s) harboring plasmid pYS1040 in the presence or absence of BCM. Bacteria were grown in LB/Cm medium for 4 h, followed by incubation in the presence of BCM or mock for 30 min. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(C) A representative 6% denaturing gel of single-round, synchronized in vitro transcription reactions performed using a DNA template generated from plasmid pMK100. The assay shown was performed in 5 mM MgCl2. Reactions were performed in triplicate, and only the relevant portion of the gel is shown. Calculation of termination efficiency and generation of an RNA sequencing ladder were performed as described in Materials and Methods.
Because the corA leader acts at the level of transcription (Fig. 2A) but contains no sequences resembling an intrinsic termination signal (Fig. 1A), we predicted that it might harbor a Rho-dependent transcription terminator. To test this hypothesis, we compared the β-galactosidase activity of wild-type Salmonella harboring plasmid pYS1040 in the presence or absence of the Rho-specific inhibitor bicyclomycin (BCM). Because Rho is essential to viability25, cells were exposed for 30 min to a sub-lethal dose. The β-galactosidase activity was 5-fold higher in cells treated with BCM compared to the mock-treated control (Fig. 2B), suggesting the presence of a Rho-dependent terminator in the corA leader. This conclusion is supported by the observation of increased RNAP occupancy directly upstream of corA in E. coli treated with BCM26.
To test directly for Rho-dependent termination in the corA leader region, we performed single-round in vitro transcription assays using purified Salmonella RNAP and a DNA template containing the corA leader and 49 nt of the coding region under the control of a constitutive promoter. In the absence of Rho, RNAP transcribed full-length RNA (Fig. 2C). Addition of purified Salmonella Rho protein resulted in accumulation of premature termination products and a significant decrease in the amount of run-off transcript, consistent with the presence of a Rho-dependent transcription terminator (Fig. 2C). Rho-dependent termination in vitro takes place at a series of sites beginning slightly upstream of the corA SD sequence and ending shortly after the corA start codon, resulting in truncated transcripts of ~230–260 nt in length (Fig. 2C). Cumulatively, these results indicate that the corA leader harbors a Rho-dependent transcription terminator.
The corA leader mRNA can adopt two mutually exclusive conformations
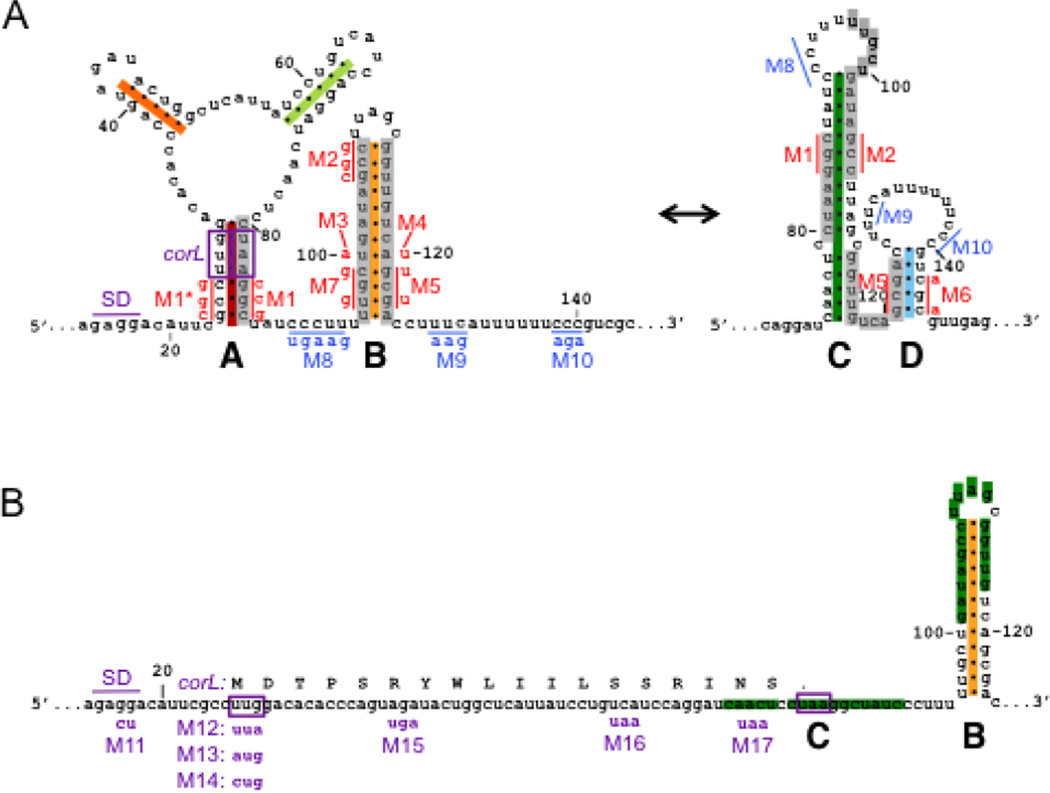
Based on its position within a leader region, we hypothesized that the function of the corA Rho-dependent terminator is regulated. To explore how the corA leader RNA might modulate the efficiency of Rho-dependent termination in the corA leader, we used the M-fold web server to analyze RNA secondary structures that the corA leader might adopt. We identified two possible RNA conformations whose regions of base pairing overlap such that they are mutually exclusive (Fig. 3A). The potential for alternative base pairing is conserved across species (Fig. 1A, compare colored bars to Fig. 3A). The previously hypothesized stem-loop C27 is predicted to be thermodynamically favored over the competing stem-loop B structure (δGC = −20.5 kcal/mol versus δGB = −16.8 kcal/mol). However, the stem-loop A+B conformation has a kinetic advantage over the stem-loop C+D conformation because nucleotides G24-G30 and C80-C86 have the opportunity to base pair before nucleotides G101-G111 emerge from RNAP (Fig. 3A).
Fig. 3. The corA leader can adopt two mutually exclusive conformations that differentially regulate expression of the associated coding region.
(A) Schematic of predicted secondary structures within the corA leader mRNA. The two possible conformations, termed stem-loops A+B and stem-loops C+D, are mutually exclusive (grey bars). Positions are numbered relative to the corA TSS. Mutations used to disrupt base pairing (red) or Rho loading (blue) are denoted next to the mutated nucleotides. Structure prediction and mutation design were aided by the M-fold Web Server (http://mfold.rna.albany.edu/?q=mfold/RNA-Folding-Form). (B) Schematic denoting the position of the short ORF corL relative to the RNA secondary structures. The corL SD sequence, start codon and stop codon are shown in purple. Translation of corL in vivo is expected to favor stem-loop B by preventing formation of stem-loops A and C. Mutations used to alter corL translation initiation or elongation are denoted below the nucleotide sequence.
To determine whether the corA leader mRNA can adopt the predicted secondary structures, in-line probing assays were performed by incubating in vitro-transcribed, 5’-radiolabeled corA leader mRNA at room temperature for 40 h and analyzing degradation products by denaturing gel electrophoresis. Base paired nucleotides generally cannot sample the in-line conformation that facilitates spontaneous cleavage of the RNA sugar-phosphate backbone and thus appear with reduced intensity on a gel28. Regions predicted to participate in secondary structures in both the A+B and C+D conformations, such as the right “ear” of stem-loop A ([A57-G62, C68-U73], Fig. 3A), displayed the expected reduction in band intensity (Fig. S1). In the case of regions predicted to base pair differentially in the A+B versus C+D conformations, the cleavage pattern suggests the presence of two RNA subpopulations, one existing in each conformation. For example, U108-C112 should be single-stranded in the A+B conformation and double-stranded in the C+D conformation, whereas the reverse is true for C92-U100 and U118-A120 (Fig. 3A), yet all three of these regions were strongly cleaved during the in-line probing reaction (Fig. S1).
To clearly define the two identified conformations, we performed in-line probing of RNAs harboring mutations anticipated to favor formation of one conformation or the other (Fig. S1). In-line probing performed with RNA containing a mutation predicted to disrupt stem-loop B (M3 [T100A], Fig. 3A) demonstrated formation of stem-loops C and D due to complete protection of nucleotides within their stems (C74-U78, C80-C90, G101-G111, G113-G117, G121-C125, G143-C147) as well as strong cleavage within their loops (C92-U100, C126-C140) and the linker between the stems (U118-A120) (Fig. S1). By contrast, in-line probing of an RNA harboring a mutation predicted to disrupt stem-loop C (M1 [G84C G85C C86G], Fig. 3A) demonstrated formation of stem-loop B due to increased cleavage at positions that should be single-stranded only in the A+B conformation (C74-C90, U108-C112) and decreased cleavage at positions that should be double-stranded only in the A+B conformation (U96-U100, U118-A120) (Fig. S1).
Cumulatively, the in-line probing results indicate that the corA leader can adopt the mutually exclusive conformations consisting of stem-loops A+B versus C+D (Fig. 3A). However, because the corL sequence is embedded in stem-loop A, corL translation is predicted to preclude formation of stem-loop A in vivo (Fig. 3B).
Two pyrimidine-rich regions flanking stem-loop B constitute the rut site
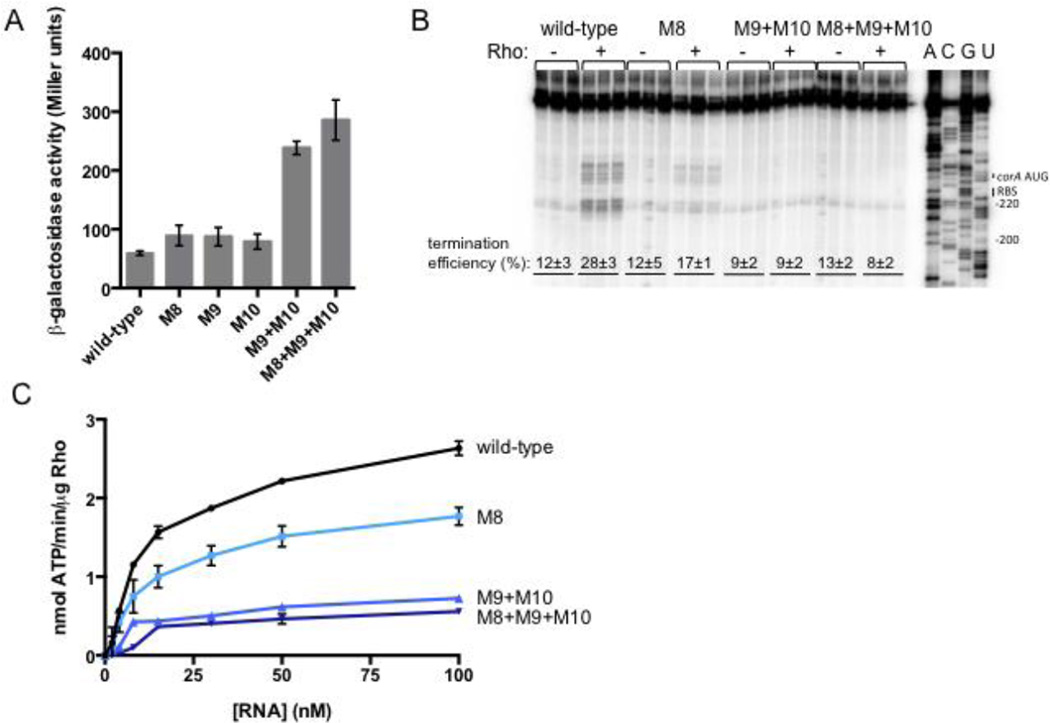
To understand how the structure of the corA leader mRNA might influence the function of the Rho-dependent terminator, we sought to characterize the rut site required for Rho-dependent termination in the corA leader. We identified two regions at positions U87-U97 and C125-C140 that have high pyrimidine content and thus might contribute to recognition of the corA leader as a Rho substrate. Mutations that reduce the pyrimidine content within these regions (M8 [C90T C91G C92 T93A T94G], M9 [T128A T129A C130G] and M10 [C138A C139G C140A], Fig. 3A) increased expression in vivo (Fig. 4A) and decreased Rho-dependent termination in vitro (Fig. 4B). The effect was largest when mutations on both sides of stem-loop B were combined (Fig. 4A,B), indicating that both pyrimidine-rich tracts are required for efficient Rho-dependent termination.
Fig. 4. Two pyrimidine-rich regions flanking stem-loop B constitute the rut site.
(A) β-galactosidase activity exhibited by wild-type Salmonella (14028s) harboring plasmid pYS1040 or derivatives containing mutations that change the indicated pyrimidines to purines (see Fig. 3A). Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(B) A representative 6% denaturing gel of single-round, synchronized in vitro transcription reactions performed using templates generated from plasmid pMK100 or derivatives containing mutations in the pyrimidine-rich regions flanking stem-loop B (Fig. 3A). The assay shown was performed in 5 mM MgCl2. Reactions were performed in triplicate, and only the relevant portion of the gel is shown. Calculation of termination efficiency and generation of an RNA sequencing ladder were performed as described in Materials and Methods.
(C) The accumulation of free phosphate was monitored to quantify Rho’s ATPase activity. Purified Rho protein was incubated with RNA corresponding to the wild-type corA leader mRNA or variants harboring mutations in the pyrimidine-rich regions. The experiment shown was performed in 0.75 mM MgCl2. Data shown correspond to mean values from two independent experiments, and error bars indicate standard deviation.
If the effects of mutations in the pyrimidine-rich regions are due to rut site disruption, then the corresponding RNAs should exhibit a decreased ability to stimulate Rho’s ATPase activity in vitro. As hypothesized, RNAs harboring mutations that reduce the pyrimidine content of U87-U97 and C125-C140 stimulated Rho’s ATPase activity to a much lesser extent than wild-type RNA (Fig. 4C). The pyrimidine-rich tract downstream of stem-loop B appears to play a larger role than the upstream one because mutation M9+M10 had a larger effect than M8, both in vitro and in vivo (Fig. 4A–C).
Rho is thought to require at least 70 nucleotides to successfully load onto a transcript10. However, the rut site nucleotides identified in the corA leader span a region of ~50 nt in length, from positions 87–140. Therefore, we examined whether other pyrimidines in the corA leader are specifically required for Rho-dependent transcription termination. Removal of pyrimidines from positions 66–68 or 74–77 actually decreased expression in vivo (Fig. S2A), which is inconsistent with participation of these nucleotides in Rho loading. Removal of pyrimidines from positions 78–80, 176–177, 186–188 or 194–196 slightly increase expression in vivo but did not reduce the ability of the corresponding RNAs to stimulate Rho’s ATPase activity in vitro (Fig. S2). These results indicate that RNA contacts made by Rho outside of positions 87–140 may not be sequence-specific. Notably, the location of the two pyrimidine-rich regions relative to stem-loops B and C (Fig. 3A) suggests that the RNA conformation adopted by the corA leader might control Rho’s ability to terminate transcription before the corA coding region.
Stem-loops C and D are required for transcription of the corA coding region
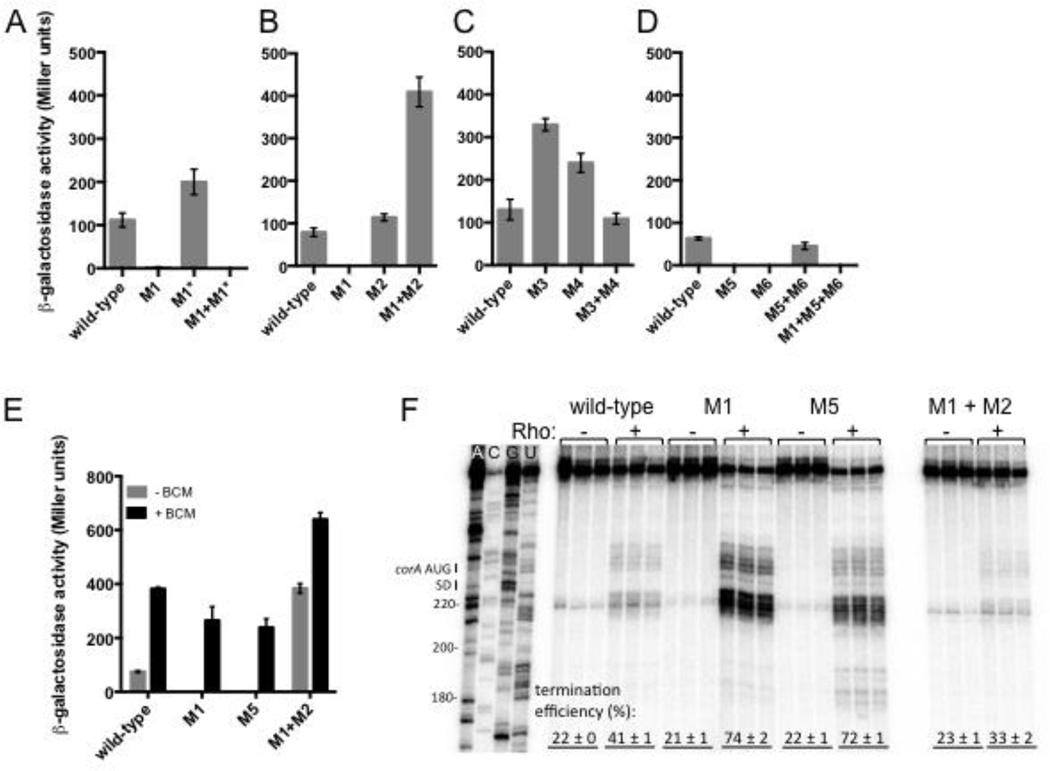
To determine the effect of the of corA leader mRNA conformation on transcription of the associated coding region, we measured the β-galactosidase activity originating from wild-type Salmonella harboring plasmid pYS1040 or derivatives containing point mutations expected to favor or disrupt a particular RNA structure. Because a single nucleotide substitution often disrupts more than one structure, both arms of a given stem-loop were disrupted independently. Moreover, by combining the mutations in both arms to restore base pairing, we could assess whether the structure itself, rather than the identity of the mutated nucleotides, was responsible for the observed behavior.
Stem-loop C promotes transcription elongation into the associated coding region because a mutation predicted to prevent formation of stem-loop C (M1, Fig. 3A) abolished β-galactosidase activity (Fig. 5A). Even though this mutation also compromises formation of stem-loop A, the observed effect is specifically due to disruption of stem-loop C because: (i) a mutation that disrupts the opposite arm of stem-loop A (M1* [G24C, C25G, C26G], Fig. 3A) did not abolish expression (Fig. 5A); (ii) a double mutation that restores stem-loop A base pairing potential while disrupting stem-loop C (M1+M1*, Fig. 3A) had the same phenotype as the M1 single mutant (Fig. 5A); and (iii) stem-loop A is unlikely to form in vivo due to translation of corL (Fig. 3A). A mutation that disrupts formation of both stem-loops B and C (M2 [G105C C106G C107G], Fig. 3A) increased expression slightly (Fig. 5B). By contrast, combination of the M1 and M2 mutations, which restores stem-loop C base pairing (and disrupts stem-loops A and B), dramatically increased expression (Fig. 5B), providing further evidence that stem-loop C favors transcription of the downstream coding region.
Fig. 5. Stem-loops C and D promote transcription of the associated coding region by hindering Rho-dependent termination.
(A–D) β-galactosidase activity (Miller units) exhibited by wild-type Salmonella (14028s) harboring plasmid pYS1040 or derivatives containing mutations that disrupt base pairing in stem-loops A, B, C and/or D. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(E) β-galactosidase activity (Miller units) exhibited by wild-type Salmonella (14028s) harboring plasmid pYS1040 or derivatives harboring mutations in stem-loops A, C and/or D, in the presence or absence of the Rho-specific inhibitor bicyclomycin (BCM). Following growth in LB/Cm for 4 h, BCM (to a final concentration of 20 µg/ml) or an equal volume of water was added and bacteria were incubated for an additional 30 min prior to measurement of β-galactosidase activity. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(F) A representative 6% denaturing gel of single-round, synchronized in vitro transcription reactions performed using templates generated from plasmid pMK100 or derivatives containing mutations in stem-loops A, C and/or D. The assay shown was performed in 1 mM MgCl2. Reactions were performed in triplicate, and only the relevant portion of the gel is shown. Calculation of termination efficiency and generation of an RNA sequencing ladder were performed as described in Materials and Methods.
Stem-loop B hinders expression because mutations that disrupt base pairing within stem-loop B (M3 [T100A] and M4 [A120T], Fig. 3A) significantly increased expression, whereas restoration of stem-loop B base-pairing potential by combining M3 with M4 resulted in wild-type expression (Fig. 5C). That the M3+M4 mutant displayed higher expression than M1 (Fig. 5A and 5C) likely reflects that the former allows formation of stem-loop C whereas the latter does not (Fig. 3A).
Stem-loop C is expected to facilitate stem-loop D formation by preventing adoption of the alternative stem-loop B (Fig. 3A). Given that stem-loop C promotes transcription of the downstream coding region, we wondered whether this was also the case for stem-loop D. Indeed, mutations that disrupt base pairing within stem-loop D (M5 [G121T G123T] and M6 [C143A C145A], Fig. 3A) abolished expression (Fig. 5D). The effect of the M5 mutation is specifically due to disruption of stem-loop D and not B because other mutations predicted to prevent formation of stem-loop D without disrupting stem-loop B (M5* [G123A], M5+M7 [T97G C99G G121T G123T], Fig. 3) also eliminated expression (Fig. S3). Restoration of base pairing within stem-loop D via combination of the M5 and M6 mutations restored wild-type expression (Fig. 5D). Cumulatively, these data indicate that stem-loop D is necessary to promote transcription into the associated coding region.
Formation of stem-loop D alone is not sufficient to promote constitutive transcription elongation because a triple mutant that retains stem-loop D but cannot form stem-loop C (M1+M5+M6, Fig. 3A) showed no expression (Fig. 5D). Taken together, the above results indicate that stem-loops C and D are both required for expression of the downstream coding region.
Stem-loops C and D promote transcription elongation by reducing Rho-dependent termination
If the lack of expression observed when stem-loops C or D are disrupted is due to Rho-promoted transcription termination, then inhibition of Rho activity should restore expression to mutants that produce RNAs unable to adopt stem-loops C or D. In agreement with this notion, BCM restored β-galactosidase activity to wild-type Salmonella harboring derivatives of plasmid pYS1040 with mutations that disrupt stem-loops C or D (M1 or M5, respectively, Fig. 5E). That BCM treatment of M1+M2 slightly increased expression (Fig. 5E) suggests that some Rho-dependent termination still occurs when the RNA adopts the stem-loop C+D conformation.
In vitro transcription experiments directly demonstrated that disruption of stem-loops C or D increases the efficiency of Rho-dependent termination (M1 and M5, Fig. 5F). By contrast, locking the RNA in the stem-loop C+D conformation reduced termination efficiency relative to the wild-type RNA (M1+M2, Fig. 5F). Together these results indicate that stem-loops C and D promote expression of the coding region by reducing the efficiency of Rho-dependent termination within the corA leader.
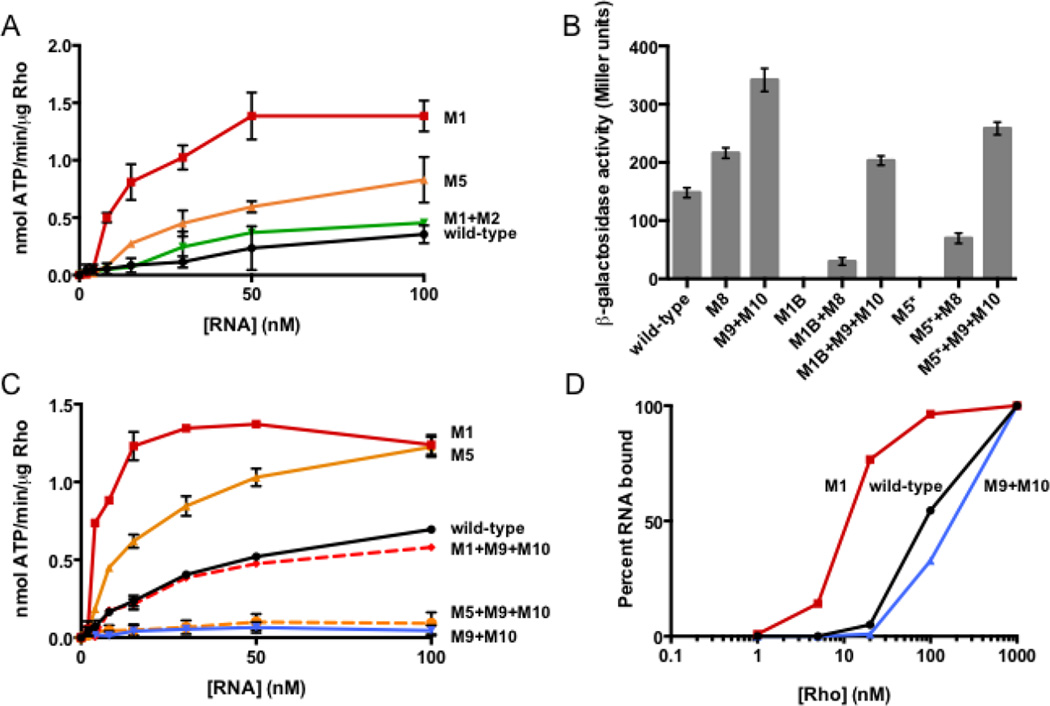
The stem-loop C+D conformation inhibits transcription termination by decreasing Rho’s ability to access the rut site
The pyrimidine-rich tracts comprising the rut site are single-stranded in the stem-loop B conformation but appear to be structurally constrained when the corA leader adopts the stem-loop C+D conformation (Fig. 3A). Thus, we reasoned that stem-loops C and D might hinder Rho-dependent termination by reducing Rho’s ability to access the rut site. In agreement with this notion, RNAs harboring mutations that disrupt stem-loops C or D (M1 or M5, respectively) stimulated Rho’s ATPase activity to a much greater extent than wild-type RNA (Fig. 6A). Conversely, an RNA locked in the stem-loop C+D conformation (M1+M2) elicited similar ATPase activity to the wild-type leader RNA (Fig. 6A). This result suggests that the wild-type RNA adopts the stem-loop C+D conformation under the assay conditions used.
Fig. 6. Stem-loops C and D hinder termination by decreasing accessibility of the rut site to Rho.
(A) The accumulation of free phosphate was monitored to quantify Rho’s ATPase activity. Purified Rho protein was incubated with RNA corresponding to wild-type corA leader mRNA or variants harboring mutations in stem-loops A-C. The experiment shown was performed in 3 mM MgCl2. Data shown correspond to mean values from two independent experiments, and error bars indicate standard deviation
(B) β-galactosidase activity exhibited by wild-type Salmonella (14028s) harboring plasmid pYS1040 or derivatives containing mutations that disrupt stem-loop C or D, reduce the pyrimidine content of the downstream pyrimidine-rich tract, or both. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(C) The accumulation of free phosphate was monitored to quantify Rho’s ATPase activity. Purified Rho protein was incubated with RNA corresponding to wild-type corA leader mRNA or variants harboring mutations that alter the RNA secondary structure, the downstream pyrimidine-rich tract, or both. The experiment shown was performed in 1.5 mM MgCl2. Data shown correspond to mean values from two independent experiments, and error bars indicate standard deviation
(D) Percentage of 5’ radiolabeled wild-type corA leader RNA (black) or mutant derivatives (M1, red and M9+M10, blue) bound by Rho and retained following vacuum filtration. Values were calculated assuming saturation at 1 mM Rho, as described in Materials and Methods. Data shown are from a single experiment representative of three independent experiments.
If the stem-loop C+D conformation hinders Rho-dependent termination by reducing rut accessibility, then mutations in the pyrimidine-rich tracts constituting the rut site should be epistatic to mutations that disrupt stem-loops C or D. As predicted, mutations in the rut site (M8, M9+M10) restored expression to mutants in which stem-loops C or D are disrupted (Fig. 6B). (In this experiment, the alternative mutations M1B (G84A G85A C88G) and M5* (G123A) were used to disrupt stem-loops C and D, respectively, because the M1 and M5 mutations introduce pyrimidines that could potentially substitute for the altered rut site nucleotides (Fig. 3A).) The restoration of gene expression can be specifically attributed to decreased Rho action because stimulation of ATPase activity by RNAs unable to adopt stem-loops C or D was also reversed when combined with a rut site mutation (Fig. 6C).
If the stem-loop C+D conformation hinders Rho access to the rut site, then its disruption is predicted to favor Rho binding to the corresponding RNA substrate. In accordance with this notion, the affinity of Rho for a mutant corA leader mRNA unable to form stem-loop C (M1) was significantly higher than that for the wild-type corA leader RNA (Fig. 6D). By contrast, Rho displayed reduced affinity for an RNA harboring a mutation in the rut site (M9+M10, Fig. 6D). These experiments indicate that the stem-loop C+D conformation inhibits Rho binding to the corA leader independently of other effects that this conformation may have on Rho translocation and associated ATP hydrolysis. We therefore conclude that stem-loops C and D favor expression of the corA coding region by sequestering a rut site, and that stem-loop B hinders expression by increasing accessibility to the rut site.
Translation of the short ORF corL hinders formation of the stem-loop C+D conformation, favoring transcription termination within the corA leader region
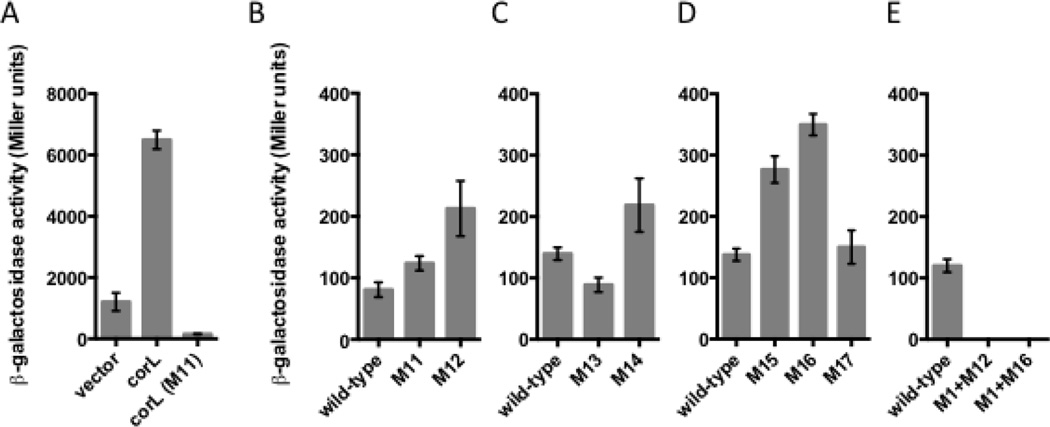
The short ORF corL overlaps sequences that participate in mutually exclusive RNA secondary structure formation, indicating that corL translation may influence the corA leader conformation (Fig. 3). To determine whether corL is actually translated in vivo, we compared the β-galactosidase activity originating from wild-type Salmonella harboring either of three plasmids: pACYC-corL-’lacZ, with a constitutive promoter driving transcription of the corA leader through the last sense codon of corL fused in frame to the ninth codon of lacZ, a derivative of pACYC-corL-’lacZ mutated in the predicted corL SD sequence (M11 [G16C G17T]) and the empty vector, in which lacZ is present but lacks an SD sequence and start codon. The β-galactosidase activity from the pACYC-corL-’lacZ-carrying strain was 42-fold higher than that of the SD mutant and 5-fold higher than that of the vector control (Fig. 7A). These results demonstrate that corL is translated in vivo in Salmonella. This conclusion is supported by ribosome profiling experiments conducted in E. coli24.
Fig. 7. Rho-dependent termination within the corA leader is inversely coupled to translation of the short ORF corL.
(A) β-galactosidase activity (Miller units) exhibited by wild-type Salmonella (14028s) harboring plasmid pACYC-’ZacZ (vector), which lacks translation initiation signals, pACYC-corL-’lacZ (corL), a translational fusion of a corA leader fragment (from the TSS to the last sense codon of corL) to the ninth codon of the E. coli lacZ gene, or a derivative containing a mutation in the corL SD sequence (corL(M11)). Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
(B–E) β-galactosidase activity (Miller units) exhibited by wild-type Salmonella (14028s) harboring plasmid pYS1040 or derivatives containing mutations that alter the efficiency of corL translation initiation (M11-M14) or introduce a premature stop codon into corL (M15-M17). Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
A ribosome occupying the corL ribosome binding site (RBS) or translating the corL open reading frame is expected to preclude stem-loop A formation, resulting in a kinetic competition between stem-loops B and C rather than stem-loops A and C. Because corL partially overlaps the left arm of stem-loop C, full translation of corL should prevent formation stem-loop C and favor stem-loop B (Fig. 3). Conversely, failure of a poised ribosome to initiate translation of corL should allow stem-loop C to dominate due to its thermodynamic stability and kinetic advantage over stem-loop B (Fig. 3). In accordance with this notion, replacement of the corL start codon with the UUA stop codon increased expression from a corA-lacZ transcriptional fusion (M12 [G29A], Fig. 7B). Mutation of the corL SD sequence de-repressed corA-lacZ transcription only 25% (M11, Fig. 7B) even though it prevented corL translation (M11, Fig. S4). These results confirm that ribosome occupancy of the corL ribosome binding site is necessary for preferential formation of stem-loop C, likely because stem-loop A can compete with stem-loop C in the absence of a ribosome at the RBS.
Because the corL start codon is a non-canonical UUG, we further explored the connection between corL translation initiation and corA transcription. Initiation of translation from UUG is significantly less efficient than from the standard AUG29. This is also the case with corL because the β-galactosidase activity from a strain harboring a corL-lacZ translational fusion was higher when the start codon was changed to AUG (Fig. S4). Conversely, alteration of the corL start codon from UUG to CUG dramatically reduced the efficiency of corL translation (Fig. S4). The AUG and CUG mutations slightly reduced and increased the β-galactosidase activity originating from strains with the corA-lacZ transcriptional fusion, respectively (Fig. 7D). Together, these results imply that corA transcription is inversely correlated with the efficiency of corL translation initiation.
The ribosome covers 12–15 nt of mRNA on both sides of its P site30. Therefore, a ribosome that stalls early on during translation of corL might not occlude the left arm of stem-loop C (Fig. 3B), leading to increased transcription of the corA coding region. To test this notion, we investigated the behavior of strains harboring derivatives of the corA-lacZ transcription fusion in plasmid pYS1040 with stop codons at various positions within the corL ORF. Stop codons at positions at least 12 nucleotides from the base of stem-loop C (M15 and M16, Fig. 3) significantly increased expression (Fig. 7D). By contrast, a premature stop codon at a position from which the ribosome should still occlude the left arm of stem-loop C (M17, Fig. 3A) had no effect on corA-lacZ transcription (Fig. 7D). The phenotypes displayed by the strains with premature stop codons in corL are likely due to their effects on corL translation (as opposed to effects on the structure of the corA leader RNA) because the amber tRNA suppressor supF partially reversed the effect of a premature amber stop codon in corL (M16 (sup), Fig. S5A). As expected, the supF-expressing plasmid did not alter corA-lacZ transcription in strains with a wild-type corL sequence or with an ochre stop codon (Fig. S5A). A corL-specified peptide does not appear to regulate corA-lacZ transcription because expression of corL in trans failed to restore normal expression to a strain harboring a premature corL stop codon (Fig. S5B).
If corL translation exerts its effect by promoting formation of particular secondary structures in the corA leader RNA, then mutations forcing the RNA to adopt a certain conformation should be epistatic over those affecting corL translation. In accordance with this notion, a mutation that disrupts stem-loop C (M1, Fig 3A) is epistatic over mutations that reduce the efficiency of corL translation initiation or prevent elongation (M12 and M16, respectively, Fig. 3 and 7). In other words, preventing the ribosome from occluding stem-loop C has no effect on transcription of the associated coding region if the RNA cannot form stem-loop C. Taken together, the above results indicate that the efficiency of corL translation initiation and elongation influence the conformation adopted by the corA leader mRNA.
DISCUSSION
Expression of the corA gene was long thought to be constitutive due to its “housekeeping” function as the primary Mg2+ transporter in Salmonella and lack of observed Mg2+-dependent regulation. However, this notion has recently been challenged by the observation that corA mRNA and protein levels do not correlate during the transition from exponential to stationary growth31. We have now established that corA is regulated at the level of transcription elongation by its 5’ leader mRNA. Through selective secondary structure formation, the corA leader modulates the accessibility of a rut site to control the function of a Rho-dependent transcription terminator. Whether the RNA adopts one conformation or the other is dictated by translation of the short ORF corL located within the leader region. Under normal conditions, corL translation favors formation of stem-loop B due to occlusion of the left arm of stem-loop C by a translating ribosome (Fig. 3B). By contrast, a decrease in the efficiency of corL translation initiation or elongation promotes adoption of stem-loop C, in turn favoring transcription elongation into the corA coding region. In sum, there is an inverse correlation between corL translation and corA coding region transcription (Fig. 8). It is likely that Salmonella shares this regulatory mechanism with other enteric bacteria because the corA leader sequence is highly conserved within this family (Fig. 1A).
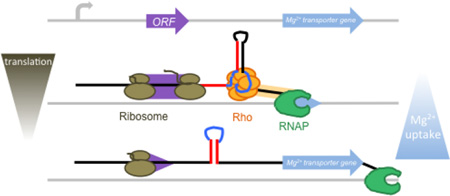
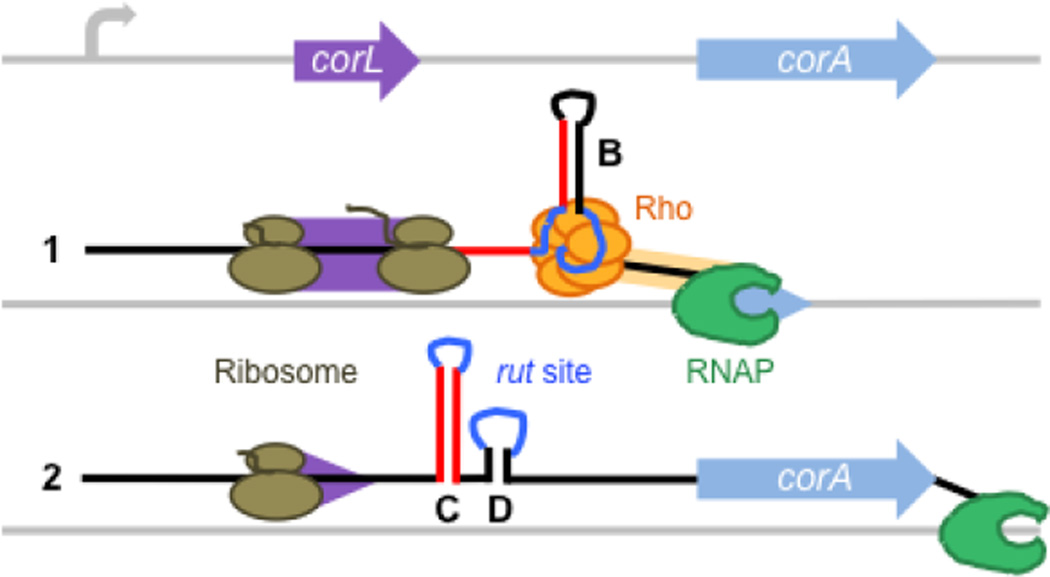
Fig. 8. Model for control of Rho-dependent transcription termination in the corA leader by translation of the short ORF corL.
When corL (purple) is translated efficiently (1), the ribosome (brown) occludes the left arm of stem-loop C, thus promoting stem-loop B formation. This RNA conformation presents an accessible rut site (dark blue), allowing Rho (orange) to load onto the corA mRNA leader and trigger RNA release by RNAP (green) prior to transcription of the corA coding region (light blue). When translation of corL is impaired (2), either by inefficient translation initiation or elongation, the kinetic and thermodynamic advantages of stem-loop C over stem-loop B lead to formation of the stem-loops C+D conformation, which sequesters the rut site and allows RNAP to continue into the corA coding region.
Surprisingly, RNAse III degrades corA mRNA in E. coli by specifically targeting stem-loop C27. Thus, adoption of the stem-loop C+D conformation is predicted to both increase transcription elongation into the coding region and decrease mRNA stability, resulting in two opposing effects on overall corA mRNA levels. Conversely, the RNAse III cleavage site is not intact if the RNA adopts stem-loop B, so this conformation is predicted to be more stable even though it facilitates premature termination of transcription by Rho. The ultimate outcome of the interplay between these opposing forces likely depends on specific cellular conditions.
The corA and mgtA leaders utilize similar strategies to regulate rut accessibility
The mutually exclusive RNA conformations adopted by the corA leader (Fig. 3A) share a strong resemblance to those within the mgtA leader mRNA, which also modulates rut site accessibility via selective secondary structure formation16, in spite of the fact that the corresponding nucleotide sequences are unrelated. In both the corA and mgtA leaders, the conformation that presents an accessible rut site contains an RNA hairpin (i.e., stem-loop B) that brings together two single-stranded pyrimidine-rich regions required for Rho loading. This function of stem-loop B is reminiscent of the ability of the boxB hairpin to clamp together the rutA and rutB sites of the λ tR1 Rho-dependent terminator32. In the rut site-accessible conformation, the corA and mgtA leaders also have the potential to form an additional three-helix structure (i.e., stem-loop A; Fig. 3A)33, although in both cases, adoption of stem-loop A in vivo is likely precluded by translation of a short ORF that overlaps it (Fig. 3B)20.
For both the corA and mgtA leader mRNAs, sequences within the right arm of stem-loop A and the left-arm of stem-loop B can alternatively base pair to form a structure (stem-loop C) that sequesters rut site nucleotides. In the case of mgtA, some of these nucleotides may participate in base pairing in the stem-loop C conformation16, whereas the analogous nucleotides within the corA leader remain single-stranded but are structurally constrained (Fig. 3A).
In the mgtA leader mRNA, the upstream portion of the rut site (R1) plays a larger role than the downstream one (R2) in loading of Rho because R1 is required for the stem-loop B conformation to repress transcription elongation into the coding region whereas R2 is not16. Similarly, the upstream rutA is far more important than the downstream rutB for Rho-dependent termination at λ tR134. By contrast, the downstream pyrimidine-rich tract in the corA leader is most important for Rho loading because mutation of this region (M9+M10) has larger effects than mutation of the upstream pyrimidine-rich tract (M8) on transcription in vivo (Fig. 4A), Rho-dependent termination in vitro (Fig. 4B) and Rho ATPase activity in vitro (Fig. 4C). The dominance of the downstream portion of the rut site in the corA leader may explain why the Rho-inaccessible conformation requires the additional stem-loop D (Fig. 3A).
Rho action establishes a regulatory link between Mg2+ homeostasis and translation
A canonical function of Rho is to implement transcriptional polarity. That is, when transcription and translation become uncoupled, cryptic rut sites normally occluded by translating ribosomes become accessible and Rho can terminate transcription3. Strikingly, the architectures of the corA (Fig. 3), mgtA16 and mgtB22 leaders reverse this relationship because Rho-dependent transcription termination within the leader is favored when translation of short ORF(s) in the leader is efficient. Regulation of Mg2+ uptake in response to translational signals appears to be a universal mechanism because it was recently shown that expression of the eukaryotic Mg2+ channel TRPM7 is controlled by translation of two short ORFs within its 5’ leader mRNA35.
Why do enteric bacteria link Mg2+ homeostasis to Rho action? After all, it is possible to inversely couple translation of a short ORF within an mRNA leader to transcription of the associated coding region using an intrinsic (i.e., Rho-independent) transcription termination mechanism36. One possibility is that a Rho-dependent mechanism might facilitate sensing aspects of translation other than availability of particular amino acids, which is used to regulate amino acid biosynthetic operons36. In support of this notion, corA transcription can be modulated by altering the efficiency with which corL translation is initiated (Fig. 7B,C). Intriguingly, both corL and mgtM use the inefficient start codon UUG, which could be especially sensitive to global changes in translation, such as ribosome availability37. Because the majority of cytosolic Mg2+ is devoted to maintenance of ribosome structure and activity, an increase in Mg2+ uptake may help the cell cope with translational stressors38. Therefore, Rho’s involvement in the control of Mg2+ transporter genes may promote Mg2+ uptake when mRNA translation is impaired.
MATERIALS AND METHODS
Bacterial strains and growth conditions
All experiments were carried out with wild-type Salmonella enterica serovar Typhimurium strain 14028s39 or its derivative MK112, in which has the sequence corresponding to the corA leader is replaced by an 85 nt scar sequence. MK112 was constructed via the one-step gene disruption method40 by transforming a PCR product generated from plasmid pKD4 using primers W332 and W333 into 14028s harboring pKD46 and selecting for KanR colonies. Following P22 transduction of the mutation into a wild-type background, the KanR cassette was excised using plasmid pCP20, which left behind the 85 nt scar sequence from pKD4. E. coli DH5α41 or XL-1 blue (Agilent) were used for cloning and plasmid DNA preparation. Bacteria were grown at 37°C in LB broth (Becton, Dickinson & Co), SOB (Becton, Dickinson & Co) or N-minimal medium (pH 7.4)42 supplemented with 0.1% casamino acids, 38 mM glycerol and either zero or 10 mM MgCl2. For plasmid maintenance, 20 µg/ml chloramphenicol (Cm), 50 µg/ml kanamycin (Kan) and/or 50 µg/ml ampicillin (Ap) were used.
Plasmid construction
Plasmid pYS1040 was constructed by cloning a PCR fragment generated from 14028s genomic DNA using primers 8446 and 8447 between the SalI and PstI sites of pYS100033. The resulting plasmid harbors a corA-lacZ transcriptional fusion in which the plac1–6 promoter drives transcription of corA leader followed by a 16 nt linker (containing PstI and XhoI sites), the lacZ ribosome binding site, the lacZ coding region and the hisT transcription terminator. As a result of the cloning process, pYS1040 and its derivatives contain two extra nucleotides between the plac1–6 promoter and the true TSS. A strain harboring a derivative of this plasmid with the correct TSS, designated pYS1040+1, produced similar β-galactosidase activity compared to the strain carrying pYS1040 (Fig. S6A). All mutations tested in the pYS1040+1 background show analogous effects to those observed in pYS1040 (Fig. S6A). Nucleotide positions are numbered relative to the true TSS. Plasmid pYS1040FL, which contains the entire corA 5’ intergenic region and lacks the plac1–6 promoter, was constructed by cloning a PCR fragment generated from 14028s genomic DNA using primers 11992 and 11987 between the SalI and XhoI sites of pYS1040.
Because some of the in vitro-mapped Rho-dependent termination sites are downstream of the corA start codon, we constructed a derivative of plasmid pYS1040 termed pYS1040ext that contains 49 nt of the corA coding region upstream of the pYS1040 lacZ ribosome binding site. Plasmid pYS1040ext was constructed by cloning a PCR fragment generated from 14028s genomic DNA using primers 8447 and W1431 between the SalI and PstI sites of pYS1040. Basal expression from pYS1040ext-carrying Salmonella is two-fold higher than the pYS1040-carrying strain (Fig. S6B). All mutations tested in the pYS1040ext background show analogous effects to those observed in pYS1040 (e.g. Fig. S6B).
Plasmid pMK100 was constructed by cloning a PCR product amplified from 14028s genomic DNA using primers W823 and W947 between the SpeI and SphI sites of pKH100. This places the corA leader and the first 49 nt of the corA coding region downstream of the λpR promoter and 26 nt C-less initial transcribed sequence and upstream of the hisT terminator43.
Plasmid pACYC-corL-’lacZ was constructed by cloning a PCR product generated from 14028s genomic DNA with primers 11903 and 11905 between the XmaI and XbaI sites of plasmid pACYC-’lacZ. This generates a translational fusion in which the constitutive plac1–6 promoter drives transcription from the beginning of the corA leader up to the last sense codon of corL fused in frame to the ninth codon of the E. coli lacZ gene.
Plasmid pcorL was constructed by cloning a PCR product generated from 14028s genomic DNA with primers 11903 and W574 into the HindIII site of pBAD1844. Correct orientation of the insert was confirmed by sequencing a PCR product generated from candidate plasmid DNA using primers 10127 and 10128.
Nucleotide substitutions in all plasmids were generated using the QuikChange II site-directed mutagenesis kit (Agilent) and primer pairs listed in Table S2.
Primer extension
10 pmol of primer 11984 was 5’-radiolabeled using [γ-32P]-ATP (Perkin Elmer) and T4 polynucleotide kinase (NEB) according to the manufacturer’s instructions for 30 min at 37°C. Labeled primer was purified on a G-50 column (GE Healthcare) and 0.4 pmol was mixed with 8 µg of total RNA extracted from Salmonella grown to mid-log phase in N-minimal media (10 mM MgCl2). The mixture was incubated at 65°C for 5 min and cooled on ice for 1 min to promote annealing. Reverse transcription was carried out using Superscript II (Life Technologies) according to the manufacturer’s instructions. Products were ethanol precipitated and mixed with 2x loading buffer II (Ambion) prior to being run on 8% polyacrylamide sequencing gels (Sequagel system, National Diagnostics). Four sequencing reactions were performed, each in the presence of one ddNTP, using Vent (exo-) DNA polymerase (NEB) according to the manufacturer’s instructions with plasmid pYS1040FL as the template.
Western blotting
1 ml N-minimal medium (10 mM MgCl2) overnight cultures were washed once and diluted 1:50 in 25 ml of the same medium. Following 2 h at 37°C with 250 rpm shaking, 1 ml was removed for measuring cell density (OD600) and 1 ml was washed with 1x TBS before freezing the pellet at −20°C. The remaining cultures were washed 2x and re-suspended in in cold N-minimal medium (no MgCl2). Incubation was continued at 37°C with 250 rpm shaking with samples collected for OD600 measurement and Western blotting every 1–2 h. Western blot samples were prepared by re-suspending cell pellets in a volume of SDS-PAGE loading buffer (2.3% v/v sodium dodecyl sulfate, 22% v/v glycerol, 0.015 g/ml Tris, 0.05 mg/ml bromophenol blue, 87 µL/ml β-mercaptoethanol) determined by the formula: loading buffer (µl) = OD600 x sample volume (µl) x 100. The samples were heated to 95°C for 5 min, diluted to 0.1X in 15 µl SDS-PAGE loading buffer and heated again to 95°C for 3 min. Samples were loaded onto 12% Bis-Tris polyacrylamide gels (Life Technologies) and run in 1x MOPS running buffer at 200 V for approximately 40 min.
Samples were transferred to nitrocellulose membranes using the iBlot system (Invitrogen). Membranes were rinsed briefly in 1X TBS and blocked with 5% skim milk in 1X TBS for 1 h. Following a brief rinse in 1X TBS-T (TBS with 0.1% Tween20), primary antibody treatment was carried out for 1 h. Primary antibodies were prepared in 1X TBS-T with 0.01% sodium azide. Rabbit anti-CorA antibody (a generous gift from Michael Maguire) was used at a dilution of 1:10,000. Mouse anti-DnaK (Enzo Life Sciences) was used at a dilution of 1:5000 as a loading control. Following one 15 min wash and three 5 min washes in 1X TBS-T, secondary antibody treatment was carried out for 1 h using horseradish peroxidase-linked anti-Rabbit or anti-Mouse IgG (GE Healthcare). Membranes were again washed as described above prior to detection using the SuperSignal ELISA Femto kit (Thermo Scientific) and visualization with LAS-4000 (GE Healthcare). Quantification was performed with ImageQuant software (GE Healthcare).
In-line probing
T7 promoter-driven transcription templates corresponding to the corA leader were generated by PCR using plasmid pYS1040 or mutant derivatives and primers 8445 and 8444. RNA was synthesized from these templates using the Megascript T7 kit (Ambion) according to the manufacturer’s instructions. 1 µg of template DNA was used and reactions were incubated at 37°C overnight. RNAs were phenol/chloroform extracted, purified on 6% TBE urea gels (Life Technologies) and ethanol precipitated. 50 pmol of RNA was dephosphorylated by treating with calf intestinal alkaline phosphatase (NEB) according to the manufacturer’s instructions for 30 min at 37°C. Dephosphorylated RNA was labeled with [γ-32P]-ATP (Perkin Elmer) using T4 polynucleotide kinase (NEB) according to the manufacturer’s instructions for 30 min at 37°C. Labeled RNA was purified on a 6% TBE urea gel and ethanol precipitated.
In-line probing reactions were set up as described45. Briefly, 1–2 µl labeled RNA was incubated in in-line buffer (50 mM Tris-HCl (pH 8.3), 200 mM KCl, 40 mM MgCl2) for 40 h at room temperature. Control RNase T1 and alkaline hydrolysis reactions were carried out using the RNase T1 (1U/µl) kit (Ambion) according to the manufacturer’s instructions. Denaturing 7% polyacrylamide sequencing gels (Sequagel system, National Diagnostics) were pre-run at 65 W for 1.5 h or until the gel reached 55°C, and then 8 µl of each sample was loaded and run for 1.5 or 3 h. After drying for 2 h at 80°C, the gel was exposed to a phosphoscreen overnight, visualized with a Typhoon FLA 9000 laser scanner (GE Healthcare) and bands were quantified with ImageQuant software (GE Healthcare).
β-galactosidase assays
0.8 ml aliquots of LB/Cm were inoculated with single wild-type Salmonella (14028s) colonies harboring plasmid pYS1040 or mutant derivatives and incubated overnight. Cells were washed once in LB and used to inoculate fresh 1 ml LB/Cm cultures in test tubes at a 1:100 dilution. Following incubation at 37°C for 4 h with 250 rpm shaking, 50 µl chloroform and 20 µl 0.1% SDS were added and cultures were vortexed vigorously for 1 min. After allowing the debris to settle for 15 min, β-galactosidase activity was measured as described46. Briefly, two 100 µl aliquots of each culture were transferred to a 96-well plate and 100 µl of 1.32 mg/ml ONPG was added to each well. Absorbance at 405 nm was recorded every 30 sec for 20 min at 30°C and the Vmax during a linear increase in absorbance was divided by cell density (absorbance at 562 nm) to yield a value in Miller units. Sterile media was used as a blank. Data shown correspond to mean values from at least three independent experiments performed in duplicate, and error bars indicate standard deviation.
For experiments testing the effect of BCM (a generous gift from Max Gottesman), 1.5 ml cultures were used. After 4 h, two 600 µl aliquots were taken and water or 20 µg/ml BCM was added prior to incubation for an additional 30 min before treating the cells with chloroform/SDS and measuring the β-galactosidase activity.
For experiments using plasmids pBAD1844 or pcorL, cells were grown in LB/Cm/Ap/0.4% L-arabinose to induce corL expresion in trans. For experiments using plasmids pUHE21-2lacIq20 or pUH-supF, cells were grown in LB/Cm/Ap/1mM IPTG.
In vitro transcription termination assays
Transcription templates were prepared by performing PCR with plasmid pMK100 or mutant derivatives as template and primers IA17 and IA256. Purified Salmonella RNA polymerase (RNAP) core enzyme and σ70 (courtesy of Kerry Hollands and Anastasia Sevostyanova) were mixed at a 1:3 molar ratio in RNAP storage buffer (50% v/v glycerol, 10 mM Tris pH 7.9, 0.1 M NaCl, 0.1 mM EDTA, 0.1 mM DTT) and allowed to associate by incubating at 30°C for 30 min.
Single round, synchronized in vitro transcription reactions were carried out as described43. 5 pmol DNA template was mixed with 5.6 pmol σ70+RNAP in a volume of 100 µl containing 100 mM Tris HCl (pH 8.0), 100 mM KCl, 50 mM MgCl2, 5 µM each of ATP and UTP, 1 µM GTP, 100 µM ApU (TriLink) and 1.5 µl [α-32P]-GTP (3000 Ci/mmol, Perkin Elmer). This mixture was incubated at 37°C for 15 min to generate halted transcription elongation complexes (hTEC). hTEC were divided into aliquots and incubated at 37°C for 3 min. To initiate synchronized transcription elongation, a 5X chase +/− Rho was added (100 mM Tris HCl (pH 8.0), 100 mM KCl, 0.1mg/ml rifampicin, 1mM NTPs, with 600 nM Rho or an equal volume of Rho buffer). Reactions were incubated at 37°C for 5 min, terminated by addition of 2X stop solution (80% v/v formamide, 50 mM EDTA, 0.1% xylene cyanol, 0.1% bromophenol blue) and placed on ice. After heating at 95°C for 3 min, transcription products were resolved on denaturing 6% polyacrylamide sequencing gels (Sequagel system, National Diagnostics) and visualized using a Typhoon FLA 9000 laser scanner (GE Healthcare).
To map transcription termination sites, a portion of hTEC were used for RNA sequencing reactions as described43. Band intensities were measured using ImageQuant software (GE Healthcare) and normalized by subtracting the intensity of a blank region of the same area. Termination efficiency was calculated using the formula: 100 – 100* (runoff band intensity/total lane intensity).
Rho ATPase assays
Rho ATPase assays were performed as described16 using the EnzChek Phosphate Assay kit (Invitrogen). RNA was synthesized as described for in-line probing. 400 nM RNA in 0.75, 1.5 or 3 mM MgCl2 (as indicated in the figure legends) was denatured by heating to 95°C and allowed to re-fold at room temperature for 1 h. The RNA was diluted in the same concentration of MgCl2 to a final concentration of 0, 2, 4, 8, 15, 30, 50 or 100 nM in a 45 µl volume. 49 µl of master mix (50mM TrisHCl pH7.5, 0.1mM sodium azide, 0.4 mM MESG, 0.02 U/ml purine nucleoside phosphorylase, 0.75, 1.5 or 3 mM MgCl2, 10 nM Rho protein) was added. The reactions were briefly centrifuged and left at room temperature for 10 m before adding 5 µl of 20 mM ATP. A SpectraMax Plus microplate reader (Molecular Diagnostics) was used to measure absorbance at 360 nM every 15 sec for 100 min at 37°C. Vmax was taken to be the slope during a 10 min window with the largest linear change in absorbance. A standard curve was generated from reactions using known concentrations of inorganic phosphate and the slope was used to convert Vmax to nmol ATP/min/µg Rho. Data shown correspond to mean values from two independent experiments and error bars indicate standard deviation.
The experiment shown in Fig. 6A was performed in 3 mM rather than 0.75 mM MgCl2 because the M1 and M5 RNAs showed a decrease in ATPase activity at high RNA concentrations when the reactions were performed in 0.75 mM MgCl2, suggesting that saturating activity was reached under this condition. Raising the MgCl2 concentration to 3 mM reduced ATPase activity stimulated by all RNAs and returned the M1 and M5 mutants to an asymptotic curve, allowing for better resolution of differences between mutants. The experiment shown in Fig. 6C was performed in an intermediate MgCl2 concentration of 1.5 mM to allow for resolution of mutants that both increase and decrease ATPase activity.
Filter binding assays
Rho-RNA binding was measured using the nitrocellulose binding method47. RNAs synthesized and 5’ radiolabeled as described for in-line probing were diluted to 0.1 nM in binding buffer (50 mM KCl, 1 mM MgCl2, 20 mM HEPES pH 7.9, 0.1 mM EDTA), heated at 80°C for 3 min, and allowed to re-nature at room temperature for 10 min. Yeast RNA was added to 0.05 mg/ml to prevent non-specific binding. 10 nM to 10 µM dilutions of Rho were prepared in Rho dilution buffer (10 mM Tris-HCL pH 7.9, 100mM KCL, 0.1 mM DTT, 0.1 mM EDTA, 50% v/v glycerol). 100 µl RNA aliquots (10 fmol) were mixed with 10 µl of each Rho dilution, or Rho dilution buffer, and incubated at room temperature for 10 min. Under vacuum, 0.45 µM nitrocellulose filters (Millipore) were washed with 5 ml binding buffer. Following application of the Rho-RNA samples, the filters were immediately washed with 5 ml binding buffer, air dried, and quantified using a Typhoon FLA 9000 laser scanner and ImageQuant software (GE Healthcare). To calculate the percentage of RNA bound, spot intensities were normalized to samples lacking Rho protein and then divided by the intensity of the highest Rho concentration used, which was a 10,000-fold excess and thus assumed to be saturating.
Supplementary Material
Highlights.
The widespread Mg2+ channel CorA was thought to be constitutively expressed
Salmonella corA leader mRNA harbors a Rho-dependent transcription terminator
The corA leader can adopt two conformations that control Rho access to the mRNA
Translation of a short ORF in the corA leader dictates the mRNA conformation
Rho links expression of all Salmonella Mg2+ transporters to translational signals
Acknowledgments
We thank Kerry Hollands and Anastasia Sevostyanova for insightful discussions, technical advice and purified Salmonella Rho, RNA polymerase and σ70 proteins, Max Gottesman for providing bicyclomycin, and Michael Maguire for providing anti-CorA antibody. This work was supported by NIH training grants 5T32AI007640-10 and 5T32GM007223-38 and the Howard Hughes Medical Institute. E.A.G. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- hTEC
halted transcription elongation complex
- nt
nucleotide
- ORF
open reading frame
- PBS
primary binding site
- RNAP
RNA polymerase
- RT
reverse transcription
- rut
Rho utilization
- RBS
ribosome binding site
- SD
Shine-Dalgarno
- TSS
transcription start site
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peters JM, Vangeloff AD, Landick R. Bacterial transcription terminators: the RNA 3’-end chronicles. J Mol Biol. 2011;412:793–813. doi: 10.1016/j.jmb.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boudvillain M, Figueroa-Bossi N, Bossi L. Terminator still moving forward: expanding roles for Rho factor. Curr Opin Microbiol. 2013;16:118–124. doi: 10.1016/j.mib.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Richardson JP, Grimley C, Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975;72:1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adhya S, Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- 5.Modrak D, Richardson JP. The RNA-binding domain of transcription termination factor rho: isolation, characterization, and determination of sequence limits. Biochemistry. 1994;33:8292–8299. doi: 10.1021/bi00193a016. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee S, Chalissery J, Bandey I, Sen R. Rho-dependent transcription termination: more questions than answers. J Microbiol. 2006;44:11–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Bogden CE, Fass D, Bergman N, Nichols MD, Berger JM. The structural basis for terminator recognition by the Rho transcription termination factor. Mol Cell. 1999;3:487–493. doi: 10.1016/s1097-2765(00)80476-1. [DOI] [PubMed] [Google Scholar]
- 8.Skordalakes E, Berger JM. Structure of the Rho transcription terminator: mechanism of mRNA recognition and helicase loading. Cell. 2003;114:135–146. doi: 10.1016/s0092-8674(03)00512-9. [DOI] [PubMed] [Google Scholar]
- 9.Graham JE. Sequence-specific Rho-RNA interactions in transcription termination. Nucleic Acids Res. 2004;32:3093–3100. doi: 10.1093/nar/gkh630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan WD, Bear DG, von Hippel PH. Rho-dependent termination of transcription. II. Kinetics of mRNA elongation during transcription from the bacteriophage lambda PR promoter. J Biol Chem. 1983;258:9565–9574. [PubMed] [Google Scholar]
- 11.Ciampi MS. Rho-dependent terminators and transcription termination. Microbiology. 2006;152:2515–2528. doi: 10.1099/mic.0.28982-0. [DOI] [PubMed] [Google Scholar]
- 12.Wei RR, Richardson JP. Identification of an RNA-binding Site in the ATP binding domain of Escherichia coli Rho by H2O2/Fe-EDTA cleavage protection studies. J Biol Chem. 2001;276:28380–28387. doi: 10.1074/jbc.M102444200. [DOI] [PubMed] [Google Scholar]
- 13.Kim DE, Patel SS. The kinetic pathway of RNA binding to the Escherichia coli transcription termination factor Rho. J Biol Chem. 2001;276:13902–13910. doi: 10.1074/jbc.M011043200. [DOI] [PubMed] [Google Scholar]
- 14.Rabhi M, Gocheva V, Jacquinot F, Lee A, Margeat E, Boudvillain M. Mutagenesis-based evidence for an asymmetric configuration of the ring-shaped transcription termination factor Rho. J Mol Biol. 2011;405:497–518. doi: 10.1016/j.jmb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Epshtein V, Dutta D, Wade J, Nudler E. An allosteric mechanism of Rho-dependent transcription termination. Nature. 2010;463:245–249. doi: 10.1038/nature08669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollands K, Proshkin S, Sklyarova S, Epshtein V, Mironov A, Nudler E, Groisman EA. Riboswitch control of Rho-dependent transcription termination. Proc Natl Acad Sci U S A. 2012;109:5376–5381. doi: 10.1073/pnas.1112211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konan KV, Yanofsky C. Rho-dependent transcription termination in the tna operon of Escherichia coli: roles of the boxA sequence and the rut site. J Bacteriol. 2000;182:3981–3988. doi: 10.1128/jb.182.14.3981-3988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossi L, Schwartz A, Guillemardet B, Boudvillain M, Figueroa-Bossi N. A role for Rho-dependent polarity in gene regulation by a noncoding small RNA. Genes Dev. 2012;26:1864–1873. doi: 10.1101/gad.195412.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa-Bossi N, Schwartz A, Guillemardet B, D’Heygère F, Bossi L, Boudvillain M. RNA remodeling by bacterial global regulator CsrA promotes Rho-dependent transcription termination. Genes Dev. 2014;28:1239–1251. doi: 10.1101/gad.240192.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Cromie MJ, Lee EJ, Groisman EA. A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell. 2010;142:737–748. doi: 10.1016/j.cell.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EJ, Groisman EA. Tandem attenuators control expression of the Salmonella mgtCBR virulence operon. Mol Microbiol. 2012;86:212–224. doi: 10.1111/j.1365-2958.2012.08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sevostyanova A, Groisman E. An RNA motif advances transcription by preventing Rho-dependent termination. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1515383112. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kröger C, Dillon SC, Cameron AD, Papenfort K, Sivasankaran SK, Hokamp K, Chao Y, Sittka A, Hébrard M, Händler K, Colgan A, Leekitcharoenphon P, Langridge GC, Lohan AJ, Loftus B, Lucchini S, Ussery DW, Dorman CJ, Thomson NR, Vogel J, Hinton JC. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A. 2012;109:E1277–E1286. doi: 10.1073/pnas.1201061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh E, Becker AH, Sandikci A, Huber D, Chaba R, Gloge F, Nichols RJ, Typas A, Gross CA, Kramer G, Weissman JS, Bukau B. Selective ribosome profiling reveals the cotranslational chaperone action of trigger factor in vivo. Cell. 2011;147:1295–1308. doi: 10.1016/j.cell.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das A, Court D, Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976;73:1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters JM, Mooney RA, Kuan PF, Rowland JL, Keles S, Landick R. Rho directs widespread termination of intragenic and stable RNA transcription. Proc Natl Acad Sci U S A. 2009;106:15406–15411. doi: 10.1073/pnas.0903846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim B, Sim SH, Sim M, Kim K, Jeon CO, Lee Y, Ha NC, Lee K. RNase III controls the degradation of corA mRNA in Escherichia coli. J Bacteriol. 2012;194:2214–2220. doi: 10.1128/JB.00099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandal M, Breaker RR. Gene regulation by riboswitches. Nat Rev Mol Cell Biol. 2004;5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 29.Reddy P, Peterkofsky A, McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985;82:5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laursen BS, Sørensen HP, Mortensen KK, Sperling-Petersen HU. Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev. 2005;69:101–123. doi: 10.1128/MMBR.69.1.101-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papp-Wallace KM, Maguire ME. Regulation of CorA Mg2+ channel function affects the virulence of Salmonella enterica serovar typhimurium. J Bacteriol. 2008;190:6509–6516. doi: 10.1128/JB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vieu E, Rahmouni AR. Dual role of boxB RNA motif in the mechanisms of termination/antitermination at the lambda tR1 terminator revealed in vivo. J Mol Biol. 2004;339:1077–1087. doi: 10.1016/j.jmb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 34.Graham JE, Richardson JP. rut Sites in the nascent transcript mediate Rho-dependent transcription termination in vivo. J Biol Chem. 1998;273:20764–20769. doi: 10.1074/jbc.273.33.20764. [DOI] [PubMed] [Google Scholar]
- 35.Nikonorova IA, Kornakov NV, Dmitriev SE, Vassilenko KS, Ryazanov AG. Identification of a Mg2+-sensitive ORF in the 5’-leader of TRPM7 magnesium channel mRNA. Nucleic Acids Res. 2014;42:12779–12788. doi: 10.1093/nar/gku951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981;289:751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- 37.Khudyakov YuE, Neplyueva VS, Kalinina TI, Smirnov VD. Effect of structure of the initiator codon on translation in E. coli. FEBS Lett. 1988;232:369–371. doi: 10.1016/0014-5793(88)80771-3. [DOI] [PubMed] [Google Scholar]
- 38.Pontes MH, Sevostyanova A, Groisman EA. When too much ATP is bad for protein synthesis. J Mol Biol. 2015 doi: 10.1016/j.jmb.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 42.Snavely MD, Gravina SA, Cheung TT, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J Biol Chem. 1991;266:824–829. [PubMed] [Google Scholar]
- 43.Artsimovitch I, Henkin TM. In vitro approaches to analysis of transcription termination. Methods. 2009;47:37–43. doi: 10.1016/j.ymeth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regulski EE, Breaker RR. In-line probing analysis of riboswitches. Methods Mol Biol. 2008;419:53–67. doi: 10.1007/978-1-59745-033-1_4. [DOI] [PubMed] [Google Scholar]
- 46.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. Assay of B-galactosidase. [Google Scholar]
- 47.Carey J, Cameron V, de Haseth PL, Uhlenbeck OC. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983;22:2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.