Abstract
We investigated the relationship between high-grade cervical disease (cervical intraepithelial neoplasia [CIN] 2, CIN3 or adenocarcinoma in situ) and persistent infection with HPV16 and/or HPV18 (HPV16/18) among 3970 women who received placebo in 3 clinical trials of a quadrivalent HPV vaccine. Statistical analysis (odds ratios, sensitivity, specificity, negative and positive predictive values, negative and positive likelihood ratios) showed that patients with a persistent infection with HPV16/18 had a much greater risk of HPV16/18-related high-grade cervical disease. Furthermore, subjects without a persistent infection with HPV16/18 were unlikely to have HPV16/18-related high-grade cervical disease. These results suggest that persistent infection with HPV16/18 meets the criteria for a surrogate endpoint for HPV16/18-related high-grade cervical disease and may be used as such in future clinical studies with prophylactic HPV vaccines and in natural history studies.
Keywords: cervical intraepithelial neoplasia, cervical cancer, statistics, HPV, Prentice criteria, surrogate endpoint
Introduction
There are several potential endpoints for HPV-related high-grade cervical disease in clinical studies of vaccines or natural history studies. Using cancer as an endpoint is unethical and impractical. Most invasive cancers take 2-3 decades to occur after initial infection with oncogenic HPV and there are effective secondary prevention strategies to treat the obligate intra-epithelial precursors to cervical cancer, that is, cervical intraepithelial neoplasia (CIN) grade 2-3. CIN2-3 and adenocarcinoma in situ (AIS) (abbreviated as CIN2+) have been accepted by the US. Food and Drug Administration and the World Health Organization as an endpoint in clinical trials of HPV vaccines.1-3 CIN1 or a single detection of HPV infection are not considered acceptable endpoints, as CIN1 usually clears spontaneously and a single detection of HPV infection usually does not progress to HPV-related high-grade cervical disease.4 A meta-analysis of 86 studies found that persistent infection with high-risk HPV was the strongest risk factor for high-grade cervical precancers.5 An earlier meta-analysis of 41 studies found that HPV persistence was consistently and strongly associated with CIN2-3/high-grade squamous intraepithelial lesions.6
The evidence is robust that persistent infection with HPV is the major risk factor for HPV-related high-grade cervical disease.5,6 The justification of using persistent infection with HPV16 and/or HPV18 (HPV16/18) as a surrogate endpoint for high-grade cervical disease (CIN2+) related to those HPV types is the focus of this communication.
Results
The proportion of subjects who received placebo, who were naïve to HPV16/18 at day 1, and subsequently had HPV16/18-related persistent infection of 6- or 12-months duration was 368/3689 (10.0%) or 249/3689 (6.7%), respectively (Table 1). Thirty-nine of 3689 subjects (1%) who received placebo were diagnosed with HPV16/18-related high-grade cervical disease within the follow-up period of the study (Table 1). The majority (38/39) of subjects diagnosed with HPV16/18-related high-grade cervical disease were HPV16/18 DNA positive in cervical swabs on at least one occasion prior to diagnosis (Fig. 1 and Table 1). The median duration of HPV16/18-related persistent infection prior to diagnosis of HPV16/18-related high-grade cervical disease was 8.3 months (mean = 12.0 months; interquartile range = 5.8 to 17.7 months). One patient was diagnosed with HPV16-related CIN2+ and this CIN2+ lesion was also coinfected with HPV39 and HPV52. This patient had no prior HPV16 DNA positivity in swabs or tissue samples before the diagnosis of HPV16-related CIN2+, but was positive for HPV39 in swabs at each visit of the study starting from Day 1. Thus, it is reasonable to infer that HPV39 was the causative type for disease in that patient. Note that 211 subjects with 12-month HPV16/18-related persistent infections and 330 subjects with 6-month HPV16/18-related persistent infections did not develop HPV16/18-related CIN2+ during the follow-up period (Table 1); the difference comprises subjects clearing the infection or those with less than 12 months persistence by the end of the study.
Table 1.
HPV 16/18-related CIN2+ by prior 6-month or 12-month HPV16/18 persistent infection. Subjects received placebo and were naïve to the relevant HPV type at day 1.
| Duration of Prior Persistent Infectiona | Total Number of Subjects | Number of Subjects with HPV16/18-Related CIN2+ | Number of Subjects Without HPV16/18-Related CIN2+ |
|---|---|---|---|
| 12 month | 249 | 38b | 211 |
| None | 3440 | 1c | 3439 |
| 6 month | 368 | 38b | 330 |
| None | 3321 | 1c | 3320 |
Prior persistent infection with same type detected in CIN2+.
32 subjects with HPV16-related CIN2+; 6 subjects with HPV18-related CIN2+.
HPV16-related CIN2+.
Figure 1.
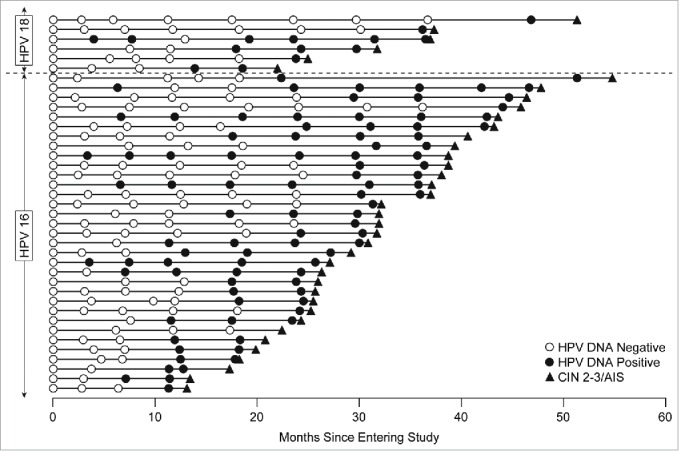
Timeline of HPV16/18 positivity in subjects who developed HPV16/18-related CIN2+. Each horizontal line corresponds to one of the 39 cases; the horizontal axis shows time since entering the study. Circles indicate cervical swab sampling visits: open circles are shown when the subject was HPV DNA negative to the type which was later present in the disease; closed circles show DNA positivity to that HPV type. The closed triangle indicates the diagnosis of HPV16/18-related CIN2, CIN3 or AIS. Though the definition of persistent infection allowed for a swab or biopsy sample to be positive for the same HPV type after a pathology panel diagnosis of disease, in all of the cases shown in Figure 1, the persistent infection was detected prior to definitive therapy.
Prentice proposed 4 statistical conditions which a valid surrogate endpoint should satisfy.7 These are: 1) the treatment has a significant effect on the surrogate endpoint; 2) the treatment has a significant effect on the true endpoint; 3) the surrogate endpoint has a significant effect on the true endpoint; and 4) the full effect of the treatment upon the true endpoint is captured by the surrogate. The case of HPV16/18-related persistent infection as a surrogate for high-grade disease using criteria 1 and 2 are satisfied, as the vaccine is highly efficacious for cervical disease related to vaccine types8,9 and efficacy for 6-month and 12-month persistent infection for HPV16/18 in the 3 studies combined was 96.2% (95% CI: 92.9, 98.2) and 95.8% (95% CI: 91.1, 98.3) in the per-protocol population.8,9 Criterion 3 is satisfied, as shown by the analyses summarized in Table 2. Crude odds ratios were high (250–750) for both 6-month and 12-month persistent infections, indicating a much greater risk of disease for those who have persistent infection. Negative predictive values were all 1.0, indicating that it is virtually impossible to develop HPV16/18-related disease without a preceding HPV16/18 infection. Positive predictive values ranged from 0.052 to 0.165, indicating that a small proportion of subjects with an HPV16/18-related persistent infection will develop HPV16/18-related high-grade cervical disease, although this is dependent upon disease prevalence and duration of follow-up. Specificity was 91–98%, indicating that there were a high proportion of subjects without high grade cervical HPV16/18-related disease who also did not have an HPV16/18-related persistent infection. High sensitivity (97–100%) indicated that there were few false negatives (subjects who had high-grade cervical disease but did not have HPV16/18 persistent infection). Finally, the high positive likelihood ratios indicated that HPV16/18-related persistent infection may be a strong predictor of HPV16/18-related high-grade cervical disease.
Table 2.
Statistical summary measures of the relationship between HPV16/18-related persistent infection (6-month and 12-month) and HPV16/18-related CIN2+.
| Duration of Prior HPV Persistent Infectiona | HPV Type | Odds Ratio (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 6-month | HPV16/18 | 257.9 (50.3, 1322.9) | 97% (93, 100) | 91% (90, 92) | 0.103 (0.072, 0.134) | 1.00 (0.99, 1.00) | 10.8 (9.4, 11.4) | 0.03 (<0.01, 0.20) |
| HPV16 | 250.1 (48.4, 1291.8) | 97% (91, 100) | 92% (91, 93) | 0.113 (0.076, 0.150) | 1.00 (0.99, 1.00) | 12.2 (10.5, 13.3) | 0.03 (0.01, 0.23) | |
| HPV18 | 408.6 (22.9, 7298.8) | 100% (NA) | 97% (96, 98) | 0.052 (0.012, 0.093) | 1.00 NA | 32.6 (27.5, 39.9) | 0.00 (NA) | |
| 12-month | HPV16/18 | 417.4 (81.2, 2145.6) | 97% (93, 100) | 94% (94, 95) | 0.153 (0.108, 0.197) | 1.00 (0.99, 1.00) | 16.9 (14.4, 18.6) | 0.03 (NA) |
| HPV16 | 397.3 (76.7, 2057.1) | 97% (91, 100) | 95% (94, 96) | 0.165 (0.113, 0.217) | 1.00 (0.99, 1.00) | 18.8 (15.8, 21.1) | 0.03 (NA) | |
| HPV18 | 750.0 (41.8, 13464.4) | 100% (NA) | 98% (98, 99) | 0.091 (0.022, 0.160) | 1.00 NA | 59.2 (46.7, 78.7) | 0.00 (NA) |
Prior HPV persistent infection with same HPV type detected in CIN2+.
CI = Confidence Interval
NA = Not applicable
Criterion 4, the most stringent criterion for surrogacy, was assessed by modeling the true endpoint (high-grade cervical disease) as a function of treatment, with or without the surrogate in the model. If persistent infection is a good surrogate, the size of the vaccine effect will be much reduced, and not statistically significant. The “proportion explained' is the percentage reduction in the treatment effect after adjustment for the surrogate. Regardless of infection duration, the addition of persistent infection to the model rendered the vaccine effect non-significant, and the magnitude of the vaccine effect was reduced by 75–83%. This suggests the effect of the vaccine on high-grade cervical disease is mediated through it's impact on persistent infection.
Discussion
Our findings suggest that HPV16/18-related persistent infection may be a suitable surrogate marker for HPV16/18-related high-grade cervical disease because persistent infection met all of the 4 Prentice criteria for surrogacy.7 In addition to the established impact of HPV vaccines on both persistent infection and high grade cervical disease, this paper demonstrates that persistent infection is strongly associated with high-grade cervical disease; and that efficacy against high-grade cervical disease is fully mediated through the effect on persistent infection. Thus, all the statistical criteria for a surrogate endpoint of HPV persistent infection were fulfilled.
The Prentice criteria are stringent and the evaluation of surrogate endpoints cannot be assessed using only statistical evidence, but should also consider clinical and biological outcomes.11 One of the greatest advances in cancer research in the last 2 decades has been the demonstration that infection with certain types of HPV is a necessary cause of cervical cancer.12,13 Using surrogate endpoints in the development of prophylactic vaccines is necessary, as the use of a cervical cancer as endpoint is unethical. Using persistent infection instead of disease endpoints would allow for smaller clinical studies and faster development. However, the use of persistent infection rather than the currently accepted endpoint of CIN2+ will require a definition of HPV persistent infection to be established and agreed upon: should it be 6 months, 12 months or some other duration? In this analysis, some measures of association (specificity; positive predictive values; positive likelihood ratios) were stronger for 12-month HPV persistent infection compared to 6-month persistent infection but other measures (sensitivity and negative predictive values) were similar for 6-month and 12-month persistent infections. Our study has limitations: it is not clear if the strong relationship reported here between persistent infection and CIN2+ for HPV16/18 will apply equally to other high-risk HPV types. It will be difficult to assess the relationship for less prevalent HPV types, due to the scarcity of related CIN2+ cases. In addition, if a virological end-point is to be used as a primary end-point in vaccine clinical trials, there must be high sensitivity, specificity, and reproducibility of the genotyping methods.14 One of the studies included here was in women aged 24–45 years; the results in that study are consistent with those in younger women, but the relative scarcity of cases (8 out of 39 total) prevent definitive conclusions. With follow-up periods longer than those used in these studies (4 or 5 years), it is likely that some of the agreement measures reported here would be even stronger, as women continued to develop high-grade cervical disease if infected. Nonetheless, our study suggests that persistent infection with HPV16/18 may serve as a clinically useful endpoint in clinical studies of prophylactic HPV vaccines.
Material and methods
Analyses
This study analyzed the relationship between persistent HPV16/18-related infection and subsequent development of HPV16/18-related high-grade cervical disease among 3970 women who were randomized to the placebo arm of one of 3 clinical trials of a quadrivalent HPV vaccine. The number of women who received a placebo in each of the 3 trials were 1907 women 24 to 45 y of age (Protocol-019, a Phase III study),15 1788 women 16 to 26 y of age (Protocol-012, a substudy of a Phase III study [FUTURE I] ),8 and 275 women 16 to 23 y of age (Protocol-007, a Phase II study).16 Women were followed up for 4 y (Protocols-019 and −012) or 5 y (Protocol-007).
In each study, comprehensive anogenital examinations were conducted at each scheduled visit at which time an endo/ectocervical swab (one specimen) and a combined labial/vulvar/perineal plus a perianal swab (pooled to become second specimen) were collected. The swabs were tested for 14 HPV types (6/11/16/18/31/33/35/39/45/51/52/56/58/59) using a PCR-based assay.16-18 Cerivcal biopsy and excisional specimens (i.e. endocervical curettage, loop electrosurgical excision procedure, or conization [cold knife/laser]) were formalin fixed, paraffin embedded, and cut into 4-μm thin sections, as previously described.8,9 Adjacent histologic sections of each sample were read for end-point determination (i.e., CIN2, CIN3, AIS, or cervical cancer) by a panel of 4 pathologists who were blinded to treatment group and HPV status. The disease was determined to be HPV16/18-related if HPV16/18 DNA was detected in an adjacent section from the same tissue block, as previously described.16-18 Persistent infection and disease were determined specific to each type, without reference to other HPV types that may have been detected in the same subject or specimen. Subjects infected with HPV16/18 at enrollment were excluded.
Definition of persistent infection
In the context of the clinical trials, persistent infection was defined as detection of HPV by PCR testing over multiple visits covering a specified time interval with no intervening negative results. For the most part, infection was detected from the swab samples collected at 6-monthly scheduled visits.16 However, in order to comprehensively capture all infections from all specimens collected in the studies, a more complex definition was developed, incorporating tissue samples (i.e. cervical biopsy and excisional specimens) as well as swabs. In this study, persistent infection was defined as either (i) detection of the same HPV type (i.e., HPV16 or HPV18) in genital swabs or tissue samples at ≥2 consecutive visits spaced ≥6 or ≥12 months apart; or (ii) a biopsy showing high-grade disease associated with HPV16 or HPV18, with DNA for that same type found in the swab or tissue sample obtained at the visit directly before or after the biopsy (regardless of interval). ‘Duration of infection’ as presented here is strictly duration prior to the diagnosis of CIN2+: the time from first PCR positive sample to the biopsy showing CIN2+. No post-diagnosis period of infection (if it existed) was used.
The relationship between persistent infection and high-grade cervical disease was quantified by odds ratios, sensitivity and specificity,19 predictive values,20 and likelihood ratio statistics.10 If a zero cell count made a statistic undefined, 0.5 was added to each cell in the table. Confidence intervals (CI) for likelihood ratios were generated by bootstrapping; where this failed to produce an interval, the method of Simel et al.21 was used. Adequacy of persistent infection as a surrogate endpoint for CIN2+ was assessed via the Prentice criteria.7 The fourth Prentice criterion was assessed by both a Cox proportional hazards model using time to HPV16/18-related CIN2+ as the dependent variable, and by a logistic regression model using incidence of HPV16/18-related CIN2+ or worse as the dependent variable.14 In both models we adjusted for age.
Abbreviations
- AIS
adenocarcinoma in situ
- CIN
cervical intraepithelial neoplasia
Funding
This study was supported by Merck & Co., Inc., Whitehouse Station, NJ.
Disclosure of potential conflicts of interest
D. Radley is a former employee of Merck & Co., Inc., Whitehouse Station, NJ, holds stock and/or stock options, and is now at Pfizer. A. Saah is an employee of Merck & Co., Inc., Whitehouse Station, NJ, and holds stock and/or stock options. M. Stanley received speaker fees from Merck Sharp & Dohme, MSD, and was a consultant for Sanofi Pasteur MSD and GlaxoSmithKline Biologicals.
Acknowledgments
All authors were involved in the collection, analysis, or interpretation of the data; revision of the manuscript; and the decision to submit the manuscript for publication. The assistance of Frank Dutko (Merck & Co., Inc.) in writing the manuscript and the technical assistance of Karyn Davis (Merck & Co., Inc.), Scott Vuocolo (Merck & Co., Inc.) and Sheila Erespe (Merck & Co., Inc.) are very much appreciated. All authors vouch for the completeness and accuracy of the data and analyses. The views expressed herein are those of the authors and do not reflect the official policy or position of Merck & Co., Inc.
References
- [1].Pagliusi SR, Aguado TA. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine 2004; 23:569-78; PMID:15630792; http://dx.doi.org/ 10.1016/j.vaccine.2004.07.046 [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization Guidelines to assure the quality, safety and efficacy of recombinant human papillomavirus virus-like particle vaccines. In: WHO Expert Committee on Biological Standardization. 57th Report Geneva 2006. Accessed Nov. 4, 2015, http://www.who.int/biologicals/vaccines/hpv/Annex_1_WHO_TRS_962-2.pdf?ua=1 [Google Scholar]
- [3].World Health Organization International Agency for Research on Cancer Primary end-points for prophylactic HPV vaccine trials. IARC Working Group Report 2014; 7. Accessed June 30, 2015. Available at http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk7/Prophylactic_HPV_VaccineTrials.pdf [PubMed] [Google Scholar]
- [4].Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev 2013; 22:553-60; PMID:23549399; http://dx.doi.org/ 10.1158/1055-9965.EPI-12-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C, Smith JS. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int J Cancer 2013; 133:1271-85; PMID:22961444; http://dx.doi.org/ 10.1002/ijc.27828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol 2008; 168:123-37; PMID:18483125; http://dx.doi.org/ 10.1093/aje/kwn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431-40; PMID:2727467; http://dx.doi.org/ 10.1002/sim.4780080407 [DOI] [PubMed] [Google Scholar]
- [8].Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, et al.. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928-43; PMID:17494926; http://dx.doi.org/ 10.1056/NEJMoa061760 [DOI] [PubMed] [Google Scholar]
- [9].FUTURE II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915-27; PMID:17494925; http://dx.doi.org/ 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- [10].Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ 2004; 329:168-9; PMID:15258077; http://dx.doi.org/ 10.1136/bmj.329.7458.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alonso A, Molenberghs G, Burzykowski T, Renard D, Geys H, Shkedy Z, Tibaldi F, Abrahantes JC, Buyse M. Prentice's approach and the meta-analytic paradigm: a reflection on the role of statistics in the evaluation of surrogate endpoints. Biometrics 2004 Sep; 60:724-8; PMID:15339295; http://dx.doi.org/ 10.1111/j.0006-341X.2004.00222.x [DOI] [PubMed] [Google Scholar]
- [12].Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- [13].Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518-27; PMID:12571259; http://dx.doi.org/ 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- [14].World Health Organization International Agency for Research on Cancer Primary end-points for prophylactic HPV vaccine trials. IRAC Working Group Report 2014; 7. Accessed June 30, 2015. Available at http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk7/Prophylactic_HPV_VaccineTrials.pdf. [PubMed] [Google Scholar]
- [15].Castellsague X, Munoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al.. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer 2011; 105:28-37; PMID:21629249; http://dx.doi.org/ 10.1038/bjc.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, Skjeldestad FE, et al.. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271-8; PMID:15863374; http://dx.doi.org/ 10.1016/S1470-2045(05)70101-7 [DOI] [PubMed] [Google Scholar]
- [17].International patent numbers WO 2003/019143 A2, WO 2006/116276 A2, and WO 2006/116303 A2. Available at http://www.wipo.int/portal/en [Google Scholar]
- [18].Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, Alvarez FB, Bautista OM, Jansen KU, Barr E. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol 2006; 107:18-27; PMID:16394035; http://dx.doi.org/ 10.1097/01.AOG.0000192397.41191.fb [DOI] [PubMed] [Google Scholar]
- [19].Altman DG, Bland JM. Diagnostic tests. 1: sensitivity and specificity. BMJ 1994; 308:1552; PMID:8019315; http://dx.doi.org/ 10.1136/bmj.308.6943.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ 1994; 309:102; PMID:8038641; http://dx.doi.org/ 10.1136/bmj.309.6947.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 1991; 44:763-70; PMID:1941027; http://dx.doi.org/ 10.1016/0895-4356(91)90128-V [DOI] [PubMed] [Google Scholar]


