ABSTRACT
Rotavirus is the most common cause of severe diarrhea leading to hospitalization or disease-specific death among young children. Effective vaccines have recently been approved and successful vaccination program implemented. The aim of this study was to evaluate the cost effectiveness of mass rotavirus vaccination program in Iran. We developed a Markov model that reflects key features of rotavirus natural history. Parameters of the model were assessed by field study or developed through literature search and published data. We applied the model to the 2009 Iranian birth cohort and evaluated the cost-effectiveness of including the rotavirus vaccine (Rotarix®) into Iranian expanded immunization program (EPI). With an estimated hospitalization rate of 0.05 and outpatient rate of 0.23 cases per person-year, vaccinating cohort of 1231735 infants in Iran with 2 doses of (Rotarix®), would prevent 32092 hospitalizations, 158750 outpatient visits, and 1591 deaths during 5 y of follow-up. Under base-case assumption of $10 cost per course of vaccine, the vaccination would incur an extra cost of $1,019,192 from health care perspective and would avert 54680 DALYs. From societal perspective, there would be $15,192,568 saving for the society with the same averted DALYs. The incremental cost effectiveness ratio showed a cost of $19 US dollars per averted DALY from health care perspective and a saving of $278 US dollars for each averted DALY from societal perspective. Introducing rotavirus vaccine into EPI program would be highly cost-effective public health intervention in Iran.
Keywords: Cost-effectiveness, Iran, rotavirus, rotavirus incidence, vaccination
Introduction
Rotavirus is a leading cause of morbidity among children less than 5 y in both developed and developing countries. 2008, the burden of diarrhea due to rotavirus was estimated 453000 deaths and 27 million medical visits and majority of these took place in low and middle income countries.1,2 In low income countries the median age at the primary rotavirus infection ranges from 6 to 12 months whereas in high income countries, the first episode may be delayed for 2 to 3 y.2,3,4 induces partial immunity and subsequent (secondary and tertiary) infections are presented clinically mild or asymptomatic.5 Introduction of rotavirus vaccination in 2006 has had major effect on reducing the burden of rotavirus diarrhea in countries with mass vaccination program.6 At present, there are 2 vaccines licensed (Rotarix® by GlaxoSmithKline and RotTeq® by Merk) and their field effectiveness documented in the range of 50% in some African countries to more than 90% in European countries.2,7 Both vaccines are recommended by WHO and provide similar benefits at similar cost for low and middle income countries. Rotavirus vaccine has been introduced in national immunization program of many countries such as Mexico, Brazil, USA, Australia, and Belgium, South Africa.8-11 Studies of cost effectiveness of rotavirus vaccine in many countries have proven to be cost-effective from both societal and health care perspective12-17 however the mass vaccination program has been shadowed by the high price of vaccine18 and the variability of vaccine's field effectiveness due to frequently evolving capability of rotavirus genome.7,19 In this paper we present the result of the cost effectiveness study of vaccination in Iran from both health care and societal perspective.
Result
Health outcome and utilization
In the absence of vaccination at base case scenario, 5% of children less than 5 y will be hospitalized for rotavirus diarrhea with mean ± sd(standard deviation) duration of 3.0 ± 1.9 d and 23% of children less than five years need outpatient care for rotavirus diarrhea. In average, each case of rotavirus diarrhea contributes a mean ± sd of 4.3 ± 4.1 d of productivity loss. With vaccination coverage of 97% and vaccine efficacy at base case scenario, the hospitalization rate will decrease 13 times (from 50 to 3 case per 1000 children per year) and the outpatient care will decrease 4 times (from 228 to 64 cases per 1000 children per year). Considering the cohort of 1,231,735 infants born in 2009 to Iranian population, the vaccination at base case scenario would prevent 32,092 cases of hospitalizations, 158,750 cases of outpatient visits, and 1,591 deaths due to rotavirus infection (Table 2). Translating the health outcomes into summary measures of healthy life, vaccination would avert 54,680 DALYs (discounted at 3% per annum) among the cohort born in 2009 (Table 2).
Table 2.
Result of the cost effectiveness analysis for a cohort of 1231735 children born in 2009 in Iran.
| Not Vaccinated | Vaccinated | Differences | ||
|---|---|---|---|---|
| Utilization | ||||
| Hospitalization | 42310 | 3217 | 32092 | |
| Out Patient visit | 211802 | 53053 | 158750 | |
| Death | 1725 | 156 | 1591 | |
| Cost (in US dollar) | ||||
| Health Care | 11,878,982 | 12,898,174 | −1,019,192 | |
| Societal | 31,633,769 | 16,441,202 | 15,192,568 | |
| Effectiveness (in DALY) | 59957 | 5277 | 54680 | |
Cost prospect
The rotavirus infection will incur major direct hospitalization and out-patient costs to the health care system and major indirect cost related to productivity loss, transportation, and accommodation to the society. In the absence of vaccination, the rotavirus infection for the cohort of infant born in 2009, would incur cost of 11,878,982 US dollars for health care perspective and 31,633,769 US dollars for societal perspective for 5 y follow-ups of the cohort. Vaccination program with the base case scenario would cost 1,019,192 dollars (discounted at 3% annum) for ministry of health.
The effectiveness
In the absence of vaccination, the average DALY lost to diarrhea among the cohort of infant born in 2009 would be 49 DALYs per 1000 children per year and vaccination will lower this rate to 4.3 DALYs per 1000 children per year.
The cost-effectiveness
The incremental cost effectiveness ratio (ICER) at the base case showed a cost of 19 US dollars for each DALY averted from the perspective of health care and a saving of 278 US dollars for each DALY averted from the societal perspective. Considering the WHO recommendation criteria for being cost-effective, the ICER from both perspectives was far lower than the estimated Gross Domestic Product (GDP) for the year 2009 (the World Bank estimated GDP for Iran in 2009 was $4,931 us dollars) indicating that the vaccination is highly cost effective in our population.
Uncertainty analysis
For both perspective (health care and societal), the ICER was more sensitive to the incidence of diarrhea, vaccine price, and the cost of hospitalization (Fig. 2. diagram A and B). example, when incidence was varied between 0.16 to 0.70 cases of diarrhea per person-year, the incremental cost-effectiveness ratio ranged from a cost of $75 to a saving of $286 per averted DALY from heath care perspective (Fig. 2. diagram A). the societal perspective, the corresponding ICER was $114 saving for lower level of incidence and a saving of $1022 for upper level for each DALY averted (Fig. 2. diagram B).
Figure 2.
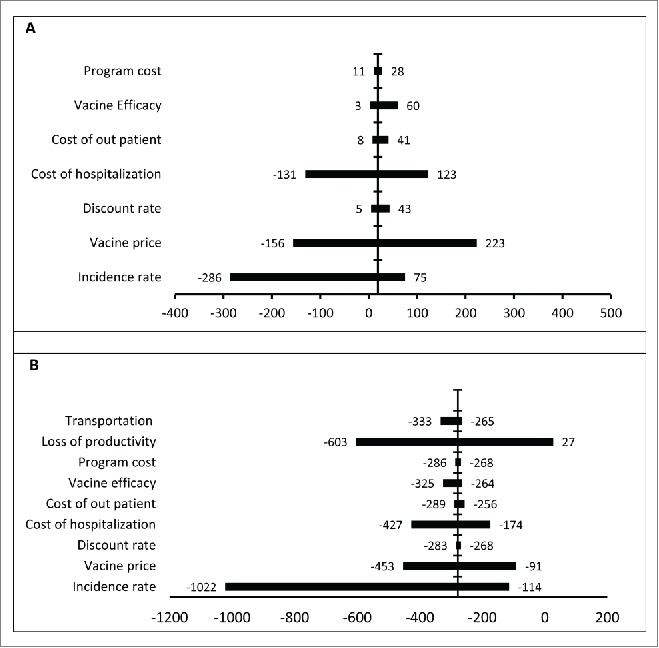
Results of one-way sensitivity analyses. This graph presents results of univariate sensitivity analyses from the health care (A) and societal perspective (B). The x-axis represents the ranges of the incremental cost-effectiveness ratios of vaccination in Iranian children when the baseline estimates of key parameters were varied over plausible ranges. The vertical line represents the base case incremental cost-effectiveness ratio of rotavirus vaccination. The negative values indicate saving per averted DALY.
Vaccine price was another variable that its variability had a major effect on the ICER from both perspectives. the price of vaccine was set at $1 per dose, the incremental cost-effectiveness ratio was dominant indicating saving of $156 and $453 for health care and societal perspectives, respectively (Fig. 3). From societal perspective, the ICER remains in a saving state for all values of the vaccine price (Fig. 3). From the health care perspective, the saving would continue up to when the price of vaccine passes $9 after which there is cost for increasing the price of vaccine (Fig. 3).
Figure 3.
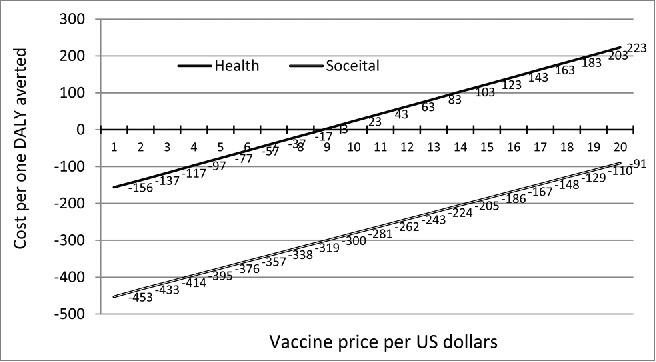
The incremental cost- effectiveness ratio (ICER) for different price of vaccine from both health care and societal perspective.
In the sensitivity analysis from the societal prospective, the loss of productivity had a large effect in cost effectiveness; the ICER ranged from a cost of $27 to a saving of $603 dollars per averted DALY (Table 1 and Figure 2 diagram B). The cost of hospitalization had large effect in health care perspective; the ICER ranged from a cost of $123 to a saving of $131 per DALY averted for low and high values, respectively. At the societal perspective, the low and high values of the cost of hospitalization did not change the saving state of the program (Fig. 2 diagram B). The ICER was robust to vaccine efficacy; discount rate, vaccine wastage, and program cost for both perspectives (Fig. 2 diagrams A and B).
Table 1.
Model input parameter values and ranges.
| Variable | Estimate Base | Low | Hi | Source | |
|---|---|---|---|---|---|
| Hospitalization rate (primary) Age group (in month) | |||||
| 2–5 | 0.005 | 0.004 | 0.016 | Estimated refer to appendix 1 | |
| 6–11 | 0.018 | 0.013 | 0.055 | ||
| 12–23 | 0.020 | 0.014 | 0.062 | ||
| 24–35 | 0.004 | 0.003 | 0.013 | ||
| 36–59 | 0.004 | 0.003 | 0.011 | ||
| Out Patient Rate (primary) | |||||
| 2–5 | 0.023 | 0.012 | 0.053 | ||
| 6–11 | 0.080 | 0.043 | 0.185 | ||
| 12–23 | 0.091 | 0.048 | 0.211 | ||
| 24–35 | 0.018 | 0.010 | 0.042 | ||
| 36–59 | 0.016 | 0.009 | 0.037 | ||
| Mortality rate from Rotavirus | |||||
| 2–5 | 0.0003 | 0.0003 | 0.0004 | ||
| 6–11 | 0.0001 | 0.0010 | 0.0012 | ||
| 12–23 | 0.0006 | 0.0006 | 0.0007 | ||
| 24–35 | 0.0001 | 0.0001 | 0.0001 | ||
| 36–59 | 0.0001 | 0.0001 | 0.0001 | ||
| Costs in Dollars per case | |||||
| Hospitalization | 214 | 73 | 416 | Estimated | |
| Out-Patient Visit | 16 | 13 | 19 | Estimated | |
| Loss of productivity (hospitalized) | 161 | 3 | 834 | Estimated | |
| Lose of productivity (outpatient) | 54 | 3 | 278 | Estimated | |
| Transportation (Hospitalized) | 11 | 3 | 40 | Estimated | |
| Transportation (outpatient) | 4 | 2 | 13 | Estimated | |
| Other societal cost | 27 | 3 | 120 | Estimated | |
| Vaccine cost per case | 10 | 1 | 20 | [16] | |
| Program cost per case | 0.25 | 0.05 | 0.5 | [16] | |
| Other Epidemiologic parameters | |||||
| Ratio of second infection VS first | 0.62 | 0.5 | 0.83 | [6] | |
| Ratio of third infection VS first | 0.4 | 0.28 | 0.59 | [6] | |
| Effectiveness parameters | |||||
| Discount rate | 0.03 | 0 | 0.05 | [50] | |
| Disability weight | 0.119 | 0.086 | 0.152 | [51] | |
| Vaccine Efficacy in percent | |||||
| Hospitalization | 93 | 75 | 95 | [16] | |
| first year | 93 | 75 | 95 | [16] | |
| at or after 1 y of age | 93 | 75 | 95 | [16] | |
| Outpatient | |||||
| first year | 78 | 75 | 95 | [16] | |
| at or first year of age | 75 | 75 | 95 | [16] | |
| Death | |||||
| first year | 75 | 75 | 95 | [16] | |
| at or first year of age | 93 | 75 | 95 | [16] | |
| Vaccine other parameters | |||||
| Coverage | 0.966 | 0.85 | 0.99 | [34] | |
| Wastage | 0.05 | 0.02 | 0.15 | [34] | |
Discussions
This study showed that the rotavirus vaccination program in Iran appears to be highly cost effective from both health care and societal perspective using the WHO criterion of having a cost per averted DALY below GDP. The robustness of results of our study depends on the assumptions of our model and the validity of our estimated parameters. The natural history of rotavirus infection is very complicated and this complication is highlighted with the fact that infection does not produce a long lasting protection and the chance of subsequent infection exists though this chance is lower and the severity infection is milder. The uncertainty in the magnitude and duration of partial protection can have great effect on validity of our results. Our model addressed the chance of subsequent infection and included up to 2 subsequent infections. In the light of the fact that the number of cases after second infection was very low, including just 2 subsequent infections (instead of 3 or 4 as some studies did 20) has not compromised validity of our result.
There was no community based study to estimate the incidence of confirmed cases of rotavirus in Iran. The Sentinel Hospital-based Surveillance of Rotavirus Diarrhea in Iranian (SHSRD) was not a through sample of all occurring cases and its reported figures was not qualify to estimate the incidence of even hospitalization in our population. Our estimated figures for incidence were in line with other studies. In a Meta analysis of reported incidence of rotavirus infection, the pooled estimate was 0.24 (0.17, 0.5) per person year of observation for children of less than 2 y Our base-case estimate of 0.24 for the first 2 y (based on the Table 1) is almost the same as the point of estimate for reported pooled estimate. There are no studies to report the incidence of rotavirus in countries of Middle East except one community based study from Egypt that reported incidence of 0.19 per person per year among the under 5 y which is close to our estimate.21 In addition, the many epidemiologic parameters used in our study (especially the proportions used in the incidence estimates) are in agreement with the pooled estimates of the same parameters reported in a recent systematic review by Mark A, et al.22 who pooled studies of rotavirus diarrheal in countries of Middle East.
In cost effectiveness analysis of rotavirus diarrhea hospitalization is a major contributing factor to overall cost effectiveness of vaccination. Our rate of 43 hospitalizations per 1000 is very close to the estimated regional rate of 45.9 ± 25% hospitalizations per 1000 children for countries middle income in Asia13 and close to the estimate from the community based study in Egypt 21 (30 hospitalizations per 1000 children per year).
Another major and determining factor in estimating cost effectiveness is how accurate is the cost estimates for different component of the model. Our estimate of the cost for hospitalization was based on field study that was design for the purpose of this study. One limitation for this part of our estimates was the fact that the studied population may not be a good representative of the whole country (at least from a socio economic stand point) but as costs of many services delivered by the government are similar all over the country this lack of representativeness would not have major effect in our overall result from both perspectives.
Our base-case estimate of $214 for hospitalization and $16 for outpatient is in the range of WHO CHOICE23 estimates for the year of 2009 for Iran, however they are higher than estimates for countries of similar income by Laura Jean Podewils et al.13 and estimates by Javanbakht M, et al.17 that estimated 174.52 for inpatient and 4.69 for outpatients cost for government perspective. The difference in our estimates from Javanbakht M, et al. estimates originates from differences in methodology of estimation; in Javanbakht M, et al., the outpatient cost (where high discrepancy is seen) estimate was based on expert opinions while ours was based on national utilization surveys and government tariff. Both studies (the Javanbakht M, et al. and our study) used the same methodology to estimate inpatient costs (both study used patient's medical records from hospitals to estimate), however our study used patient's records from many hospitals while they used just one hospital. In addition, the 2 studies had different definitions and cost components (for example we included loss of productivity in our societal perspectives while they did not) that explain the discrepancies seen in cost estimates and cost per averted DALYs between 2 studies.
Our study has its own limitations among the most important ones are; 1) the estimate of burden of rotavirus that was not based on a well defined community based study, 2) lack of locally generated data on the vaccine efficacy as the efficacy has been heterogeneous across different countries,7 3) the fact that we did not include indirect effect of vaccination in our Marko model, 4) the fact that our analysis did not include a probability sensitivity analysis, and 5) and the fact that we did included data on adverse effect of vaccination in our analysis.
Conclusion
Introducing rotavirus vaccine would be highly cost-effective public health intervention in Iran.
Methods
The economic model
An age structured cohort model was used to estimate the cost effectiveness of vaccination. The cohort of children born in 2009 to Iranian population was followed for 5 y (model's time horizon) based on 2 scenarios: 1) vaccinated with 2 doses of vaccine (at 2 and 4 months of age), and 2) not vaccinated. The model was structured as a Markova model with a cycle length of 2 weeks. In each cycle, a child is at risk of getting infection with rotavirus with 3 outcomes (hospitalized or needing outpatient care or dies of rotavirus), or survives without infection, or dies of other causes. The cohort entered into each scenario at 2 months of age (we assumed that no infection could happened before 2 months of age due to maternal antibodies.24 The model structure is detailed in the Figure 1. We assumed that a child can get infection up to 3 times and after the third infection she/he will be no longer at risk of infection that requires medical care. We assumed that the first exposure does produce partial immunity that the chance of second or third infection after first infection will be lower compared to first infection and the clinical presentation will be moderate and just requires outpatient care (subsequent infection does not require hospitalization). The model was implemented using Microsoft Excel spreadsheet.
Figure 1.

The decision tree model. In this figure patient follow is shown. The first node represents the decision between the not vaccinated and vaccinated strategy (displayed as square). After the decision node a Markov node follows, where possible Markov-states are defined.
Burden of disease
The incidence
Since there was no data on the community based incidence of rotavirus related diarrhea in Iran, we used a comprehensive search of literature in English and Farsi (local language) in order to identify any relevant published estimates. The search was performed on 2 databases 1) the Pubmed and 2) the IranMedex (a database indexing published studies in Farsi language). The literature search identified; 1) 2 major community surveys that reported the prevalence of all cause diarrhea, 2) one community based study that reported proportion of cause specific rotavirus diarrhea among outpatients with all cause diarrhea, 3) 9 hospital based studies25-34 that reported the proportion of cause specific rotavirus diarrhea among the hospitalized all cause diarrheal patients. The two community surveys included; a World Bank funded research that measured prevalence of diarrhea in a sample of 5 cities throughout the country,35 and the other one reported the result of the Multiple-Indicator Demographic and Health Survey (MIDHS) performed in 2010.36 The World Bank study estimated the rate of hospitalization and outpatient visit among the surveyed diarrheal patients. estimate the incidence of cause specific rotavirus diarrhea, we applied the hospitalization and outpatient rate from the World Bank study to the proportion of diarrhea reported by the MIDSH study to estimate the proportion of hospitalization and outpatient among all cause diarrheas. we applied the proportion of rotavirus among the outpatient all cause diarrheal from the community based study to estimate the rate of rotavirus cause specific outpatient diarrhea in the MIDSH survey. To estimate the rate of rotavirus hospitalization in the MIDSH survey, we performed a fixed effect meta-analysis of the proportion of rotavirus diarrhea reported in the 9 hospital-based studies. Then the pooled estimate was applied to the proportion of hospitalized all cause diarrhea in the MIDSH survey to estimate the rate of rotavirus hospitalization. The results estimated a crude hospitalization rate of 0.05 and a crude outpatient rate of 0.23 cases per children less than 5 y per year. To estimate the age specific incidence, the age distribution of rotavirus diarrhea reported by the “Sentinel Hospital-based Surveillance of Rotavirus Diarrhea in Iranian (SHSRD)” study 25 was used and applied in the estimation process (Table 1). The SHSRD study did not include all cases in the country and it just included cases referred to pediatric ward of major hospitals in selected cities (disqualifying the study to directly estimate the incidence of rotavirus hospitalization). Appendix 1 provides the details of the analysis done to estimate the incidence. As the model included chance of second and third infection, the risk of subsequent infection after first infection was reduced by 62% and 40% for second and third infection, respectively. The ratios were based on the study by Velazquez et al.5 who reported the subsequent risk of infection after first and second infections.
The mortality
The all cause mortality for under 5 y in Iran accounted for 25 cases per 1000 child per year in 2009.36 The Sentinel Hospital-based Surveillance of Rotavirus Diarrhea in Iranian (SHSRD)in Iran25 did not report any death among 1298 confirmed cases of rotavirus during the one year follow-up. In our analysis, the number of death due to rotavirus gastroenteritis in Iran estimated by the WHO for the year 2008 was obtained from the WHO'Immunization, Vaccines and Biologicals web site.37 The estimated number of deaths was applied to the estimated population of under 5 y to estimate the crude mortality rate of rotavirus gastroenteritis for children less than 5 y The crude mortality was converted to transition probabilities using the same age distribution as hospitalized and the mathematical function converting rate to probability (Appendix 1 includes the details of the analysis).
The cost
The cost was estimated for both health care and societal perspectives. We used the “WHO guidelines38 for estimating the economic burden of diarrheal diseases due to rotavirus” to estimate costs whenever a reliable estimate was not available in the literature. For this purpose, we used the population living in the central and eastern part of the province of Tehran (geographically located 1300 km north of Persian Gulf) to measure the cost. This population consists of close to 4 million people living in both urban and rural areas. The cost component of health care perspective included direct cost of hospitalization and outpatient visit and the cost of societal perspective included; cost of hospitalization, outpatient visit, transportation, loss of productivity, and patient visit at hospital. All costs were measured in Rials (local currency) and converted to US dollar based on the mid-year of 2009 currency exchange rate declared by the Central Bank of the I.R. of Iran. All costs were discounted at rate of 3% per year.
Cost of hospitalization
To estimate the cost of hospitalization, the medical records of all cases of acute diarrhea referred to 4 general hospitals in the catchment area of the study population during the year of 2009 were obtained. Detailed information about the duration of hospitalization, diagnostic, treatment, and other related utilization and their costs were abstracted from the patient's medical record. A patient medical record included a detailed cost of all diagnostic and treatment procedures (hospital stay, diagnostic procedures, treatment cost, and nursing cost). Among the 157 cases of hospitalized diarrhea referred to the 4 hospitals, 64 cases were coded as viral gastroenteritis (with ICD 10 coding of A08.4, and A08.3). The data from the viral coded cases was used to estimate the hospitalization cost.
Out patient cost
To estimate the average cost of outpatient visit; the pattern of care seeking for diarrheal disease reported by the National Integrated Monitoring and Evaluation Survey of Family Health (IMESFH)39 in 2006 provided the proportion of different facilities (rural health post, urban outpatient clinic, stand alone pediatrician or general physician) providing outpatient care for diarrheal patients, the prescribing indicators from the report of the National Essential Drug Monitoring Program (NEDMP)40 provided the prescribing pattern of medication for diarrheal diseases, and the government visiting tariff 41 for general physician, pediatrician, or other axillaries provided the cost of paying for care giver. The combination of these parameters estimated an average cost of 16 US dollars per each outpatient visit.
Societal cost
To estimate the societal cost, a sample of 69 patients from the 157 cases were randomly selected and the cost of transportation, days of absence from work for both mother and father, and the cost for relative visiting patient while in hospital was obtained by telephone interviews. The sample of 69 cases out of 157 was enough to account for expected variations on health care utilization and socioeconomic status among the patients for accurate cost estimation. To calculate the cost due to loss of productivity, the average number of days of loss productivity was multiplied by the average household income reported by the Statistical Center of Iran42 for the year 2009.
The vaccine
Two oral rotavirus vaccines are available to mass vaccination programs; Rotarix, a monovalent P1A G1 vaccine7 (GlaxoSmithKline), and RotaTeq, a pentavalent bovine-human reassortant vaccine (Merck).2,7 Rotarix is administered in a 2-dose schedule along the first and second dose of Diphtheria-Tetanus-Pertussis (DTP) vaccine. RotaTeq requires a 3-dose schedule with an interval of 4–10 weeks between doses.7 In this study we included just Rotaix because of its 2-dose schedule compared to RotaTeq which requires 3 doses (saving administrative resources). We assumed that the vaccine will be introduced into the national program and administered along with the DPT vaccine schedule.
The cost of vaccine
Iran is not an eligible country to benefit from the WHO-UNICEF Global Immunization Vision and Strategy (GIVS) Alliance rotavirus vaccine pricing program.43 The GlaxoSmithKline that manufactures the Rotrix, has recently reduced the price of its vaccine to 2.5 US dollars per dose18 if large volume of purchase is ordered. It is estimated that the price of vaccine will decline to a value as low as 1 US dollars per doses due to price maturation by 2025.44 For our base case scenario, we assumed a cost of $5 dollars per dose of vaccine with $0.25 US dollars per dose as program cost (Table 1). These values have been recommended by other investigators for countries with similar economic level.13
The vaccine efficacy, coverage, wastage
The vaccine efficacy has been heterogonous in different countries and regions of the world. The highest reported efficacy has been reported in Europe with a value of 95% and the lowest in Malawi with a value of 45%.7 Vaccine efficacy has been related to the genotype frequency,45 severity of diarrhea, and the partial immunity due to either infection or vaccination.7,29 In addition, WHO-UNICEF suggests efficacy would follow the pattern of under-5 mortality in a country and recommends that countries with similar mortality rate should use similar efficacy figures for economic evaluation of rotavirus vaccination.43 Considering the genotype frequency and WHO recommendation, the efficacy in the base case scenario was considered 93% for hospitalization and death and 78% for out-patient visit with reduction in efficacy after the first year of vaccination (Table 1). The efficacy figures used in our model are figures that have been used to project the cost effectiveness of rotavirus vaccination in Asian countries.13
The same rate of coverage and wastage for the DPT in the EPI national program were used in our analysis. The EPI reported a coverage rate of 97% and a wastage rate of 5% for DPT in the year 200936 (Table 1).
Effectiveness
Disability-adjusted life-years (DALY) were used to measure the effectiveness of the vaccination using the recommended formula by JA Fox-Rushby and K Hanson.46 We calculated Years of lost life (YLL) for each case of death due to rotavirus and Years of life lived with disability (YLD) for each case of outpatient and hospitalization. In calculating DALY, the age weighting parameter of 0.04, disability weight of 0.119,47 diarrhea duration of 5 days, and the life expectancy based on the WHO life expectancy estimates for Iran for the year 2009 were used.48 We used DALY with age-weighting scheme to have our result comparable with other studies. DALYs were discounted at 3% per year.
Uncertainty analysis
Sensitivity analysis was conducted on all the variables in the model that were considered to be uncertain. Tornado diagram was used to assess the magnitude of uncertainties and their effects on the cost-effectiveness ratio. With the price maturation and market variability of the price of vaccine, we run a cost effectiveness threshold analysis on varying prices of vaccine.
Supplementary Material
Funding
This study was funded by a grant from the office of Vaccine Preventable Diseases, Communicable Disease Control Dept., Ministry of Health, I. R. of Iran.
Disclosure of potential conflicts of interest
The authors declare there were no potential conflicts of interest to disclose.
References
- [1].Rheingans RD, Antil L, Dreibelbis R, Podewils LJ, Bresee JS, Parashar UD. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis 2009/November/1; 200 Suppl 1:S16-S27; PMID:19817595; http://dx.doi.org/ 10.1086/605026 [DOI] [PubMed] [Google Scholar]
- [2].WHO Rotavirus vaccines WHO position paper- January 2013. Weekly Epidemiological Record 13 AD/1/2; 88:49-64 [Google Scholar]
- [3].Ogilvie I, Khoury H, El Khoury AC, Goetghebeur MM. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: serotype distribution and burden of illness. Hum Vaccin 2011/5; 7:523-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM. Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect Dis 2011; 11:9; PMID:21214934; http://dx.doi.org/ 10.1186/1471-2334-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996/October/3; 335:1022-8; PMID:8793926; http://dx.doi.org/ 10.1056/NEJM199610033351404 [DOI] [PubMed] [Google Scholar]
- [6].Kuehn BM. High rotavirus vaccination rates continue to pay off. JAMA 2014/July/2; 312:18; PMID:25058201; http://dx.doi.org/ 10.1001/jama.2014.7939 [DOI] [PubMed] [Google Scholar]
- [7].Vesikari T. Rotavirus vaccination: a concise review. Clin Microbiol Infect 2012/10; 18 Suppl 5:57-63; PMID:22882248; http://dx.doi.org/ 10.1111/j.1469-0691.2012.03981.x [DOI] [PubMed] [Google Scholar]
- [8].Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, Poulhazan E, Jossens A, Auzanneau L, Tran A, et al.. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 2011/May/12; 29:3753-9; PMID:21443962; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.035 [DOI] [PubMed] [Google Scholar]
- [9].Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium: policy and impact. Pediat Infect Dis J 2011; 30:S21-4; PMID:21183836; http://dx.doi.org/ 10.1097/INF.0b013e3181fefc51 [DOI] [PubMed] [Google Scholar]
- [10].Giaquinto C, Dominiak-Felden G, Van DP, Myint TT, Maldonado YA, Spoulou V, Mast TC, Staat MA. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin 2011/7; 7:734-48 [DOI] [PubMed] [Google Scholar]
- [11].Fernandes EG, Sato HK, Leshem E, Flannery B, Konstantyner TC, Veras MA, Patel MM. Impact of rotavirus vaccination on diarrhea-related hospitalizations in Sao Paulo State, Brazil. Vaccine 2014/June/5; 32:3402-8; PMID:24736002; http://dx.doi.org/ 10.1016/j.vaccine.2014.04.015 [DOI] [PubMed] [Google Scholar]
- [12].Atherly D, Dreibelbis R, Parashar UD, Levin C, Wecker J, Rheingans RD. Rotavirus vaccination: cost-effectiveness and impact on child mortality in developing countries. J Infect Dis 2009; 200 Suppl 1:S28-38; PMID:19817610; http://dx.doi.org/ 10.1086/605033 [DOI] [PubMed] [Google Scholar]
- [13].Podewils LJ, Antil L, Hummelman E, Bresee J, Parashar UD, Rheingans R. Projected cost-effectiveness of rotavirus vaccination for children in Asia. J Infect Dis 2005/September/1; 192 Suppl 1:S133-S45; PMID:16088797; http://dx.doi.org/ 10.1086/431513 [DOI] [PubMed] [Google Scholar]
- [14].Sigei C, Odaga J, Mvundura M, Madrid Y, Clark AD. Cost-effectiveness of rotavirus vaccination in Kenya and Uganda. Vaccine 2015/May/7; 33 Suppl 1:A109-A18; PMID:25919149; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.079 [DOI] [PubMed] [Google Scholar]
- [15].Diop A, Atherly D, Faye A, Lamine SF, Clark AD, Nadiel L, Yade B, Ndiaye M, Fafa CM, Ba M. Estimated impact and cost-effectiveness of rotavirus vaccination in Senegal: a country-led analysis. Vaccine 2015/May/7; 33 Suppl 1:A119-A25; PMID:25919151; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.065 [DOI] [PubMed] [Google Scholar]
- [16].Ahmeti A, Preza I, Simaku A, Nelaj E, Clark AD, Felix Garcia AG, Lara C, Hoestlandt C, Blau J, Bino S. Cost-effectiveness of rotavirus vaccination in Albania. Vaccine 2015; 33 Suppl 1:A201-8; PMID:25919162; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.075 [DOI] [PubMed] [Google Scholar]
- [17].Javanbakht M, Moradi-Lakeh M, Yaghoubi M, Esteghamati A, Mansour GR, Mahmoudi S, Shamshiri AR, Zahraei SM, Baxter L, Shakerian S, et al.. Cost-effectiveness analysis of the introduction of rotavirus vaccine in Iran. Vaccine 2015/May/7; 33 Suppl 1:A192-A200; PMID:25919160; http://dx.doi.org/ 10.1016/j.vaccine.2014.12.035 [DOI] [PubMed] [Google Scholar]
- [18].Madsen LB, Ustrup M, Fischer TK, Bygbjerg IC, Konradsen F. Reduced price on rotavirus vaccines: enough to facilitate access where most needed? Bull World Health Organ 2012/July/1; 90:554-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jain S, Vashistt J, Changotra H. Rotaviruses: is their surveillance needed? Vaccine 2014/June/5; 32:3367-78; PMID:24793942; http://dx.doi.org/ 10.1016/j.vaccine.2014.04.037 [DOI] [PubMed] [Google Scholar]
- [20].Kim SY, Goldie SJ, Salomon JA. Cost-effectiveness of Rotavirus vaccination in Vietnam. BMCPublic Health 2009; 9:29; PMID:19159483; http://dx.doi.org/ 10.1186/1471-2458-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ortega O, El-Sayed N, Sanders JW, bd-Rabou Z, Antil L, Bresee J, Mansour A, Adib I, Nahkla I, Riddle MS. Cost-benefit analysis of a rotavirus immunization program in the Arab Republic of Egypt. JInfectDis 2009/November/1; 200 Suppl 1:S92-S8; PMID:19817621; http://dx.doi.org/ 10.1086/605057 [DOI] [PubMed] [Google Scholar]
- [22].Malek MA, Teleb N, bu-Elyazeed R, Riddle MS, Sherif ME, Steele AD, Glass RI, Bresee JS. The epidemiology of rotavirus diarrhea in countries in the Eastern Mediterranean Region. JInfectDis 2010/September/1; 202 Suppl:S12-S22; PMID:20684691; http://dx.doi.org/ 10.1086/653579 [DOI] [PubMed] [Google Scholar]
- [23].World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE). Available at http://www.who.int/choice/en/ [Google Scholar]
- [24].Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet 2006/July/22; 368:323-32; PMID:16860702; http://dx.doi.org/ 10.1016/S0140-6736(06)68815-6 [DOI] [PubMed] [Google Scholar]
- [25].Eesteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M, Yaghini F, El MH, Patel M, Klena JD, et al.. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. JInfectDis 2009/November/1; 200 Suppl 1:S244-S7; PMID:19821714; http://dx.doi.org/ 10.1086/605050 [DOI] [PubMed] [Google Scholar]
- [26].Hamkar R, Yahyapour Y, Noroozi M, Nourijelyani K, Jalilvand S, Adibi L, Vaziri S, Poor-Babaei A, Pakfetrat A, Savad-Koohi R. Prevalence of rotavirus, adenovirus, and astrovirus infections among patients with acute gastroenteritis in, Northern Iran. Iran JPublic Health 2010; 39:45-51; PMID:23113006 [PMC free article] [PubMed] [Google Scholar]
- [27].Khalili B, Cuevas LE, Reisi N, Dove W, Cunliffe NA, Hart CA. Epidemiology of rotavirus diarrhoea in Iranian children. JMedVirol 2004/6; 73:309-12; PMID:15122809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Modaress S, Rahbarimanesh AA, Edalat R, Sohrabi A, Modarres S, Gomari H, Motamedirad M, Sayari AA. Human rotavirus genotypes detection among hospitalized children, a study in Tehran, Iran. ArchIran Med 2011/1; 14:39-45 [PubMed] [Google Scholar]
- [29].Modarres S, Rahbarimanesh AA, Karimi M, Modarres S, Motamedi-Rad M, Sohrabi A, Nasiri-Oskoii N. Electrophoretic RNA genomic profiles of rotavirus strains prevailing among hospitalized children with acute gastroenteritis in tehran, iran. ArchIran Med 2008/9; 11:526-31; PMID:18759520 [PubMed] [Google Scholar]
- [30].Modarres S MS, Nassiri Oskoii N. Rotavirus Infection in Infants and young children with acute gastroenteraritis in the Islamic Republic of Iran. Eastern Mediterranean Health Journal 1995; 7:210-4 [Google Scholar]
- [31].Emamghorashi F, Rajabi SH, Shadman A. Frequency of Rotavirus Infection in Children with Acute Gastroenteritis in Jahrom, South of Iran. Iran J Med Sci 2008; 33:84-7. [Google Scholar]
- [32].Sadeghian A, Hamedi A, Sadeghian M, Sadeghian H. Incidence of rotavirus diarrhea in children under 6 years referred to the Pediatric Emergency and Clinic of Ghaem Hospital, Mashhad, Iran. Acta MedIran 2010/7; 48:263-5 [PubMed] [Google Scholar]
- [33].Kargar M, Akbarizadeh AR. Prevalence and molecular genotyping of group a rotaviruses in Iranian children. Indian JVirol 2012/6; 23:24-8; PMID:23729998; http://dx.doi.org/ 10.1007/s13337-012-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kargar M, Khodadadi P, Najafi A, Ansari H. Predominance of rotavirus G8 genotype in hospitalized children with acute gastroenteritis in Yasuj, Iran. EurRevMedPharmacolSci 2014; 18:699-702 [PubMed] [Google Scholar]
- [35].Kolahi AA, Rastegarpour A, Abadi A, Gachkar L. An unexpectedly high incidence of acute childhood diarrhea in Koot-Abdollah, Ahwaz, Iran. Int J InfectDis 2010/7; 14:e618-e21; PMID:20116314; http://dx.doi.org/ 10.1016/j.ijid.2009.10.001 [DOI] [PubMed] [Google Scholar]
- [36].Rashidian A, Khosravi A, Khabiri R, Khodayari-Moez E, Elahi E, Arab M, Radaie Z. Islamic Republic of Iran's Multiple-Indicator Demographic and Health Survey (IrMIDHS) 2010. Tehran, Iran: Ministry of Health and Medical Education; 2012 [Google Scholar]
- [37].World Health Organization. Estimated rotavirus deaths for children under 5 years of age: 2008. Available at http://www.who.int/immunization/monitoring_surveillance/burden/estimates/rotavirus/en/ [Google Scholar]
- [38].World Health Organization. Guidelines for estimating the economic burden of diarrhoeal disease with focus on assessing the cost of rotavirus diarrhoeal. Geneva, Switzerland: World Health Organization, Department of Immunization, Vaccines and Biologicals; 2005 [Google Scholar]
- [39].Motlagh ME, Heidarzadeh A, Hashemian H, Dosstdar M. Patterns of care seeking during episodes of childhood diarrhea and its relation to preventive care patterns: national integrated monitoring and evaluation survey (IMES) of Family Health. Islamic Republic of Iran. Int JPrevMed 2012/1; 3:60-7 [PMC free article] [PubMed] [Google Scholar]
- [40].Cheraghali AM, Nikfar S, Behmanesh Y, Rahimi V, Habibipour F, Tirdad R, Asadi A, Bahrami A. Evaluation of availability, accessibility and prescribing pattern of medicines in the Islamic Republic of Iran. Eastern Mediterranean Health J = La Revue de Sante de la Mediterranee Orientale = al-Majallah al-sihhiyah li-sharq al-Mutawassit 2004; 10:406-15 [PubMed] [Google Scholar]
- [41].Iranian Medical Council, Prescribing tariff, Medical Council of I.R.Iran. Available at http://irimc.org/DynamicContentaspx?ci=6c4f49db-aaaa-4e0c-8692-d3fea9e9fdf5 (site in Farsi Language). [Google Scholar]
- [42].Statistical Center of Iran. Average annual income of an Urban/Rural household. Available at http://www.amar.orgir/Defaultaspx?tabidD496 [Google Scholar]
- [43].Wolfson LJ, Gasse F, Lee-Martin SP, Lydon P, Magan A, Tibouti A, Johns B, Hutubessy R, Salama P, Okwo-Bele JM. Estimating the costs of achieving the WHO-UNICEF global immunization vision and strategy, 2006-2015. Bull World Health Organ 2008/1; 86:27-39; PMID:18235887; http://dx.doi.org/ 10.2471/BLT.07.045096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jit M, Yuzbashyan R, Sahakyan G, Avagyan T, Mosina L. The cost-effectiveness of rotavirus vaccination in Armenia. Vaccine 2011/11/8; 29:9104-11; PMID:21945959; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.127 [DOI] [PubMed] [Google Scholar]
- [45].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006/1/5; 354:11-22; PMID:16394298; http://dx.doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- [46].Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan 2001/9; 16:326-31; PMID:11527874; http://dx.doi.org/ 10.1093/heapol/16.3.326 [DOI] [PubMed] [Google Scholar]
- [47]. Murray CJL, Lopez AD. Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. World Health Organization, 1996. [Google Scholar]
- [48].World Health Organization. Global estimates of burden of disease caused by the environment and occupational risks, 2009. Available at http://appswho.int/gho/data/?themeDmain&vidD60760/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


