Abstract
Background:
The use of herbals in the treatment of diabetes mellitus is a well-established practice in traditional medicine. The medicinal plant Prosopis farcta has some antioxidant activity, which may be useful in diabetic patients. Since, there is no report on the antidiabetic effect of the P. farcta, this study evaluated antidiabetic activity of P. farcta bean extract (PFE) in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods:
Hyperglycemia was induced in male albino Wistar rats by intraperitoneal injection of STZ (55 mg/kg body weight [BW]), after which, the animals were randomly allocated into six experimental groups as follows: Group 1: Normal rats (received normal saline), Groups 2 and 3: Normal rats received PFE; (50 and 75 mg/kg BW), Group 4: Diabetic control rats, Group 5: Diabetic rats received PFE (50 mg/kg BW), Group 6: Diabetic rats received PFE (75 mg/kg BW). Three days after induction of diabetes, rats were received an extract of PFE orally for 12 days. Blood samples were collected by cardiac puncture to determine liver enzymes; aspartate aminotransferase and alanine aminotransferase (AST and ALT), cholesterol, triglyceride (TG), high and low density lipoproteins (HDL and LDL).
Results:
The administration of PFE (50 and 75 mg/kg) in STZ-induced diabetic rats significantly reduced the blood glucose levels when compared with the STZ-control group (227.2 ± 12.00 and 259.6 ± 7.03 vs. 454.6 ± 12.66, P < 0.001). PFE in diabetic groups had no significant effect on the levels of cholesterol, TG, HDL, LDL, AST, and ALT compare to the STZ-control group.
Conclusion:
P. farcta could reduce blood glucose in diabetic rats.
Keywords: Diabetes, glucose, Prosopis farcta
INTRODUCTION
Diabetes mellitus is a complex endocrinological, metabolic disorder characterized by hyperglycemia due to disturbance of carbohydrate, fat and protein metabolism associated with a defect in insulin secretion and/or impaired target cell responsiveness to insulin.[1] Reactive oxygen species (ROS) are implicated in the clinical condition of diabetes. The most common exogenous factors originating ROS are included environmental pollutants, drug metabolism (side effects), smoking, alcohol, inadequate nutrition, and excess solar radiation.[2]
Many plant species have been used for treating diabetes in different countries.[3,4,5] Medicinal plants are rich sources of natural antioxidants. These plants use in traditional medicine for the control and treatment of many diseases. More than 800 kinds of plants estimated as traditional folk medicine use to treat diabetes. Without any academic confirmation, the Prosopis farcta are used to treat diabetes by the tribal people in Jordan.[3] Leaves and beans of P. farcta have been used as a traditional medicine.[4] P. farcta is native in Asia, distributed from India to Iran[6] and mostly well adapted to drought and warm weather. Some of the beneficial effects of Prosopis are antitumour activity,[7] antioxidant capacity,[8] antiparasitic and antimicrobial activity,[9] increase high-density lipoprotein (HDL) cholesterol and decrease low-density lipoprotein (LDL) cholesterol in ostriches,[10] and hepatoprotective potential in Wista rats.[11] Since the medicinal plant P. farcta has antioxidant activities which may be useful in diabetic patients, and there is no report on the effect of this plant against diabetes, the objective of this study was to evaluate the hypo- and anti-hyperglycemic effects of P. farcta beans in normal and streptozotocin (STZ)-diabetic rats.
MATERIALS AND METHODS
Animals
Albino Wistar rats (200–250 g) were kept in a well-ventilated cage under standard laboratory conditions (temperature 25°C ± 2°C with 12/12 h dark/light cycle). They were fed pellet diet and water ad libitum. The animals were accustomed to laboratory conditions for one-week prior to the experiment.
Induction of diabetes
Diabetes was induced by intraperitoneal injection of STZ (55 mg/kg BW) dissolved in citrate buffer, pH 4.5 (0.1 mol/L trisodium citrate, 0.1 mol/L citric acid). Nondiabetic animals were sham injected with buffer only. Diabetes was confirmed by measuring blood glucose levels 2–3 days after the STZ-injection. Animals with plasma glucose level higher than 250 mg/dl were classified as diabetic.[12]
Preparation of extract
The fruit of P. farcta was powdered in an electrical grinder and then 50 g of powder dissolved in 1000 cc ethanol 80% for 24 h at room temperature. After 24 h, the solution is passed through a filter paper. To remove the solvent, the solution was filtered and placed in the oven for 1–2 days at 40°C. After evaporation of the solvent, the samples maintained at − 20°C. A stock solution of 1 g/ml was then prepared for the experiments.[11]
Experimental design
The animals were divided into six groups (n = 5) and supplemented diets commenced 3 days after injection of STZ. Both healthy and diabetic rats were given a different concentration of P. farcta bean extract (PFE) (50 mg/kg and 75 mg/kg body weight [BW]) orally for 12 days.[11] Rats divided into six groups:
Groups 1, 2 and 3 were nondiabetic, and the remaining groups were diabetic:
Group 1: Control rats were given only physiological saline;
Group 2: PFE 50 mg/kg BW
Group 3: PFE 75 mg/kg BW
Group 4: Diabetic rats
Group 5: Diabetic rats + PFE 50 mg/kg BW.
Blood sampling and measurement of some biochemical factors
Twelve days posttreatment, blood samples were collected directly from the heart after intraperitoneal injection of ketamine into a heparinized syringe. The blood samples were then analyzed for different biochemical parameters.
RESULTS
The hypoglycemic effect of PFE on the fasting blood sugar levels is shown in Figure 1. STZ led to an approximately 4.5-fold elevation of fasting blood glucose level as compared to normal control group (454.6 ± 12.66 vs. 103.2 ± 4.48, P < 0.0001). The administration of PFE (50 and 75 mg/kg) in STZ-induced diabetic rats reduced markedly the fasting blood glucose levels when compared with the STZ-control group (227.2 ± 12.00 and 259.6 ± 7.03 vs. 454.6 ± 12.66, P < 0.001).
Figure 1.
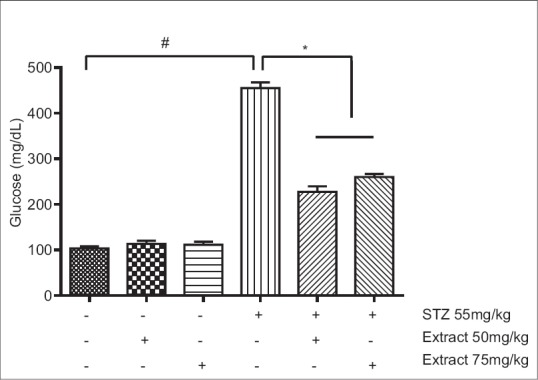
Inhibitory effect Prosopis farcta extract on glucose levels in normal and diabetic rats. Each value is mean ± standard error (n = 5). *P < 0.001, #P < 0.0001
As demonstrated in Table 1, STZ and PFE had no significant effect on the levels of cholesterol, triglyceride (TG) and HDL compared to the control group, although two former parameters enhanced numerically, and the last one declined. STZ diabetes remarkably increased LDL levels (21.52 ± 1.64 vs. 11.96 ± 1.58, P < 0.05) compared to normal control and treatment with PFE (50 and 75 mg/kg) in diabetic rat effectively prevented LDL increase (11.96 ± 1.21 and 11.56 ± 3.63 vs. 21.52 ± 1.64, P < 0.05).
Table 1.
The effect of PFE on lipid profile in STZ-induced diabetic rats
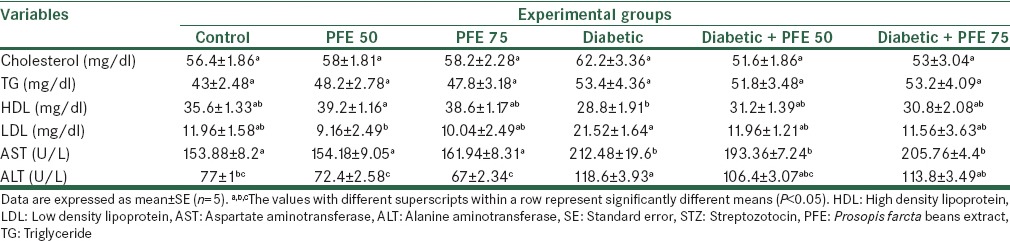
Diabetic control rats showed significant higher aspartate aminotransferase (AST) (153.88 ± 8.2 vs. 212.48 ± 19.6) and alanine aminotransferase (ALT) (77 ± 1 vs. 118.6 ± 3.9) activity than that of normal group (P < 0.05, Table 1). The level of AST and ALT activity in PFE (50 and 75 mg/kg) treated groups were (193.36 ± 7.24 and 205.76 ± 4.4 U/L) and (106.4 ± 3.07 and 113.8 ± 3.49 U/L and 58 ± 10 U/L), respectively, which was not significantly different from that of diabetic control rats.
DISCUSSION
This study is the preliminary assessment of the antihyperglycemic activity of a hydroalcholic extract of PFE in normal and STZ-induced diabetic rats. The results of the study revealed that P. farcta induced a significant decrease in serum glucose level of STZ-induced diabetic rats as compared to the diabetic control group [Figure 1].
In the present study, STZ was used as a diabetogen. It induces diabetes by penetration into pancreatic beta cells and moderately destroying these cells, through the production of relative oxygen species as well as DNA alkylation. The role of ROS has been proven in the pathophysiology of diabetes by different studies in animal models and human.[13,14,15,16]
There is increasing evidence that oxidative stress has a key role in the development and progression of diabetes which is accompanied by increased production of free radicals or impaired antioxidant defences.[13,15,16] A number of plants have been reported to have antidiabetic effects. The bioactive antidiabetic effect of these herbal plants could be related to phenolic, flavonoids, sterols and alkaloids. These compounds that are part of P. farcta ingredient have the ability to serve as antioxidants. Quercetin, as a flavonoidal component of PF with antioxidant properties, has been demonstrated to regenerate the pancreatic islets and probably increases insulin release in streptozocin-induced diabetic rats.[17] On the other hand, quercetin is an excellent radical scavenger and protect cellular damage against oxidative stresses induced by ROS.[18,19,20]
Induction of diabetes with STZ is associated with a characteristic loss of BW, which is attributed to increased muscle wasting and degeneration of the adipocytes.[21] It has been documented that hyperglycemia and hyperlipidemia are the common characteristics of STZ-induced diabetes in the experimental rats.[22] In this study, total cholesterol and TG just numerically enhanced, and PFE brought down the elevated serum total cholesterol and TG levels in diabetic rats. In addition, PFE significantly declined LDL elevation in diabetic rats. The observed hypolipidemic effect by PFE may be due to decreased hepatic cholesterogenesis and fatty acid synthesis or increased plasma lipid uptake by the liver and adipose tissue. Insulin deficiency in diabetes increases the generation of cholesterol and TG by enhancement of the levels 3-hydroxy-3-methylglutaryl coenzyme A reductase enzyme that is, accountable for the synthesis of cholesterol. This, found as a constituent of PFE, activity may contribute to flavonoid quercetin which has an antidiabetic effect like metformin.[23] Moreover, this effect has been related to increasing in insulin absorption, glucose uptake[24] and/or insulin release.[17] In addition, Narasimhacharya et al. demonstrated the similar effect by Prosopis juliflora in hypercholesterolemic male albino rats.[25]
In our study, aminotrasferases are elevated in diabetic rats, and PFE consumption did not have a significant effect on these enzymes levels although the insignificant decrease in ALT and AST levels was observed.
The findings of the present investigation specify that oral administration of PFE to diabetic rats significantly lower the blood glucose level. Further comprehensive pharmacological and biochemical investigations are needed to elucidate the exact mechanism of hypoglycemic action of the PFE.
Financial support and sponsorship
The authors gratefully acknowledge the financial support for this work as a part of the thesis No. 2143244 that was provided by University of Birjand.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to Ms. Yousefi for doing the biochemical measurements.
REFERENCES
- 1.Talubmook C, Forrest A, Parsons M. Streptozotocin-induced diabetes modulates presynaptic and postsynaptic function in the rat ileum. Eur J Pharmacol. 2003;469:153–8. doi: 10.1016/s0014-2999(03)01722-9. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med. 1991;91:S14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 3.Al-Aboudi A, Afifi FU. Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm Biol. 2011;49:221–39. doi: 10.3109/13880209.2010.501802. [DOI] [PubMed] [Google Scholar]
- 4.Ali-Shtayeh MS, Jamous RM, Al-Shafie’ JH, Elgharabah WA, Kherfan FA, Qarariah KH, et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J Ethnobiol Ethnomed. 2008;4:13. doi: 10.1186/1746-4269-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 6.Pasiecznik NM, Harris PJ, Smith SJ. Identifying Tropical Prosopis Species: A Field Guide. Coventry, United Kingdom HDRA Publishing. 2004 [Google Scholar]
- 7.Robertson S, Narayanan N, Raj Kapoor B. Antitumour activity of Prosopis cineraria (L.) Druce against Ehrlich ascites carcinoma-induced mice. Nat Prod Res. 2011;25:857–62. doi: 10.1080/14786419.2010.536159. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Garg V, Paul A. Antihyperglycemic, antihyperlipidemic and antioxidative potential of Prosopis cineraria bark. Indian J Clin Biochem. 2010;25:193–200. doi: 10.1007/s12291-010-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman AA, Samoylenko V, Jain SK, Tekwani BL, Khan SI, Jacob MR, et al. Antiparasitic and antimicrobial isoflavanquinones from Abrus schimperi. Nat Prod Commun. 2011;6:1645–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Omidi A, Ansari nik H, Ghazaghi M. Prosopis farcta beans increase HDL cholesterol and decrease LDL cholesterol in ostriches (Struthio camelus) Trop Anim Health Prod. 2013;45:431–4. doi: 10.1007/s11250-012-0234-x. [DOI] [PubMed] [Google Scholar]
- 11.Asadollahi A, Sarir H, Omidi A, Torbati MB. Hepatoprotective potential of Prosopis farcta beans extracts against acetaminophen-induced hepatotoxicity in wister rats. Int J Prev Med. 2014;5:1281–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–39. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48:927–32. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 14.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–96. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- 16.Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:146–54. doi: 10.1053/j.ackd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 18.Anjaneyulu M, Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31:244–8. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- 19.Mira L, Fernandez MT, Santos M, Rocha R, Florêncio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T. Safety of quercetin for clinical application (Review) Int J Mol Med. 2005;16:275–8. [PubMed] [Google Scholar]
- 21.Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes. Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–4. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SR, Dwivedi SK, Swarup D. Hypoglycaemic, antihyperglycaemic and hypolipidemic activities of Caesalpinia bonducella seeds in rats. J Ethnopharmacol. 1997;58:39–44. doi: 10.1016/s0378-8741(97)00079-2. [DOI] [PubMed] [Google Scholar]
- 23.Kannappan S, Anuradha CV. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin and metformin in a rat model. Indian J Med Res. 2009;129:401–8. [PubMed] [Google Scholar]
- 24.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes. 2005;542:S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhacharya A, Nitesh KV, Desai TN. Prosopis juliflora as an antihypercholesterolemic agent. J Herb Med Toxicol. 2010;4:31–4. [Google Scholar]


