Abstract
Recently prevention strategies for breast cancer are focused on lifestyle modification such as diet. Some dietary factors such as Conjugated linoleic acid (CLA) can lower the risk of breast cancer, metastasis and some factors concerning this malignancy. Many studies have been established in this field, but their results are inconsistent. Therefore, we evaluated this association based on systematic review among published scientific literature. We performed an electronic search using PubMed, Cochrane, Scopus, Google Scholar and Persian database (Iran Medex, magiran) to identify relevant studies. We summarized the findings of 8 papers in this review. Although, three cohort studies were not overall identified a protective effect of CLA dietary intake or CLA content in breast tissue on breast cancer incidence, metastasis and death, one of them showed an inverse association after adjusting for age. Also, among case-control studies a weak inverse association between breast cancer risk and CLA dietary intake and serum levels among post-menopausal women was reported. Besides, a clinical trial showed that some indicator of breast tumor decreased after CLA administration among women with breast adenocarcinoma. Lacking published evidence suggested inconsistent results. So, further well-designed studies are required, particularly in considering the main breast cancer risk factors.
Keywords: Breast cancer, conjugated linoleic acid, systematic review
INTRODUCTION
Globally, Breast cancer is a major health problem in developing and developed countries that affect 1 in 8 women during their life span.[1] Diet modification is the most important preventive and treatment strategy against breast cancer.[2,3,4,5,6] Although dietary fat is associated with increased risk of mammary tumor, some types of fats such as omega-3 and conjugated linoleic acids (CLA) can have protective effects.[7,8]
The relation between this fatty acid intake and breast cancer risk was investigated in a small numbers of studies.[8,9,10,11,12,13,14,15] Therefore, we carried out this systematic review to discuss evidence between incidence, metastasis, death and other related factors of breast cancer and CLA dietary intake or CLA serum levels or CLA content in breast fat tissue.
MATERIALS AND METHODS
Search strategy
In order to collect data for this systematic review, we performed a systematic search through the PubMed, Cochrane, Scopus, Google scholar, Iran Medex and Magiran database. The following keywords were used in this searching: “Breast cancer”, “breast tumor”, “breast neoplasm”, “mammary tumor”, “breast carcinoma”, “conjugated linoleic acids”. All studies that covered the association between CLA dietary intake and breast cancer incidence, metastasis or mortality have been collected until November 2013. Also, we manually searched through the references from relevant articles where necessary. We didn’t use any filtration in our searches but study selection was limited to articles published in the English or Persian languages.
Inclusion and exclusion criteria
According to our inclusion criteria, we considered human studies with cancer incidence, metastasis, and any cancer marker such as Fatty acid synthase (FASN), Lipoprotein lipase (LPL) and Spot 14, a modifier protein in breast cancer cell's growth as the outcome and CLA as the exposure. We found 394 papers from database search that were screened by the two investigators based on study title and abstract. At the first, we omitted duplicated studies (n = 255). Then irrelevant papers (research other CLA outcomes, lack of data and not agree to our objective) were excluded (n = 55). After that we removed in vitro and animals studies (n = 75), nine articles remained that one of them was written in other languages and we didn’t access to one full text of them. Not only we classified these seven articles as three cohort, three case-control and one clinical trial, but also we evaluated them based on CLA dietary or serum levels or content in breast fat tissue. Figure 1 shows the flow of studies through the selection process.
Figure 1.
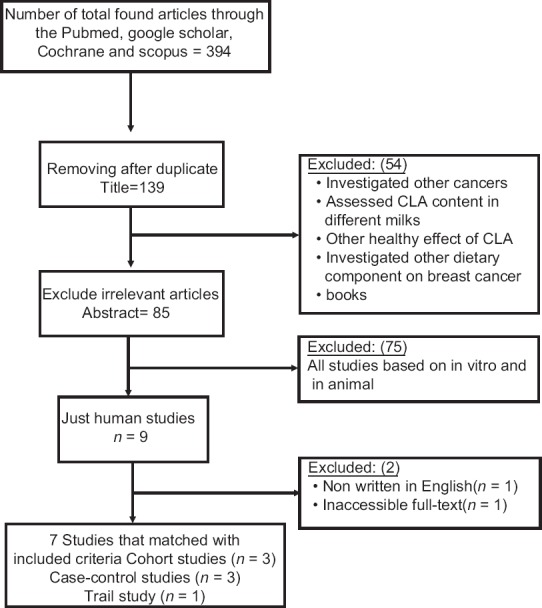
Flow chart of systematic literature search
Data extraction
From each included study we collected data on these characteristics: The first author's name, published year, country, participant's age, dietary assessment, duration of follow up, duration of intervention, study population, study type, event followed, types of CLA, amount of CLA and adjustment for confounders. Tables 1–4 show the abstracted information of data extraction.
Table 1.
Characteristics of cohort studies about breast cancer risk and CLA
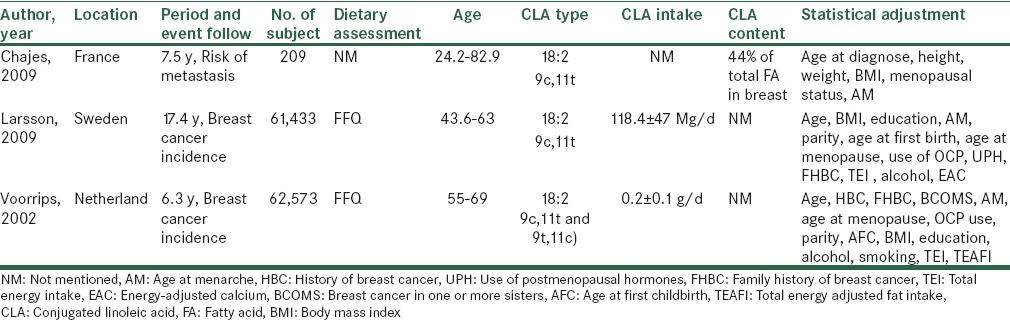
Table 4.
Characteristics of case-control study about breast cancer risk and conjugated linoleic acids
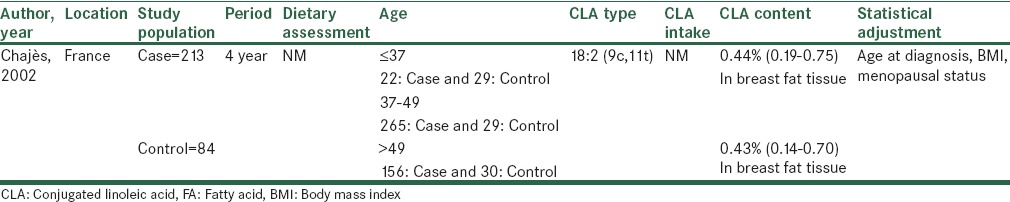
Table 2.
Characteristics of clinical trial about breast cancer risk and CLA
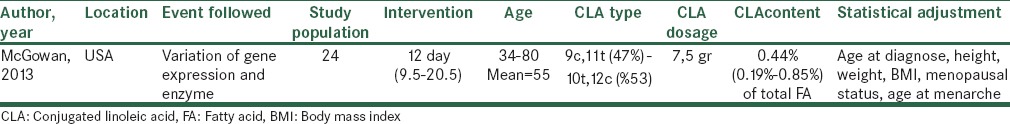
Table 3.
Characteristics of case-control studies about breast cancer risk and conjugated linoleic acids
Quality assessment
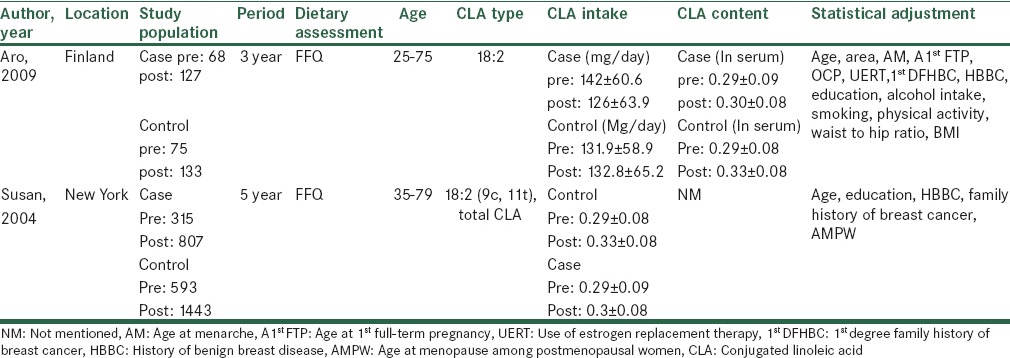
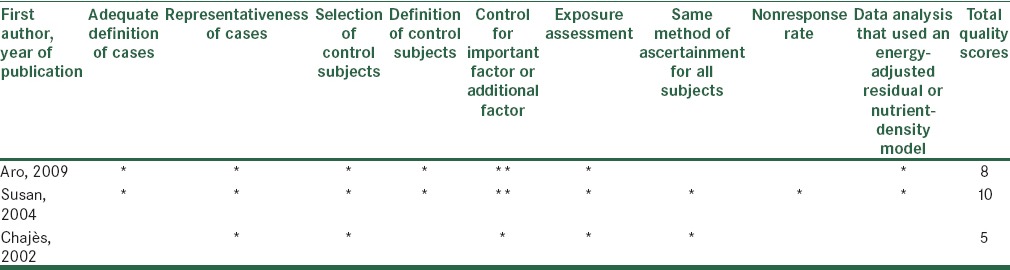
In order to assess the quality of selected studies, we used a system based on Newcastle-Ottawa Scale. The full score was 10 and the high or median quality study was defined as a study with 7 or 4-7 respectively.[16] The quality scores of studies summarized in Tables 5 and 6.
Table 5.
Methodological quality of cohort studies included in the systematic review
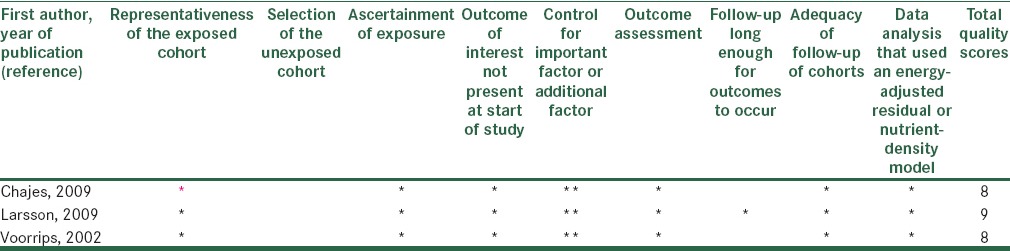
Table 6.
Methodological quality of case-control studies included in the systematic review
RESULTS
As previous mentioned, in this field, studies categorized in two groups. First group focused on the role of CLA on incidence of breast cancer and second group evaluated the role of CLA in breast cancer after diagnosis and its effects on metastasis. We divided our result to three parts according to types of studies and also in each part, studies belong to one of the two groups.
Cohort studies
3 cohorts were included in this study that 1 of them the CLA content in breast fat tissue on the time of surgery was investigated and patients follow up to metastasis and death incidence that no relation was seen. Others studies tried to find a relationship between CLA dietary intake and risk of breast cancer that didn’t find at all.
In a prospective cohort study Swedish cancer-free women have been followed up during 17.4 years and 2952 incident cases of breast cancer were diagnosed.[14] In order to diet assessment, a validated food-frequency questionnaire (FFQ) was used twice, at baseline and ten years later. Also, CLA dietary intake calculated with the use of published data on the CLA concentration of total fat in various foods.[17,18] Overall, no correlation between breast cancer incidence and CLA dietary intake was presented. Moreover, in comparison the highest with the lowest quintile of CLA dietary intake, the interaction of CLA and estrogen receptor/progesterone receptor (ER/PR) status on breast cancer incidence weren’t significantly approved. Adjustment for different factors done in this study but the results remained without change.
In the second cohort study,[8] women without cancer follow up in 6.3 years. At the beginning and end of this study, FFQ for examining dietary intake was used in blinded manner to minimize observer bias. Although 941 cases of breast cancer were found, but no relation was observed with CLA dietary intake. While this effect become manifest when CLA dietary intake was adjusted for age, adjusting for other confounding factors such as energy and fat intake had not same results. Furthermore, when different source of CLA was categorized, it was shown the most dietary intake of CLA (29%) in this population was from butter that it had a significant inverse association with breast cancer incidence.
In the last cohort study, Chajès focused on the CLA content in adipose tissue of invasive breast carcinoma patients with mean age 55 years and the risk of metastasis during 7.5 years. Forty-three percent of patients were premenopausal. High performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) used to extract and measure CLA content of adipose tissue. Measurement of CLA was reported as a percentage of total fatty acid. Finally, the analysis showed no considerable association between CLA levels in adipose tissue and risk of metastasis. The same findings were seen about metastasis and death risk related to CLA level in this tissue. To explain the findings, it should be stated that the amount of CLA in breast adipose tissue might be too low to induce the protective effect on tumor.[10]
Generally in three cohort studies the hypothesis that CLA can reduce the risk of death, metastasis or incidence of breast neoplasm wasn’t observed.
Case–control studies
Among three case-control studies, one study investigated CLA content in breast tissue and its relation to breast cancer risk that no relation was seen. Another study measured CLA dietary intake and no association was seen but, in the other reaserch both CLA dietary intake and serum levels assessed that an inverse association between both variables with reduction of breast cancer risk among postmenopausal women was absorved.[9]
In a case-control survey, the efficacy of total CLA dietary intake and its isomer (9:11) has been examined in 1166 women with breast cancer as case and 2036 healthy subjects as control. They were matched by age, race and the county of residence. At the baseline dietary assessment was carried out by self-administered FFQ. Although, this study categorized participants to pre and post-menopausal women, no significant difference was detected at all. The extracted data of ER evaluation illustrated a marginally significant decrease breast cancer rate among patients with ER-negative tumor in highest compared to lowest quartile for CLA dietary intake. The important point in this study is its high sample size. However, assessment of some cofounders factors like physical activity and use of estrogen replacement therapy were ignored in this study.[15]
In the other study, Chajès investigated CLA content in breast adipose tissue and the risk of breast cancer metastasis. The objectives in the case and control groups include 241 women with invasive breast carcinoma and 88 women with benign breast pathologies respectively that weren’t matched. There was no change observed in comparison third tertile to first tertile of CLA levels in this tissue with the malignancy metastasis while it was adjusted for BMI and menopausal states. Some limitations should also be indicated such as lack of assessment of physical activity, family history of breast cancer, estrogen replacement therapy and menarche age. Other limitations are, not CLA dietary intake assay and not enough participants in both groups. Furthermore, the control group included women with benign breast disease that were more at risk for breast cancer than healthy women.[10]
Results of another research suggested an inverse association between incidence of breast tumor and CLA serum and dietary intake in postmenopausal women with breast cancer. In this survey dietary intake and serum CLA level in Finnish women aged 25-75 was assessed. Case and control groups comprised premenopausal and postmenopausal women that were individually matched to the breast cancer cases for age and residence.[9]
Clinical trial study
Based on a clinical trial study,[14] 24 women with resectable invasive breast adenocarcinoma were asked to consume seven and half gr of CLA as capsule gel include 50:50 ratio of 9c, 11 t and 10 t, 12 c per day. The mean duration of intervention was 12 days (range 9.5-20.5) before tumor resection. The CLA concentration in plasma was obtained before initiation of intervention and on the day of resection. Also, some enzymes such as FAS, LPL, FAS regulator protein and spot 14 proteins (S14), before and after CLA administration were examined. These two enzymes can provide fatty acids for tumor growth.[19] S14 mediates the induction of lipogenesis by progestin in breast cancer cells and accelerates their growth. High expression of S14 in primary invasive breast cancer is conspicuously recurrence prediction.[20] The measurement showed the higher plasma concentration of 9c, 11t CLA at baseline and after treatment in compared with 10t, 12c CLA. Although, the findings indicated that S14 protein expression decreased after CLA administration that resulted in reduction of proliferation index, no significant change in FAS or LPL expression was observed.
DISCUSSION
This systematic review shows no consistent findings to support the concept that CLA consumption or serum CLA levels or CLA content in breast tissue is associated with a lower risk of breast cancer, metastasis or death. As mentioned previous, among case-control studies, only one of them showed an inverse relation between breast cancer risk and serum level and dietary intake of CLA.[9] Also, among cohort studies, only one survey provided marginally inverse association[8] and only one clinical trial of this research reported a positive effect of CLA on some mediators and enzymes concerning breast cancer. Despite controlling of some cofounders in these studies, results were low reliable due to type of studies and its bias such as sampling and recall bias.
In addition, differences in CLA assessment, dietary intake, serum and tissue, caused difficulty in clear result. These comparisons showed that among two studies CLA content in breast tissue didn’t have any relationship with breast cancer incidence, metastasis and death.[10,11] Furthermore, in one of four studies that assessed the risk of breast cancer against dietary CLA intake, an inverse association was seen.[9] Also, one trial was declared that high CLA plasma concentration had favorite effects on tumor indices.[14]
It should be state that in these surveys, average of CLA dietary intake may be lower than CLA amounts which have protective effects.[8,13] According to some studies anticancer effects of CLA were seen in 2.8 g/d of CLA for a 70 Kg person that it was different rather than usual dietary intake in human. So it could be concluded that CLA supplement could supply higher and beneficial effects than dietary CLA.[21]
Also, it is important to considerate that dietary CLA and its effects may be modified by some dietary factors such as fruits, vegetables and other kinds of fats that maybe effective on breast cancer risk and metastasis risk.[22] One considerable confounder factor is that all studies except one of them, omitted the vaccinic acid assessment that can convert to CLA in body.[9]
Although dairy products and meat products are the sources of CLA, the protective impacts between these products with breast carcinoma didn’t illustrated.[23,24] Due to some heterocyclic amines and polyaromatic hydrocarbons created in the cooking of red meat, it can alter the favorable effects of CLA. So, dietary patterns which represent a complex integration of food and nutrients, should be concurrently used with food item assessment to explore the association between CLA and the risk of breast cancer, metastasis and death.
Other limitations in these studies include small population, measurement and report bias, loss of CLA measurement in serum, and investigation between CLA in serum and dietary intake and some confounding variables such as body fatness, high-energy intake, physical activity, and family history of breast cancer, estrogen replacement therapy and age at menarche. Also, it's necessary to remind some poor-quality and heterogeneous studies maybe cause misjudge about conclusion.
Further, for their nature, systematic reviews have limitation that definitely our article is not separated from them, like publication, reporting, performance, attrition and detection biases.
Several mechanisms involved in the biological activity of this fatty acid on malignant cells. The inhibitory effects of CLA on breast cancer cells may result from decreased estrogen receptors that it can decrease proliferation of ER-positive cells selectively.[25] ER is over expressed in around 70% of breast cancer cases that is called estrogen receptor positive (ER+). It can stimulate proliferation of mammary cells via increasing in cell division and DNA replication leading to mutation. Also, PR is related to epithelial cell growth. Furthermore, estrogen metabolism produces genotoxic waste like Quinone derivatives that decay DNA base and cause to mutation and malignancy.[26]
It has been shown that CLA may induce apoptosis in ER+ breast epithelial cells via down regulation of anti-apoptosis protein, B-cell lymphoma 2(BCL-2) that result in reduction of breast cancer risk.[27] Also, it seems that this fatty acid influence on these tumors via changing the lipid metabolism in breast fat tissue by reduction of FAS. This enzyme is essential for nourishing the cancer cells.[28] Additionally, some articles revealed the increased activity and expression of FAS caused to disturb the cell cycle controlling so it could not induce apoptosis in S phase.[29] Suppressing of S14 gene expression, a nuclear protein that regulates expression of FAS in mammary epithelial and adipose tissue cells, as well as FAS can be exerted by CLA. Altogether, inhibition of spot 14 proteins and FAS genes expression can lead to prevention of breast cancer cells growth.[30] As well, CLA was proposed as aLXR agonist, inducing genes implicated in cholesterol efflux and increasing the mRNA levels of LXR target genes and HMG-CoA-reductase, as a rate limiting enzyme in the cholesterol biosynthesis process. Therefore, LXR activation by CLA leads to cholesterol cell deprivation by stimulating its efflux resulting to inhibition of cell proliferation and stimulation of apoptosis.[31] Beside mentioned mechanisms, CLA also have effect on caveolae lipid composition and function. Caveolae are specialized plasma membrane structures that effect on cancer cell functions including cell signaling, growth and apoptosis. This report mentions that CLA can incorporate into caveolae and modulate its function in breast tumor cells.[32]
Another antitumor activity of CLA is related to stearoyl-CoA desaturase (SCD) enzyme. The high protein expression of stearoyl-CoA desaturase-1 (SCD1), which is a synthetic enzyme of MUFA, was shown in the cancerous areas of breast. The cis-9, trans-11 and trans-10, cis-12 CLA isomers regulate human SCD by reducing SCD protein levels. So it can be protective dietary agent against breast cancer.[33]
CONCLUSION
Evidences in this systematic review are inconsistent and suggested CLA consumption might not lead to a significant change in risk of breast cancer. Narrow ranges of CLA dietary intake, measurement and report bias, confounding variables such as body fatness, high-energy intake, other dietary agents, physical activity, and family history of breast cancer, estrogen replacement therapy and age at menarche are probable explanations for these inconsistent results. As well as, main source of CLA, meat and dairy are rich in saturated fatty acids and other components involved in carcinogenic pathway. So, further well designed studies are required, particularly in considering the main breast cancer risk factors.
Financial support and sponsorship
This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
The authors report no conflicts of interest concerning this study.
REFERENCES
- 1.Friedman EB, Chun J, Schnabel F, Schwartz S, Law S, Billig J, et al. Screening prior to Breast Cancer Diagnosis: The More Things Change, the More They Stay the Same. Int J Breast Cancer 2013. 2013 doi: 10.1155/2013/327567. 327567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Q, Chen ML, Qin Y, Zhang QY, Xu HX, Zhou Y, et al. Isoflavone consumption and risk of breast cancer: A dose-response meta-analysis of observational studies. Asia Pac J Clin Nutr. 2013;22:118–27. doi: 10.6133/apjcn.2013.22.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Farsinejad-Marj M, Talebi S, Ghiyasvand R, Miraghajani M. Adherence to Mediterranean diet and risk of breast cancer in premenopausal and postmenopausal women. Arch Iran Med. 2015;18:786–92. [PubMed] [Google Scholar]
- 4.Golpour S, Rafie N, Safavi SM, Miraghajani M. Dietary isoflavones and gastric cancer: A brief review of current studies. J Res Med Sci. 2015;20:893–900. doi: 10.4103/1735-1995.170627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: A systematic review. J Res Med Sci. 2015;20:910–8. doi: 10.4103/1735-1995.170634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafie N, Golpour Hamedani S, Ghiasvand R, Miraghajani M. Kefir and Cancer: A Systematic Review of Literatures. Arch Iran Med. 2015;18:852–7. [PubMed] [Google Scholar]
- 7.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 8.Voorrips LE, Brants HA, Kardinaal AF, Hiddink GJ, van den Brandt PA, Goldbohm RA. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: The Netherlands Cohort Study on Diet and Cancer. Am J Clin Nutr. 2002;76:873–82. doi: 10.1093/ajcn/76.4.873. [DOI] [PubMed] [Google Scholar]
- 9.Aro A, Mannisto S, Salminen I, Ovaskainen ML, Kataja V, Uusitupa M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr Cancer. 2000;38:151–7. doi: 10.1207/S15327914NC382_2. [DOI] [PubMed] [Google Scholar]
- 10.Chajes V, Lavillonniere F, Ferrari P, Jourdan ML, Pinault M, Maillard V, et al. Conjugated linoleic acid content in breast adipose tissue is not associated with the relative risk of breast cancer in a population of French patients. Cancer Epidemiol Biomarkers Prev. 2002;11:672–3. [PubMed] [Google Scholar]
- 11.Chajes V, Lavillonniere F, Maillard V, Giraudeau B, Jourdan ML, Sebedio JL, et al. Conjugated linoleic acid content in breast adipose tissue of breast cancer patients and the risk of metastasis. Nutr Cancer. 2003;45:17–23. doi: 10.1207/S15327914NC4501_2. [DOI] [PubMed] [Google Scholar]
- 12.Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J Nutr. 2007;137:2599–607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Bergkvist L, Wolk A. Conjugated linoleic acid intake and breast cancer risk in a prospective cohort of Swedish women. Am J Clin Nutr. 2009;90:556–60. doi: 10.3945/ajcn.2009.27480. [DOI] [PubMed] [Google Scholar]
- 14.McGowan MM, Eisenberg BL, Lewis LD, Froehlich HM, Wells WA, Eastman A, et al. A proof of principle clinical trial to determine whether conjugated linoleic acid modulates the lipogenic pathway in human breast cancer tissue. Breast Cancer Res Treat. 2013;138:175–83. doi: 10.1007/s10549-013-2446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCann SE, Ip C, Ip MM, Mc Guire MK, Muti P, Edge SB, et al. Dietary intake of conjugated linoleic acids and risk of premenopausal and postmenopausal breast cancer, Western NewYork Exposures and Breast Cancer Study (WEBStudy) Cancer Epidemiol Biomarkers Prev. 2004;13:1480–4. [PubMed] [Google Scholar]
- 16.Wei G, Liang J, Chen B, Zhou C, Ru N, Chen J, et al. Comparison of transforaminal verse interlaminar epidural steroid injection in low back pain with lumbosacral radicular pain: A meta-analysis of the literature. Int Orthop. 2016 doi: 10.1007/s00264-016-3220-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Bonanno A, Tornambè G, Bellina V, De Pasquale C, Mazza F, Maniaci G, et al. Effect of farming system and cheesemaking technology on the physicochemical characteristics, fatty acid profile, and sensory properties of Caciocavallo Palermitano cheese. J Dairy Sci. 2013;96:710–24. doi: 10.3168/jds.2012-5973. [DOI] [PubMed] [Google Scholar]
- 18.Chin SF, Liu W, Storkson JM, Ha YL, Pariza MW. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Compost Anal. 1992;5:185–97. [Google Scholar]
- 19.Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–36. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinlaw WB, Quinn JL, Wells WA, Roser-Jones C, Moncur JT. Spot 14: A marker of aggressive breast cancer and a potential therapeutic target. Endocrinology. 2006;147:4048–55. doi: 10.1210/en.2006-0463. [DOI] [PubMed] [Google Scholar]
- 21.Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–5. [PubMed] [Google Scholar]
- 22.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. In fluence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127:23–31. doi: 10.1007/s10549-011-1467-5. [DOI] [PubMed] [Google Scholar]
- 24.Missmer SA, Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, et al. Meat and dairy food consumption and breast cancer: A pooled analysis of cohort studies. Int J Epidemiol. 2002;31:78–85. doi: 10.1093/ije/31.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Durgam VR, Fernandes G. The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Lett. 1997;116:121–30. doi: 10.1016/s0304-3835(97)00192-4. [DOI] [PubMed] [Google Scholar]
- 26.Yue W, Wang JP, Li Y, Bocchinfuso WP, Korach KS, Devanesan PD, et al. Tamoxifen versus aromatase inhibitors for breast cancer prevention. Clin Cancer Res. 2005;11(2Pt2):925s–30. [PubMed] [Google Scholar]
- 27.Wang LS, Huang YW, Liu S, Yan P, Lin YC. Conjugated linoleic acid induces apoptosis through estrogen receptor alpha in human breast tissue. BMC Cancer. 2008;8:208. doi: 10.1186/1471-2407-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song HJ, Sneddon AA, Heys SD, Wahle KW. Regulation of fatty acid synthase (FAS) and apoptosis in estrogen-receptor positive and negative breast cancer cells by conjugated linoleic acids. Prostaglandins Leukot Essent Fatty Acids. 2012;87:197–203. doi: 10.1016/j.plefa.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7. [PubMed] [Google Scholar]
- 30.Donnelly C, Olsen AM, Lewis LD, Eisenberg BL, Eastman A, Kinlaw WB. Conjugated linoleic acid (CLA) inhibits expression of the S pot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells. Nutr Cancer. 2009;61:114–22. doi: 10.1080/01635580802348666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Roz A, Bard JM, Huvelin JM, Nazih H. The anti-proliferative and pro-apoptotic effects of the trans9, trans11 conjugated linoleic acid isomer on MCF-7 breast cancer cells are associated with LXR activation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:265–72. doi: 10.1016/j.plefa.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Huot PS, Sarkar B, Ma DW. Conjugated linoleic acid alters caveolae phospholipid fatty acid composition and decreases caveolin-1 expression in MCF-7 breast cancer cells. Nutr Res. 2010;30:179–85. doi: 10.1016/j.nutres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Park Y, Storkson JM, Pariza MW, Ntambi JM. Inhibition of stearoyl-CoA desaturase activity by the cis-9, trans-11 isomer and the trans-10, cis-12 isomer of conjugated linoleic acid in MDA-MB-231 and MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2002;294:785–90. doi: 10.1016/S0006-291X(02)00554-5. [DOI] [PubMed] [Google Scholar]


