abstract
In Germany, vaccination of infants against hepatitis B is recommended since 1995. However, data on long-term immunity is sparse and the necessity of a booster dose remains uncertain. Aims of this study were to assess the long-term persistence of antibodies to the hepatitis B surface antigen (anti-HBs) after immunization during infancy and the effect of a subsequent hepatitis B booster vaccination during adolescence on anti-HBs levels. Patients from a private pediatric practice who had received a full vaccination course of hepatitis B as infants and who were quantitatively tested for anti-HBs during adolescence (pre-booster levels) were included. In those participants who received a hepatitis B booster, post-booster anti-HBs levels were measured. Univariate analyses were conducted to determine factors associated with pre- and post-booster anti-HBs levels, respectively. 106 participants (53% male) were included in the study. At an average of 13.7 y after primary vaccination, 14% of participants had an anti-HBs level of ≥100 IU/l, while 46% were at 10–99 IU/l and 40% had anti-HBs levels of <10 IU/l. In total, 34 received a booster vaccination. Of those, 97% (33/34) had post-booster anti-HBs levels ≥ 100 IU/l, which were independent from pre-booster levels. No other patient characteristics were associated with pre-booster or post-booster anti-HBs≥ 100 IU/l. Although almost half of study participants showed low anti-HBs levels at follow-up, robust responses to booster vaccination suggest that adolescents who received the full vaccination course during infancy are still protected against hepatitis B infection.
Keywords: hepatitis B, vaccination, infant, adolescent, booster, immune memory persistence, anti-HBs
Routine vaccination against hepatitis B during infancy is recommended by World Health Organization (WHO) and many National Immunization Technical Advisory Groups.1,2 In Germany, the Standing Committee on Vaccinations (STIKO) recommends vaccination against hepatitis B for all infants since 1995.3 However, the exact duration of protection after infant vaccination is not well-known: While some authors suggest that life-long protection exists after a full course of vaccination during infancy,4 others have suggested that protection lasts for about 10 to 15 y.5 Consequently, WHO and other authorities currently do not recommend routine hepatitis B booster vaccinations for healthy individuals who have received primary hepatitis B vaccinations in infancy.6
Serum antibody to the hepatitis B surface antigen (anti-HBs) has long been established as a marker of vaccine-induced protection against hepatitis B. An anti-HBs level of ≥ 10 IU/l has been suggested to indicate protection against hepatitis B disease.7 However, some countries including Germany, Switzerland, England and Ireland recommend a more conservative cut-off of ≥100 IU/l.8-10 Anti-HBs levels gradually decrease over time and 20–50% of children who were immunized as infants show low (i.e. ≤ 10 IU/l) anti-HBs levels 4–10 y after primary immunization.11,12 However, it is widely accepted that protection against clinical hepatitis disease outlasts the presence of detectable antibodies.5 The presence of an immunological memory can be confirmed by challenging the immune system with a hepatitis B booster dose and measuring the subsequent anti-HBs response in comparison to pre-booster anti-HBs levels. An anamnestic response (defined as a fourfold increase of the anti-HBs level) would suggest the presence of immune memory.13
Since only limited data are available for European countries, we performed a retrospective cohort study with the primary aim to assess the long-term persistence of anti-HBs after infant hepatitis B vaccination in Germany. Secondary aims were to identify factors that might be associated with lower anti-HBs-levels at long-term follow up and to measure the effect of a subsequent hepatitis B booster vaccination on anti-HBs levels. Accordingly, the following research questions were addressed:
What is the proportion of adolescents with low anti-HBs levels (<10 IU/l or <100 IU/l) after having received a full hepatitis B vaccination course during infancy?
What are risk factors for low anti-HBs levels at follow-up (pre-booster)?
What is the proportion of adolescents with low anti-HBs levels (<10 IU/l or <100 IU/l) after booster immunization?
What are risk factors for low anti-HBs levels after booster immunization?
Study participants were recruited from a private pediatric practice in Gütersloh, Germany. The study base included all patients of the practice who had received hepatitis B immunization during infancy and subsequently attended the practice as adolescents (<18 years) in 2013. To be eligible for inclusion into the study, patients had to fulfill the following criteria: (i) received a full course of hepatitis B vaccination during infancy according to STIKO recommendations3 and (ii) had at least one blood sample drawn in adolescence to determine anti-HBs level. Patients were excluded if (i) they were born before 1996, i.e., before hepatitis B vaccination was recommended for all infants in Germany; (ii) they were registered in another clinical study; (iii) they had received the first dose of hepatitis B vaccine beyond one year of age; or (iv) the time span between first and third vaccine dose was greater than 2 y.
According to our inclusion criteria, all participants had one blood sample drawn which was quantitatively tested for anti-HBs levels (pre-booster blood sample). Patients with anti-HBs levels <100 IU/l were offered by the physician a single booster dose of hepatitis B vaccine as part of the routine service. Those who accepted the booster dose were invited to assess post-booster anti-HBs level (post-booster blood sample).
From the medical records of the pediatric practice, 2 staff members extracted data on the following variables: year of birth, sex, gestational week, birth weight, vaccine type and age at vaccination, age, body weight, height and chronic diseases at follow-up, brand and dates of pre-booster and post-booster vaccinations, and results of anti-HBs testing.
All pre- and post-booster serum samples were quantitatively tested for anti-HBs levels using the Axsym AUSAB assay (Abbott Laboratories, Illinois, USA). We defined protective antibody levels either as anti-HBs concentrations of ≥ 10 IU/l (according to the international recommendations) or as concentrations of ≥100 IU/l (according to the German recommendations). An anamnestic response to a booster vaccination was defined as a four-fold increase of the anti-HBs level after hepatitis B booster.13 Written consent for study participation was obtained from all parents and –in addition- from participants themselves if they aged 14 y or older to allow the use of their data in anonymized form.
Kruskal-Wallis test and chi-squared test were used for group comparisons. Hypothesis tests were performed 2-sided, and a p-value of <0.05 was considered statistically significant. Calculations were done using STATA 13.0 (StataCorp, College Station, TX, USA). An institutional data protection officer assured adherence with data protection laws. The study was approved by the ethics committee of Charité-Universitätsmedizin Berlin (EA2/100/13).
During routine check-up in the pediatric practice, a total of 136 adolescents obtained anti-HBs testing. Thirty adolescents were subsequently excluded for the following reasons: incomplete primary hepatitis B vaccination course (n = 10); reception of the first vaccine dose beyond the first year of age (n = 8); time span between first and third vaccination >2 y (n = 6); date of birth prior to 1996 (n = 4); participation in a clinical study (n = 1); implausible data (n = 1) (see Figure 1 for details).
Figure 1.
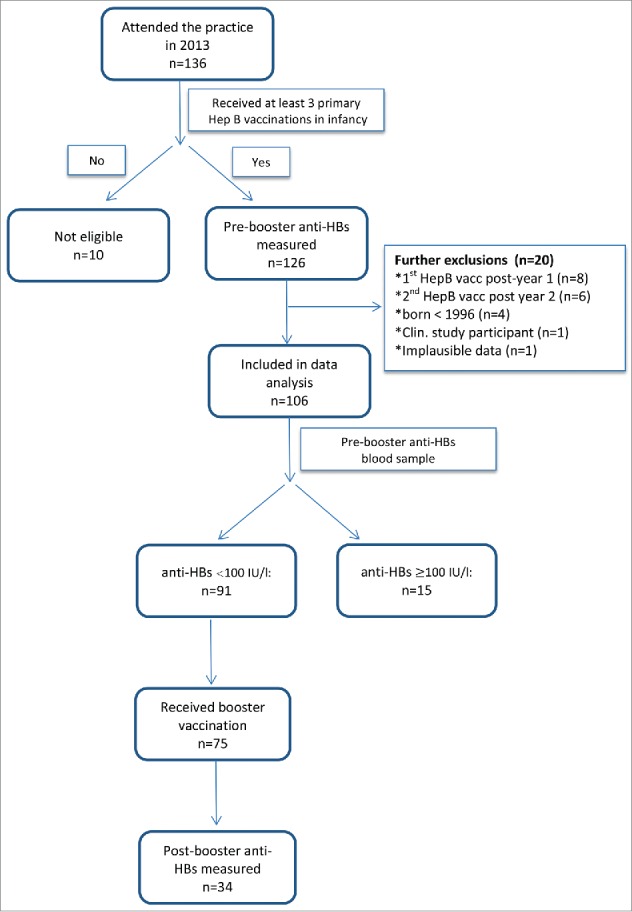
Participant flowchart.
The remaining 106 participants were included in the study. Year of birth ranged between 1996 and 2002, and 56 (53%) were male (Table 1). Of the 81 participants with information on primary immunization brands used, 68 (84%) were immunized with a monovalent (Gen H-B-Vax®-K, Pasteur Mérieux MSD), and 13 (16%) with a hexavalent vaccine including a hepatitis B component (Infanrix hexa®, GlaxoSmithKline (n = 5); unknown brand (n = 8)). Median age at first, second and third vaccination was 30, 36 and 84 weeks, respectively. The time span between first vaccination and measurement of the pre-booster anti-HBs level at follow-up examination was 13.6 y (median, range: 10.4–16.5 years). 45 children (52%) were documented as having a chronic disease (for details see footnote in Table 1).
Table 1.
Characteristics of study participants (n = 106).
| Characteristics at baseline (N = 87–106) | n or median (% or range) |
|---|---|
| Male gender | 56 (53) |
| Gestational age (weeks) | 40 (33–42) |
| Full-term pregnancy1 | 77 (89) |
| Birth weight (g) | 3460 (1890–4670) |
| Received 3-doses vaccination course | 96 (91) |
| Received 4-doses vaccination course | 10 (9) |
| Vaccine type used for infant vaccination | |
| - monovalent vaccine | 68 (64) |
| - hexavalent vaccine | 13 (12) |
| - unknown | 25 (24) |
| Age (weeks) at vaccination | |
| - first dose | 30 (1–53) |
| - second dose | 36 (4–86) |
| - third dose | 84 (20–122) |
| - fourth dose | 85 (47–104) |
| Average time between first and third dose (weeks) | 52 (12–95) |
| Average time between second and third dose (weeks) | 43 (5–90) |
| Characteristics at follow-up (N=84–106) | |
| Age at follow-up blood sample (years) | 13.6 (10.4–16.5) |
| Chronic diseases2 | 45 (52) |
| Body mass index (kg/m2) | 19.5 (15–30) |
defined as gestational age >37 weeks
includes: endocrine and metabolic, neuropsychiatric, respiratory, orthopedic, hematologic and cardiovascular diseases, congenital malformations.
Pre-booster blood testing revealed that 42 (40%) of participants had an anti-HBs level of <10 IU/l, 49 (46%) had a level of 10–99 IU/l, and 15 (14%) had a level of > 100 IU/l. Univariate analysis did not reveal statistically significant associations between pre-booster anti-HBs levels (grouped as <10 IU/l vs. 10–99 IU/l vs. >100 IU/l) and any of the demographic or medical characteristics/variables, including gender, birth weight, prematurity, age at primary vaccination, time span between first vaccination and follow-up and any chronic disease (see Table 2).
Table 2.
Associations between participant characteristics and pre-booster anti-HBs levels (n = 106).
| Pre-booster anti-HBs at follow up (IU/l) |
||||
|---|---|---|---|---|
| <10(n = 42) | 10–99 (n = 49) | ≥100(n = 15) | ||
| Characteristics at baseline | p1 | |||
| Male (gender) | 27 (64) | 20 (41) | 9 (60) | 0.07 |
| Gestational week | 40 (33–42) | 40 (33–42) | 39 (34–42) | 0.51 |
| Full-term pregnancy | 32 (91) | 33 (89) | 12 (80) | 0.50 |
| Birth weight (g) | 3490 (2070–4670) | 3450 (1990–4370) | 3600 (1890–4400) | 0.87 |
| Received 4-doses vaccination course | 2 (5) | 8 (16) | 0 (0) | 0.1 |
| Vaccine type used at baseline | 0.06 | |||
| - monovalent vaccine | 27 (64) | 29 (59) | 12 (80) | |
| - hexavalent vaccine | 1 (2) | 9 (18) | 3 (20) | |
| - unknown, n (%) | 14 (33) | 11 (22) | 0 (0) | |
| Age (weeks) at | ||||
| - first vaccination | 32 (1–52) | 29 (6–52) | 30 (13–52) | 0.48 |
| - second vaccination | 38.5 (4–61) | 35 (12–86) | 34 (17–69) | 0.33 |
| - third vaccination | 85.5 (26–116) | 82 (20–122) | 90 (30–120) | 0.47 |
| Time between first and third dose (weeks) | 51 (13–86) | 51 (12–89) | 60 (17–94) | 0.41 |
| Time between second and third dose (weeks) | 43 (8–82) | 43 (4–85) | 52 (13–90) | 0.25 |
| Characteristics at follow-up | ||||
| Age at follow-up blood sample (months) | 165 | 164 | 165 | 0.94 |
| Chronic illness | 0.2 | |||
| - none | 21 (54) | 21 (51) | 0 | |
| - endocrine and metabolic diseases | 2 (5) | 6 (15) | 2 (29) | |
| - neuropsychiatric diseases | 2 (5) | 1 (2) | 0 | |
| - respiratory diseases | 9 (23) | 10 (24) | 4 (57) | |
| - congenital malformations | 2 (5) | 0 | 0 | |
| - orthopedic diseases | 1 (3) | 0 | 0 | |
| - haemotologic diseases | 1 (3) | 3 (7) | 1 (14) | |
| - cardiovascular diseases | 1 (3) | 0 | 0 | |
| Body mass index (kg/m)2 | 20 (17–25) | 19 (15–27) | 19.5 (17–30) | 0.14 |
Numbers are n (&) or median (range)
Kruskal-Wallis test or chi-square test, as appropriate.
75 (82%) of the 91 study participants with pre-booster anti-HBs levels < 100 IU/l received a monovalent hepatitis B booster vaccination (Engerix, n = 68; Gen-H-B-Vax-K, n = 6; unknown brand, n = 1) with 34 returning to provide a final blood sample after a median time of 18 weeks (6–47) (see Figure 1 and Table 1). Twenty (58.9%) of the 34 boosted patients had been vaccinated with a monovalent vaccine and 2 (5.9%) with a hexavalent vaccine. In a further 12 patients (35.3%) the vaccine brand was not documented. Of these 34 patients, 22 (65%) had pre-booster anti-HBs levels of <10 IU/l, while the remaining 12 (35%) had pre-booster anti-HBs levels of 10–99 IU/l. All but one participants had post-booster anti-HBs levels of ≥ 100 IU/l, indicating the presence of an anamnestic response. Table 3 shows the relation between pre-booster and post-booster anti-HBs levels in these 34 study participants. Nineteen participants (56%) had post-booster anti-HBs levels of ≥1000 IU/l. Those with pre-booster anti-HBs of 10–99 IU/l were more likely to have a post-booster anti-HBs ≥1000 IU/l than those with lower pre-booster levels (p = 0.045). The only participant with post-booster anti-HBs of 10 IU/l was a female who had received primary vaccination at 18, 26 and 100 weeks of age. She had no underlying chronic disease or other abnormalities which could explain failure to respond to hepatitis B vaccination. The brand of vaccine administered to this particular patient was unknown.
Table 3.
Association between pre-booster and post-booster anti-HBs levels (p = 0.045 by Fishers exact test; n = 34).
| Post-booster anti-HBs level | Pre-booster anti-HBs <10 IU/l | Pre-booster anti-HBs 10–99 IU/l |
|---|---|---|
| <10 IU/l | — | — |
| 10–99 IU/l | 1 | 0 |
| 100–999 IU/l | 12 | 2 |
| ≥ 1000 IU/l | 9 | 10 |
To our knowledge, only a few publications addressed hepatitis B immune status after primary vaccination during infancy in children and adolescents in the German population. Using the data from the German Health Survey for children and adolescents, Jorgensen et al.14 examined anti-HBs levels in 477 children 2.4 y after primary vaccination with either Hexavac or Infanrix hexa. Another study15 investigated anti-HBs level of children who were primed in infancy at the age of 4–6 and 7–9 years, respectively. The publication by Behre et al.13 comprised results of 2 studies that investigated 301 participants at 7–8 y of age and 306 participants at 12–13 y of age. Finally, a publication reported data on a total of 300 children aged 7–8 y.16 Thereby, with age of participants spanning from 10 to 16 years, our study included participants with the longest follow-up duration in Germany so far. Furthermore, only a very limited number of studies have been published on cohorts in low endemicity countries like Germany. While other studies only reported data on either the anti-HBs threshold of >10 IU/l or >100 IU/l as a measure of protective antibody levels, we used and reported both thresholds. Moreover, in the majority of other studies only the results of descriptive analyses were reported, whereas in our study an additional risk factor analysis was conducted. Finally, nearly all published studies on this issue lack a detailed description of the prevalence of chronic diseases in the study sample which was performed in our data set.
Our study performed in a pediatric practice in Germany shows that a considerable proportion of participants who completed hepatitis B vaccination during infancy had anti-HBs levels <100IU/l after 10 to 16 y. However, the fact that 97% of adolescents showed an anamnestic response to a booster dose indicates the presence of robust immunological memory ensuring protection against hepatitis B. Nevertheless, one has to consider that different vaccine brands were used. We cannot completely exclude that this might explain the absence of a response in one participant since information was not available on vaccine brand in this particular case.
Hepatitis B vaccination during infancy has been shown to reduce the incidence of hepatitis B infection through the stimulation of immunological memory.4,6 The first 2 vaccine doses typically stimulate antibody production and prime the immune system for a secondary immune response to the third dose.6 However, anti-HBs levels decrease over time. In two studies in Germany, Jilg et al. showed that anti-HBs decreases below 10 IU/l in 22% and 39% of vaccinees over a 4-year-period after primary vaccination.17,18 It has been found that anti-HBs falls to < 10IU/l in 10–50% of vaccinees 10–15 y post-primary vaccination.5 However, loss of detectable antibodies does not necessarily indicate loss of protection against hepatitis infection or disease, due to the presence of memory B lymphocytes that are able to rapidly produce anti-HBs soon after exposure to the virus.6
Our finding of a high proportion of adolescents with anamnestic responses is in line with those by other investigators. In an Italian study 35% of teenagers vaccinated against hepatitis B during infancy had anti-HBs levels <10 IU/l at 10 y after vaccination in infancy.19 After a booster was administered 17 y post-primary vaccination, 98% exhibited a strong anamnestic response, indicating the presence of immune memory despite the low antibody titers detected. A study performed in Slovakia found that 10–11 y after a primary vaccination course with a hexavalent vaccine containing a hepatitis B component or with a monovalent hepatitis B vaccine 48% and 58% of the participants, respectively, had anti-HBs levels ≥ 10 IU/l. After a hepatitis B challenge dose, the proportion of participants with anti-HBs levels of ≥100 IU/l was 94% in both groups.19
A recent study performed in Germany showed that 72% of children had anti-HBs levels ≥ 10 IU/l seven to eight y after vaccination with a hexavalent vaccine during infancy.16 Finally, very similar to our results, only 24% of 16 to 19-year-olds in the US had anti-HBs levels ≥ 10 IU/l, but 92% responded to a booster dose.20
In our study, we did not find associations between participant characteristics and pre-booster anti-HBs levels. However, one has to consider the relatively small sample size of our study when interpreting these data. A recent systematic review and meta-analysis provides more reliable and robust data on determinants of pre-booster antibody levels after primary vaccination against hepatitis B in infancy.21 Analyzing 46 studies which included a total of >28,000 participants, these authors found that neither vaccination schedule, nor vaccine type, exact age at first vaccination or number of doses were associated with antibody levels in the long-term. Although preterm birth was found to be associated with low anti-HBs levels in some studies, results are inconclusive for birth weight independent of gestational age.14,22 When comparing studies assessing anti-HBs persistence, the local endemicity of hepatitis B has to be taken into account. This cohort study comprised children in an area of low hepatitis B endemicity. Therefore, the results cannot be generalized to high-endemic regions, where repetitive exposure to the wild virus may lead to natural boosting and subsequent longer protection against the disease.5 Other potential problems in the comparison of studies consist of the use of different vaccine formulations, dosing schedules, and different assays used for antibody quantification.
Some limitations of our study need to be taken into account. First, our study population was rather small, comprising children from only one pediatric practice in Germany. Therefore, it is unclear whether the results can be translated to the general population in Germany. However, representativeness is not a necessary condition for every epidemiological study to generate valid results.23 This argument particularly applies to risk factors analyses, as they were performed and reported in our study. Therefore, even if our study sample was not representative, the results of this analysis are still valid and could be generalized. Second, although 75 of 91 eligible adolescents received a booster vaccination, only 34 had returned for post-booster blood sampling which might have introduced selection bias. To explore this issue, we performed a missing data analysis. Comparing the baseline characteristics (gender, gestational age, birth weight, 4-dose vaccination schedule, vaccine type, age and time between vaccine doses) between those 34 patients to the remaining participants who did not return for blood sampling, we did not find significant differences (data available upon request from the authors). Third, although we excluded children who received the first vaccine dose after the age of 12 months, study participants had received their primary immunization course at varying ages, mostly later than currently recommended by STIKO. However, we believe that this study presents “real life” conditions in the ambulatory setting. Moreover, as shown in Table 2, there was no relation between age at primary vaccination and pre-booster anti-HBs. Fourth, different hepatitis B vaccine brands were used consisting of both monovalent and hexavalent combination vaccines, and primary vaccination regimens consisted of both 3 and 4 dose schemes. This might have affected anti-HBs levels differentially. Still, the number of doses was neither associated with pre-booster nor post-booster anti-HBs levels. Finally, the relatively high proportion of participants with underlying chronic illnesses might indicate selection bias. According to a representative telephone survey which was conducted between 2009 and 2012 in the German general population, 20.6% of children aged 14 to 17 y were reported to have an underlying chronic disease.24 However, our study population comprised adolescents who subsequently attended a pediatric practice due to medical reasons. Therefore, it is plausible that the prevalence of underlying diseases in this population was higher. Since chronic diseases are associated with decreased immunogenicity, our result could be interpreted as a rather conservative estimate. Moreover, the analysis presented in Table 2 shows that the presence of any of the chronic diseases was unrelated to the main outcome variable, indicating that chronic diseases did not bias the study results.
Taken together, this study shows that a large proportion of children vaccinated against hepatitis B as infants had low anti-HBs levels 10 to 16 y after primary vaccination. However, the strong immune response detected after booster administration indicates the continued presence of immunity to hepatitis B, thus indicating long-term protection. Since our data set was limited by its sample size and it is unclear whether the anamnestic response translates into protection against hepatitis B infection, further studies are needed to assess both long-term immunological response and long-term effectiveness of the vaccine against clinical disease.
Funding
The study was funded by internal funds of the Robert Koch Institute.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank all children and their parents for the participation in the study and the staff members from the pediatric practice for extracting the data.
References
- [1].Advisory Committee on Immunization Practice, Centers for Disease Control and Prevention Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control 2011; 60:1-45 [PubMed] [Google Scholar]
- [2].Publication WHO World Health Organization: Hepatitis B Vaccines: WHO position paper. Vaccine 2010; 28:589-90; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.110 [DOI] [PubMed] [Google Scholar]
- [3].Ständige Impfkommission Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: August 2014. Epidemiologisches Bulletin 34/2014. 2014 [Google Scholar]
- [4].Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011; 53:68-75; PMID:21653306; http://dx.doi.org/ 10.1093/cid/cir270 [DOI] [PubMed] [Google Scholar]
- [5].Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, Duval B, Jilg W, Bocher WO, Lu SN, et al.. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine 2005; 23:4158-66; PMID:15964484; http://dx.doi.org/ 10.1016/j.vaccine.2005.03.017 [DOI] [PubMed] [Google Scholar]
- [6].Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet 2000; 355:561-5; PMID:10683019; http://dx.doi.org/ 10.1016/S0140-6736(99)07239-6 [DOI] [PubMed] [Google Scholar]
- [7].Advisory Committee on Immunization Practice, Centers for Disease Control and Prevention: Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports :Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control, 2011:1-45 [PubMed] [Google Scholar]
- [8].Impfkommission S. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: Juli 2012. Epidemiologisches Bulletin 2012; 30:290-1 [Google Scholar]
- [9].Bundesamt für Gesundheit: Schweizerischer Impfplan 2012. Available at: http://www.bag.admin.ch/ekif/04423/04428/ [Google Scholar]
- [10].Health Do. Immunisation against infectious disease In: Department of Health L, UK, ed., 2006 [Google Scholar]
- [11].Jilg W, Schmidt M, Deinhardt F. Four-year experience with a recombinant hepatitis B vaccine. Infection 1989; 17:70-6; PMID:2714860; http://dx.doi.org/ 10.1007/BF01646879 [DOI] [PubMed] [Google Scholar]
- [12].Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C, McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population–results of a 10-year study. J Infect Dis 1997; 175:674-7; PMID:9041341; http://dx.doi.org/ 10.1093/infdis/175.3.674 [DOI] [PubMed] [Google Scholar]
- [13].Behre U, Bleckmann G, Crasta PD, Leyssen M, Messier M, Jacquet JM, Hardt K. Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccin Immunother 2012; 8:813-8; PMID:22508412; http://dx.doi.org/ 10.4161/hv.19898 [DOI] [PubMed] [Google Scholar]
- [14].Jorgensen P, Poethko-Muller C, Hellenbrand W, Jilg W, Thierfelder W, Meyer C, an der Heiden M, Schlaud M, Radun D. Low hepatitis B immunogenicity of a hexavalent vaccine widely used in Germany: results of the German Health Survey for Children and Adolescents, 2003–2006. Epidemiol Infect 2010; 138:1621-9; PMID:20233496; http://dx.doi.org/ 10.1017/S0950268810000543 [DOI] [PubMed] [Google Scholar]
- [15].Zinke M, Kappes R, Kindler K, Paulus-Koschik A, Goering U, Disselhoff J, Soemantri P, Grunert D, Laakmann KH, Gunasekaran R, et al.. Immune memory to hepatitis B virus in 4–9-year old children vaccinated in infancy with four doses of hexavalent DTPa-HBV-IPV/Hib vaccine. Hum Vaccin 2009; 5:592-8; PMID:19535920; http://dx.doi.org/ 10.4161/hv.9051 [DOI] [PubMed] [Google Scholar]
- [16].Van Der Meeren O, Bleckmann G, Crasta PD. Immune memory to hepatitis B persists in children aged 7–8 years, who were vaccinated in infancy with 4 doses of hexavalent DTPa-HBV-IPV/Hib (Infanrix hexa) vaccine. Hum Vaccin Immunother 2014; 10:1682-7; PMID:24637296; http://dx.doi.org/ 10.4161/hv.28480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: how long does protection last? Lancet 1984; 2:458; PMID:6147519; http://dx.doi.org/ 10.1016/S0140-6736(84)92926-X [DOI] [PubMed] [Google Scholar]
- [18].Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol 1988; 6:201-7; PMID:2970501; http://dx.doi.org/ 10.1016/S0168-8278(88)80032-1 [DOI] [PubMed] [Google Scholar]
- [19].Spada E, Romano L, Tosti ME, Zuccaro O, Paladini S, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, et al.. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect 2014; 20:O680-6; PMID:24528380; http://dx.doi.org/ 10.1111/1469-0691.12591 [DOI] [PubMed] [Google Scholar]
- [20].Middleman AB, Baker CJ, Kozinetz CA, Kamili S, Nguyen C, Hu DJ, Spradling PR. Duration of protection after infant hepatitis B vaccination series. Pediatrics 2014; 133:e1500-7; PMID:24843060; http://dx.doi.org/ 10.1542/peds.2013-2940 [DOI] [PubMed] [Google Scholar]
- [21].Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of Long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J 2013; 32:307-13; PMID:23249904; http://dx.doi.org/ 10.1097/INF.0b013e31827bd1b0 [DOI] [PubMed] [Google Scholar]
- [22].Kesler K, Nasenbeny J, Wainwright R, McMahon B, Bulkow L. Immune responses of prematurely born infants to hepatitis B vaccination: results through three years of age. Pediatr Infect Disease J 1998; 17:116-9; PMID:9493806; http://dx.doi.org/ 10.1097/00006454-199802000-00007 [DOI] [PubMed] [Google Scholar]
- [23].Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol 2013; 42:1012-4; PMID:24062287; http://dx.doi.org/ 10.1093/ije/dys223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Neuhauser H, Poethko-Muller C, Ki GGSSG. [Chronic and vaccine-preventable diseases in children and adolescents in Germany: results of the KiGGS study: first follow up (KiGGS wave 1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014; 57:779-88; PMID:24950827; http://dx.doi.org/ 10.1007/s00103-014-1976-6 [DOI] [PubMed] [Google Scholar]


