Abstract
Hyperphosphatemia is a risk factor for ectopic calcification and coronary artery diseases in end stage renal diseases (ESRD). The aim of this study was to assess the effect of low-dose nicotinic acid on hyperphosphatemia in patients with ESRD. This randomized, double-blind clinical trial was done on 70 ESRD patients with serum phosphoure ≥5.5 mg/dl. Patients were randomly divided into two equal groups (n = 35) and the intervention group received niacin 25 mg/day as the initial dose. After 4 weeks, in patients who did not respond to treatment, niacin dose was increased up to 50 mg/dl. At the end of week 8, in case there was no treatment effect, the dose was raised to 100 mg/day. The appropriate response to treatment was defined as serum phosphorous level reductions <5.5 mg/dl. The age was 50.5 ± 14.3 years and duration of dialysis 5.1 ± 5.3 months. In the niacin group, mean phosphorus level decreased from 6.7 ± 0.84 mg/dl at the end of the 1st month to 5.8 ± 1.0 mg/dl at the end of the 2nd month and to 4.4 ± 1.4 mg/dl at the end of the 3rd month (P = 0.004). In the placebo group, mean phosphorus level increased from 6.5 ± 1.2 mg/dl to 7.2 ± 0.91 mg/dl at the end of the 3rd month (P = 0.006). In the niacin group, high density lipoprotein (HDL) increased significantly from 45.00 ± 14.9 to 47.2 ± 11.6 (P = 0.009). We conclude that niacin (100 mg/day) decreased phosphorus serum level and increased HDL serum level in patients on dialysis.
Key words: End stage renal disease, high-density lipoprotein, hyperphosphatemia, nicotinic acid
Introduction
Chronic kidney disease (CKD) is growing worldwide and the incidence of end stage renal diseases (ESRD) is on the rise.[1] Previous studies have reported that hyperphosphatemia results in increased morbidity and mortality rates among patients with CKD. Serum phosphorus levels more than 6.5 mg/dl increase mortality rate about 27% compared to phosphorus levels <6.5 mg/dl.[2,3] Long-term inadequate phosphate control leads to secondary hyperparathyroidism, metabolic bone diseases, calcific uremic arteriolopathy, and cardiovascular calcification. Progressive increases in arterial calcification are associated with higher rates of mortality.[4] Management of hyperphosphatemia in patients with ESRD is not adequate. Calcium containing phosphate binders may sometimes result in adverse effects such as hypercalcemia.[5] Noncalcium phosphate binders, such as sevelamer and lanthanum, are expensive.[6] Several trials have shown that niacinamide and niacin are capable of remarkably reducing serum phosphate levels in patients undergoing dialysis.[7,8,9,10,11]
Other studies have shown that niacin increases high density lipoprotein (HDL) cholesterol and reduces triglyceride levels with potentially favourable cardiovascular effects.[12] Human and animal experiences in in vitro studies have indicated that niacin through inhibiting the cotransporter NaPi2a in the renal proximal tubule and cotransporter NaPi2b in the intestine results in decreased phosphate uptake.[12,13,14,15,16]
Adverse effects of high-dose niacin limit the use of this agent. This study was designed to evaluate the impact of low-dose nicotinic acid on phosphorus levels in chronic dialysate patients with ESRD as the main objective. The effect of niacin on low density lipoprotein (LDL) is investigated as the secondary objective.
Materials and Methods
In this randomized, double-blind clinical trial, 70 dialysis patients referred to the Dialysis Ward of Ashrafi Esfehani Hospital from May 2013 to April 2014 were evaluated. The participants were observed for 12 weeks. Inclusion criteria were age >18 years, ability to give informed consent, (P ≥ 5.5) mg/dl, dialysis duration for more than 3 months, adequate dialysis (Kt/V >1.2) during the study, and protocol, including calcium carbonate as phosphate binder 2 weeks prior to the study. Exclusion criteria were pregnancy, liver disease, active peptic ulcer disease, taking carbamazepine, history of niacin (or niacin as other drugs' component) sensitivity, and malignancy. Written informed consent was obtained before randomization, as per the institution's protocol. The study was approved by Ethics Committee of Shahid Beheshti University of Medical Sciences. The patients were randomly assigned to either niacinamide or placebo groups. Both participants and the study staff (site investigators and trial coordinating staff) were masked to the treatment.
Niacin and placebo were packaged in identical tablets by Pharmacy Company (Azad Pharmacology University). The patients were prescribed one tablet daily with their meal. Dosages were titrated from 25 mg/day over 12 weeks. Phosphorus level was measured pretreatment and at the end of weeks 4, 8, and 12. When serum phosphorus levels did not reach the normal reference range (P ≤ 5.5 mg/dl), the dose of nicotinic acid was increased to 50 mg/day at the end of the 1st month and to 100 mg/day at the end of the 3rd month. None of the participants was treated with sevalmer (renagel) because of economic problems. Wash-out period was not permitted by the Ethics Committee of Shahid Behshti University of Medical Sciences; thus, all patients were administered 1500 mg calcium carbonate as phosphate binder during the study. When phosphorus levels exceeded 5.5 mg/dl or decreased to less than 3.5 mg/dl, the dose of phosphate binder was changed in order to continue niacin as the descripted dose. None of the participants used statins or resins.
The patients were followed for 3 months and during this period, serum levels of P, Ca, alanine aminotransferase (ALP), HDL cholesterol, triglycerides (TG), platelet, and parathyroid hormone (PTH) were measured monthly.
Statistical analysis
Descriptive statistics that used means and standard deviations are presented as contentious variables. The Chi-square and Fisher's exact test were used to compare categorical variables. Mann–Whitney's U-test and paired t-test were used to compare continuous variables. For all the tests, (P ≤ 0.05) was considered significant. Data were analyzed using SPSS 20 (SPSS Inc., Chicago, IL, USA).
Results
In this study, 17 patients (35 male and 35 female) with the mean age of 50.5 ± 14.3 (range 16-76) years and mean duration of dialysis 5.1 ± 5.3 months were evaluated. The difference in mean age between niacin and placebo groups was not significant (49.8 ± 14.6 and 51.1 ± 14.1, respectively) (P = 0.70). At the end of the first month, the difference between two groups in calcium level was significant [Table 1]; moreover, at the end of the second month, the difference between the two groups in terms of phosphorus and calcium levels was significant [Table 2]. At the end of the third month, mean phosphorus level was significantly different between the two groups [Table 3]. The mean difference in cholesterol, TG, low-density lipoprotein (LDL), AST, ALT, platelet, and bilirubin between the two groups was not significant at the end of the first, second, and third month (P > 0.05) [Tables 1 and 3].
Table 1.
The mean and standard deviation of parameters at the end of first month in two groups (25 mg/day niacin)
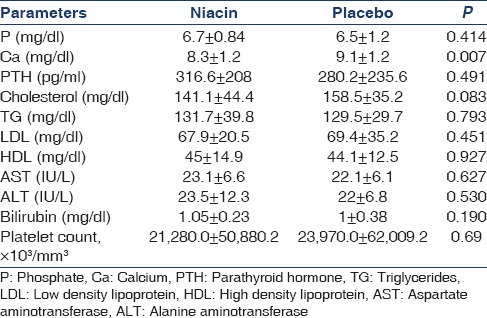
Table 2.
The mean and standard deviation of parameters at the end of second month in two groups (50 mg/day niacin)
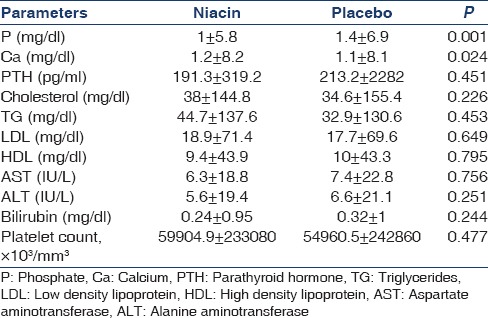
Table 3.
The mean and standard deviation of parameters at the end of third month in two groups (100 mg/day niacin)
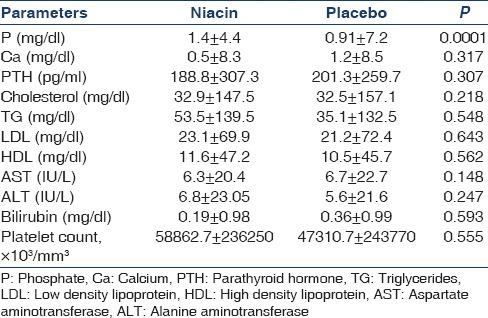
In the niacin group, mean phosphorus level significantly decreased from 6.7 ± 0.84 mg/dl at the end of the first month to 5.8 ± 1 mg/dl at the end of the second month and to 4.4 ± 1.4 mg/dl at the end of the third month (P = 0.004) [Figure 1]. Moreover, the mean of the secondary outcome (HDL) increased at the end of the third month [Table 4, Figure 2].
Figure 1.
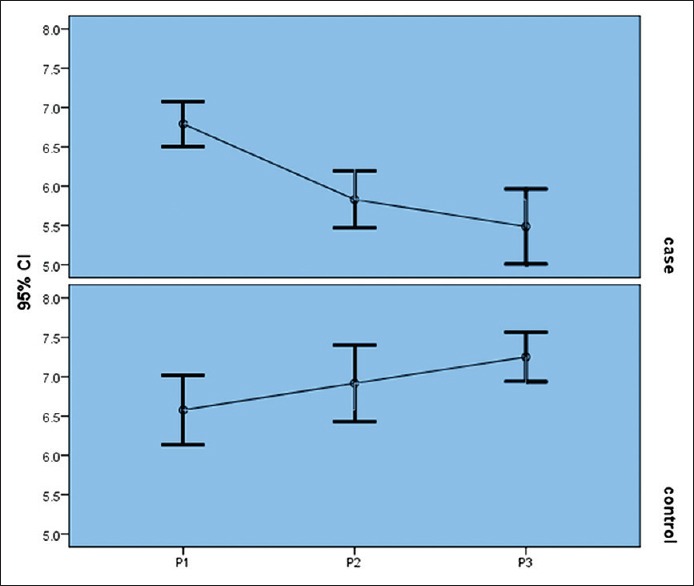
Niacin effect on serum phosphorus levels
Table 4.
The mean and standard deviation of parameters at the end of first, second and third month in Niacin group
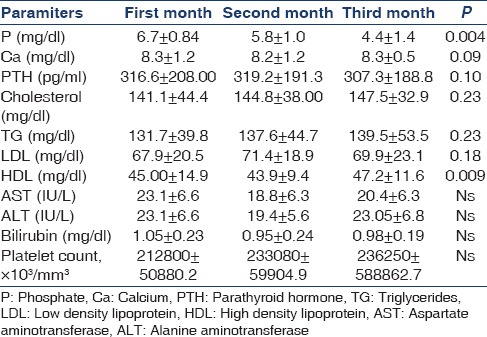
Figure 2.
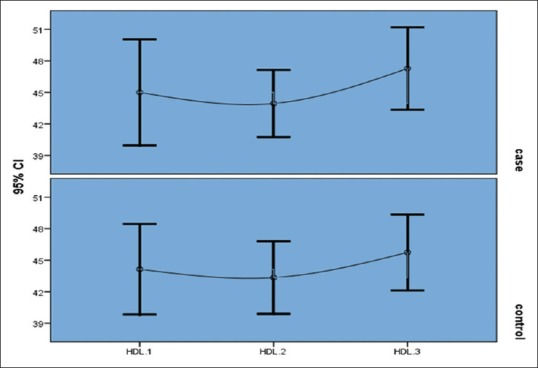
Niacin effect on high density lipoprotein
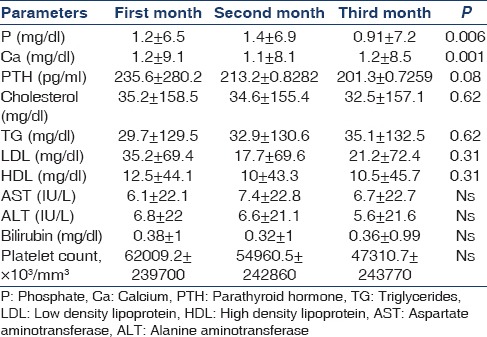
In the placebo group, mean phosphorus level significantly increased from 6.5 ± 1.2 mg/dl at the end of the first month to 6.9 ± 1.4 mg/dl at the end of the second month and to 7.2 ± 0.91 mg/dl at end of the third month (P = 0.006) [Table 5].
Table 5.
The mean and standard deviation of parameters at the end of first, second and third month in placebo group
Flushing (in one patient) and vomiting (in one patient) were detected in the niacin group during the second month.
Discussion
Recently, niacin and niacinamide derivatives as phosphate-binder agents have been used to manage hyperphosphatemia in patients with CKD. Previous experiences have indicated that nicotinamide inhibits sodium/phosphorous transport in both renal and intestinal brush borders and decreases phosphorus levels in patients undergoing dialysis.[17,18,19,20] Although several studies suggest the effectiveness of niacinamide in reducing plasma phosphorus, only one study (2013) investigated low-dose niacin (500 mg/daily).[17]
The present clinical trial evaluated the impact of 100 mg/day nicotinic acid. It was observed that after 8 weeks, mean serum phosphorus level in the niacin group significantly was different from the control group. In line with the results of this study, Vasantha et al., in an open-label study on 30 dialysis patients receiving a dose of NAM 750 mg/day, reported reductions in serum phosphorus level (2.3 mg/dl).[18] Rennick et al., in a meta-analysis reviewed seven studies that evaluated the effect of nicotinamide and nicotinic acid on phosphorus serum level in ESRD patients undergoing dialysis and indicated that in the three studies using nicotinic acid as the therapeutic intervention and four studies using nicotinamide, both nicotinic acid and nicotinamide significantly reduced serum phosphorus level.[19] In a randomized, double-blind placebo-controlled crossover trial, Cheng et al., examined the effect of niacinamide (500 mg/day to 1500 mg/day) on 33 patients with ESRD undergoing hemodialysis. The results indicated significant decreases in serum phosphorus. Moreover, in accord with our study, HDL cholesterol significantly increased in the niacin group.[9]
In the present study, no significant changes in Ca and PTH were detected. Cheng et al., observed insignificant changes in serum calcium and PTH levels in the niacin group.[9] In agreement with Kang et al., statically significant increases in HDL were observed in the present study.[17] Similarly, Shahbazian et al., in a placebo-controlled clinical study on 48 dialysis patients, showed that the administration of niacinamide 500 mg/day decreased serum phosphate, increased HDL levels and fasting glycaemia, and reduced LDL and triglyceride levels.[20] The important side effects of niacin are vasodilation and flushing;[21] however, in the present study, we detected flashing only in one patient. Although reduction in platelet count occurred in some participants, no cases of thrombocytopenia were reported in the present study.
Use of low-dose niacin is affordable due to low adverse effects and the low cost. Recent studies, suggested that by reducing side effects, nicotinamide can be an inexpensive alternative.[22] However, further studies are recommended to examine if it is an acceptable alternative to sevelamer in patients with economic problems.
The present study is novel in examining the effects of low-dose niacin (100 mg/daily). The obtained results at this low dose may be attributed to differences in nutritional and genetic backgrounds of Iranian patients, though more in vitro studies should be conducted to confirm its effects.
The main limitations of our study are small sample size and short duration of follow-up (3 months). Thus, further prospective double-blind clinical trials are recommended with longer follow-up durations and larger populations.
Conclusion
Niacin 100 mg/daily decreased phosphorus serum levels and increased HDL serum levels in patients with ESRD that undergo dialysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the nursing, administrative, and secretarial staff at the Nephrology Department and Clinic of Ashrafi Esfehani Hospital for their kind contribution.
References
- 1.Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2000-2009. Ottawa, Ont: CIHI; 2011. Canadian Institute for Health Information. [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int. 2006;70:351–7. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: Recommendations for a change in management. Am J Kidney Dis. 2000;35:1226–37. doi: 10.1016/s0272-6386(00)70064-3. [DOI] [PubMed] [Google Scholar]
- 5.Manns B, Stevens L, Miskulin D, Owen WF, Jr, Winkelmayer WC, Tonelli M. A systematic review of sevelamer in ESRD and an analysis of its potential economic impact in Canada and the United States. Kidney Int. 2004;66:1239–47. doi: 10.1111/j.1523-1755.2004.00877.x. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med. 2010;362:1312–24. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, et al. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 8.Müller D, Mehling H, Otto B, Bergmann-Lips R, Luft F, Jordan J, et al. Niacin lowers serum phosphate and increases HDL cholesterol in dialysis patients. Clin J Am Soc Nephrol. 2007;2:1249–54. doi: 10.2215/CJN.01470307. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1131–8. doi: 10.2215/CJN.04211007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampathkumar K, Selvam M, Sooraj YS, Gowthaman S, Ajeshkumar RN. Extended release nicotinic acid – A novel oral agent for phosphate control. Int Urol Nephrol. 2006;38:171–4. doi: 10.1007/s11255-006-0001-x. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo Valencia CA, Cruz J. Safety and effectiveness of nicotinic acid in the management of patients with chronic renal disease and hyperlipidemia associated to hyperphosphatemia. Nefrologia. 2008;28:61–6. [PubMed] [Google Scholar]
- 12.Katai K, Tanaka H, Tatsumi S, Fukunaga Y, Genjida K, Morita K, et al. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14:1195–201. doi: 10.1093/ndt/14.5.1195. [DOI] [PubMed] [Google Scholar]
- 13.Berndt TJ, Pfeifer JD, Knox FG, Kempson SA, Dousa TP. Nicotinamide restores phosphaturic effect of PTH and calcitonin in phosphate deprivation. Am J Physiol. 1982;242:F447–52. doi: 10.1152/ajprenal.1982.242.5.F447. [DOI] [PubMed] [Google Scholar]
- 14.Kempson SA, Colon-Otero G, Ou SY, Turner ST, Dousa TP. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest. 1981;67:1347–60. doi: 10.1172/JCI110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eto N, Miyata Y, Ohno H, Yamashita T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005;20:1378–84. doi: 10.1093/ndt/gfh781. [DOI] [PubMed] [Google Scholar]
- 16.Marcus R, Coulston AM. Water soluble vitamins. The vitamin B complex and ascorbic acid. In: Gilman G, editor. The Pharmacological Basis of Therapeutics. 9th ed. New York: McGrawhill; 1996. pp. 1555–71. [Google Scholar]
- 17.Kang HJ, Kim DY, Lee SM, Kim KH, Han SH, Nam HK, et al. Effect of low-dose niacin on dyslipidemia, serum phosphorus levels and adverse effects in patients with chronic kidney disease. Kidney Res Clin Pract. 2013;32:21–6. doi: 10.1016/j.krcp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasantha J, Soundararajan P, Vanitharani N, Kannan G, Thennarasu P, Neenu G, et al. Safety and efficacy of nicotinamide in the management of hyperphosphatemia in patients on hemodialysis. Indian J Nephrol. 2011;21:245–9. doi: 10.4103/0971-4065.83735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennick A, Kalakeche R, Seel L, Shepler B. Nicotinic acid and nicotinamide: A review of their use for hyperphosphatemia in dialysis patients. Pharmacotherapy. 2013;33:683–90. doi: 10.1002/phar.1258. [DOI] [PubMed] [Google Scholar]
- 20.Shahbazian H, Zafar Mohtashami A, Ghorbani A, Abbaspour MR, Belladi Musavi SS, Hayati F, et al. Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: A double-blind randomized clinical trial. Nefrologia. 2011;31:58–65. doi: 10.3265/Nefrologia.pre2010.Nov.10734. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Parsons WG, 3rd, Roberts LJ., 2nd Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263–74. doi: 10.1016/0090-6980(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 22.Shin S, Lee S. Niacin as a drug repositioning candidate for hyperphosphatemia management in dialysis patients. Ther Clin Risk Manag. 2014;10:875–83. doi: 10.2147/TCRM.S71559. [DOI] [PMC free article] [PubMed] [Google Scholar]


