Abstract
Prevalence of hepatitis B is still high among adults in China, athough the prevalence in children has decreased dramatically. Immunization against hepatitis B among adults is urgently required. Through analyzing the immunogenicity of different doses, schedules and booster immunization among adults, we recommend 10 or 20 μg with a 0-1-6-month schedule or a 0-1-12-month schedule for migrant adults. For immunity failure, increasing the dose or covalent vaccine is suggested to provide protective antibodies. To enhance immunity among adults, hepatitis B vaccine should be included in health insurance.
Keywords: China, hepatitis B, healthy adults, immunization, prevalence
Introduction
China has one of the highest rates of infection with hepatitis B virus (HBV) worldwide. Currently, China has ∼93 million persons infected with HBV, of whom, ∼20 million have chronic hepatitis B1. Due to the attention and efforts of government, China has made remarkable achievements in the prevention and management of hepatitis B, and made outstanding progress in decreasing its incidence, especially among younger people,1 as shown in Figure 1. The national HBV seroepidemiology survey results in 1992 and 2006 showed that the general population with HBV infection rate decreased significantly, especially children aged <15 years. The number of children infected with HBV has declined by nearly 80 million. The average of hepatitis B surface antigen (HBsAg) carrier rate has fallen from 9.75% to 7.18%, but the rate is still over 8% among adults.1 The question is how to control HBV prevalence in the general population. It seems that we should rely on vaccination of adults.
Figure 1.
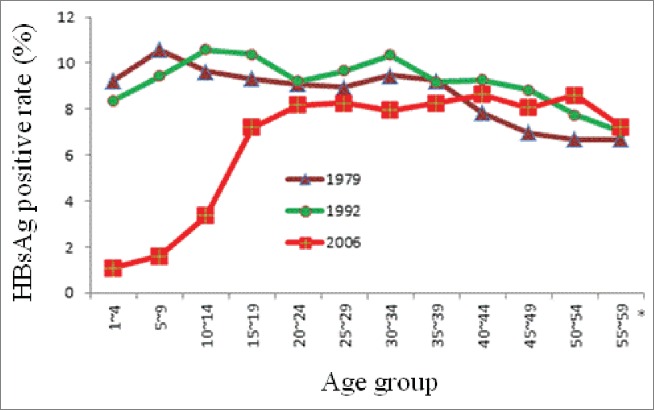
The comparison of HBsAg positive rate of population aged 1–59 years in 1979, 1992 and 2006.
Many studies2-5 have found that hepatitis B vaccination has played an important role in reducing the incidence of hepatitis B among population aged <15 years. For adults, vaccination is still the most economical and effective measure to prevent and control hepatitis B.6 Li J et al.7 also found that inoculation with hepatitis B vaccine among adults achieved good immune. Furthermore, an investigation performed by Lin bin et al.8 found that 1.8% of adults with a history of hepatitis B vaccination were positive for HBsAg, compared with 11.96% of adults without a history of hepatitis B inoculation. This indicates that administration of hepatitis B vaccine is an effective method to control HBV infection in adults. Zhang Wei et al.9 were also of the opinion that immunization with hepatitis B vaccine in adults is important in reducing hepatitis B morbidity and mortality in our country. These views are relevant to national situation. HBV infection rate is still at a higher level among adults, it will take a long time to control hepatitis B if we rely solely on implementing universal newborn vaccination. Hence, it is necessary to implement hepatitis B vaccination in adults. However, only a few countries have expanded immunization against hepatitis B to 18-year-old adults, and most countries, including China, administer routine vaccination in neonates and infants only. As the age distribution of hepatitis B prevalence in China has changed, adult immunization should be paid more attention. However, there is no unified strategy for adult hepatitis B vaccination in China. Current recommendations from the Chinese Center for Disease Control and Prevention for hepatitis B immunization of adults follow the conventional immunization programs available for infants.. Hence, more research is required on hepatitis B immunization strategies for adults to improve vaccination rate and seroconversion to hepatitis B surface antigen antibody (anti-HBs). We still must insist on the implementation of neonatal hepatitis B immunization strategies. When necessary, the adult hepatitis B immunization should be included into Expanded Program on Immunization (EPI). The health economic evaluation of adult vaccination should be performed for more scientific and rational guidance.
This review summarizes the status of adult hepatitis B vaccination in China, and discusses the appropriate dose, regimen and strategy for dealing with immune failure.
Epidemiology of hepatitis B in adults
Since hepatitis B immunization strategy for neonates was launched >20 years ago, the incidence of hepatitis B among younger population has been effectively controlled. However, the incidence of hepatitis B in adults has not changed. From 1990 to 2008, the incidence of hepatitis B in China continued to show an upward trend, with the number of reported cases rising from 0.24 million to 1.17 million. The reported cases of hepatitis B accounted for 83% of viral hepatitis.The reported incidence in the population aged 20–49 years was more than 100/100,000.10 According to the Chinese 1992 National Seroepidemiological Survey of Viral Hepatitis, hepatitis B prevalence among adults aged ≥20 years reached 58%–68%.11 In 2006, the national data showed that the HBsAg carrier rate in the population aged 15–59 years was still as high as 8.75%.12
Several studies observed a common phenomenon: the incidence of hepatitis B was clustered in young adults and farmers in China. In 2013, Henan province13 reported that the incidence of hepatitis B was 88.79/100,000. Most of them were 20–64 years old and farmer was the most common occupation. From 2007 to 2012, the incidence of hepatitis B in Liaoning province14 was 69.74/100,000. Most of those were accounted for people aged 30–59 years and farmers and agricultural workers. During the same period, 59.76% of new patients with virus hepatitis in Huangpu district of Shanghai were 40–59 years old15 Apart from the provincial investigation, cities and counties across the mainland had similar feature.16-24
Based on the above, adults are the population that should be focused on for control of hepatitis B in China. The current EPI with hepatitis B vaccine for neonates and children has been unable to achieve the goal of China becoming an aera of low hepatitis B endemicity. Apart from mother to child transmission, there are still another 2 routes of transmission for HBV, which are blood-borne and sexual transmission. Adults account for a large percentage of the total population, and many encounter several routes of transmission, such as blood, sexual activity, and close human contact. This implies that implementation of neonatal hepatitis B immunization cannot completely control the spread of HBV in the general population. In consequence, China needs to adhere to the neonatal EPI of hepatitis B vaccination, expand the coverage of hepatitis B vaccination, enhance hepatitis B immunization for adults, and improve the immunity barrier among the whole population, in the hope of becoming low-endemic area.
Adult immunization with hepatitis B vaccine
Currently, there is a universal opinion that hepatitis B vaccination in adults could reduce the number of new cases and control the spread of HBV, as an immune barrier. Therefore, vaccinations among adults are advocated. Although there have been many studies of adult immunization in China and other countries, there is no uniform strategy as to dosages, procedures, booster immunization after immune failure.
Seroconversion rates after hepatitis B vaccination
Age is related to the chance of obtaining protective antibodies after hepatitis B vaccination. Though hepatitis B vaccines were administrated with the same doses and procedures, their anti-HBs seroconversion rate and antibody titers among vaccinated children and adults vary significantly. Wen Jinsheng et al.25 found a 95.17% anti-HBs seroconversion rate in newnates after 3 5-μg doses of recombinant hepatitis B vaccine according to a 0-1-6-month schedule. The anti-HBs seroconversion rate in infants whose mothers were HBsAg negative was 97.25%. However, Cui Zhongtai et al.26 found anti-HBs seroconversion rate among students without a history of hepatitis B vaccination was 85.26% after 3 5-μg doses of recombinant hepatitis B vaccine according to a 0-1-6-month schedule. These studies indicated that hepatitis B vaccine could achieve good results among adults, athough the effect was not good as in neonates.
Immunogenicity of different doses and schedules of hepatitis B vaccine
In China, there are 2 kinds of hepatitis B vaccines used mainly for adults, recombinant (yeast) vaccine and recombinant (CHO) vaccine.27 However, the immune effects are not consistent in domestic and foreign reports. Doses of 10 and 20 μg with a 0-1-6-month schedule are recommended more often, and a 0-1-12-month schedule is also recommended for adults. (Table 1).
Table 1.
Overview of immunogenicity results according different doses, schedules and accelerated vaccination among healthy adults.
| Ref. | Immunization schedule | Dose(μg);vaccine | Tested N | Serum collected times | M/F* | Age range (years) | Seropretective rate (%) (≧10IU/L) | P | Anti-HBs geometric mean concentration (IU/L) | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 27 | 0-1-6m | 10; Chinese-1 | 593 | 1m | 0.69 | 32.45 ± 0.66 | 81.67 | >0.05 | 177.28 | >0.05 |
| 10; Chinese-2 | 465 | 0.64 | 33.69 ± 0.70 | 95.05 | 473.23 | |||||
| 10; Chinese-3 | 579 | 0.74 | 31.71 ± 0.69 | 89.64 | 246.13 | |||||
| 10;Engerix | 235 | 0.75 | 32.20 ± 1.07 | 86.81 | 332.20 | |||||
| 49 | 0-1-6m | 5; Chinese-1 | 50 | 1m | 0.94 | / | 75.00 | <0.01 | 197.4 | <0.01 |
| 10; Chinese-1 | 49 | 91.84 | 315.6 | |||||||
| 15; Chinese-1 | 49 | 95.65 | 403.6 | |||||||
| 20; Chinese-1 | 48 | 97.87 | 477.8 | |||||||
| 50 | 0-1-6m | 10; Chinese-3 | 392 | 1m | 1.08 | 18–55 | 90.56 | >0.05 | / | / |
| 20; Chinese-3 | 395 | 94.94 | ||||||||
| 28 | 0-1-6m | 10; Chinese-3 | / | 12m | / | / | 73.2 | <0.01 | 32.94 | <0.01 |
| 20; Chinese-3 | 97.1 | 87.06 | ||||||||
| 30 | 0-1-3m | 10; Chinese-1 | 159 | 1m | / | 20–46 | 88.05 | >0.05 | 91.69 | >0.05 |
| 20; Chinese-1 | 101 | 94.06 | 290.23 | |||||||
| 38 | 0-1-6m | 20;Engerix | 60 | 2m | 1.14 | 30.56 ± 10.47 | 98.33 | / | 468.45 ± 329.11 | / |
| 40;Engerix | 60 | 1.07 | 31.83 ± 11.43 | 85.0 | 427.87 ± 332.10 | |||||
| 10; Chinese-1 | 60 | 0.88 | 31.33 ± 9.76 | 65.0 | 287.97 ± 283.29 | |||||
| 20; Chinese-1 | 60 | 0.94 | 28.95 ± 10.28 | 65.0 | 229.30 ± 304.94 | |||||
| 47 | 0-1-3m | 10; Chinese-2 | 366 | 1m | 0.49 | 16–49 | 98.36 | >0.05 | 1863.60 | >0.05 |
| 0-1-6m | 10; Chinese-2 | 262 | 96.18 | 883.85 | ||||||
| 0-1-3m | 20;Engerix | 140 | 97.86 | 629.59 | ||||||
| 0-1-6m | 20;Engerix | 172 | 95.35 | 993.09 | ||||||
| 48 | 0-1-3m | 10; Chinese-1 | 190 | 1m | 0.62 | 16–49 | 88.95 | >0.05 | 94.95 | <0.05 |
| 0-1-6m | 191 | 90.05 | 145.12 | |||||||
| 32 | 0-1-2m | / | 90 | 6m | 0.8 | 25.91 ± 11.92 | 100 | >0.05 | / | / |
| 0-10-21d | / | 30 | 0.875 | 22.53 ± 9.30 | 100 | |||||
| 33 | 0-2m | /; HBV-ISS | 50 | 1w§: | 0.54 | 18–28 | 100 | / | 1603 | / |
| 0-2-6m | /; Engerix-B | 49 | 18 | 2.4 | ||||||
| 34 | 0-1m | /; HBV-ISS | 1809;2 | 2m | / | 18–55 | 95.1 | / | / | / |
| 0-1-6m | /; HBV-Eng | 606;1 | 1m | 81.1 | ||||||
| 35 | 0-1-3m | 10; Chinese-3 | 249 | 1m | 0.75 | 20–39 | 83.94 | 0.0003 | 61.19 | <0.0001 |
| 0-1-6m | 229 | 88.21 | 214.04 | |||||||
| 0-1-12m | 208 | 94.23 | 345.78 | |||||||
| 10; licensed HB vaccine | / | ≥83.0 | / | ≥110.1 | / | |||||
| 39 | 0-1-6m | 30; licensed HB vaccine | / | 1m§: | 16–60 | ≥87.1 | ≥164.0 | |||
| 60; licensed HB vaccine | ≥92.1 | ≥286.0 |
M/F: male/female ratio;
serum collected from the second dose; others after full vaccination;
m: months, d: days; w: weeks;
Chinese-1: recombinant yeast-derived hepatitis B vaccine produced by the Shenzhen Kangtai Biological Products Co., Ltd., China;
Chinese-2: recombinant yeast-derived hepatitis B vaccine produced by the Dalian High-Tech Biopharmaceutical Co., Ltd., China; Chinese-3: recombinant hepatitis B vaccine (Chinese Hamster Ovary, CHO cells) produced by the North China Pharmaceutical Company, GeneTech Biotechnology Pharmaceutical Co., Ltd.
Engerix: recombinant yeast-derived hepatitis B vaccine produced by the GlaxoSmithKline Company, UK.
HBV-Eng: licensed alum-adjuvanted vaccine.
Hepatitis B vaccine is now recommended for high-risk population worldwide, but the recommended dose varies. Chen Yinzhong et al.28 administrated 2 doses (10 and 20 μg) and 2 kinds of hepatitis B vaccine (CHO vaccine and recombinant yeast vaccine) to adults according to a 0-1-6-month schedule. They observed that there were no significant differences in immunological effects between 10 μg CHO vaccine and recombinant yeast vaccine, but 20 μg recombinant yeast vaccine had better effects than 10 μg vaccine. These results indicated that a high dose of recombinant vaccines has a good immune effect in adults. A meta-analysis done by An Shuyi et al.29 showed that 10 μg recombinant yeast hepatitis B vaccine induced a higher seroconversion rate than 5 μg recombinant yeast hepatitis B vaccine vaccination after 3 full doses according to a 0-1-6-month schedule. Li J et al.30 found that both 10 and 20 μg doses of hepatitis B vaccine had a good immunological effect, as their anti-HBs seroprotection rates (anti-HBs ≥10 mIU/mL indicated seroprotection) after the third dose were 88.05% and 94.06% respectively. Ren JJ et al.27 concluded that domestic hepatitis B vaccine after 3 10-μg doses according to a 0-1-6-month schedule yeilded an average anti-HBs seroconversion rate of 88.29%. All of these results demonstrate that 3 full 10-μg doses of recombinant hepatitis B vaccine according to a 0-1-6-month schedule achieved better protection for adults in China, and could enhance their immunity against HBV infection. Europe and the United States recommended 3 doses of 20 μg hepatitis B recombinant yeast vaccine for adults. Based on our own experience and that of others, we recommended 10 or 20 μg hepatitis B vaccine for adults.
At the present time, 3 doses of hepatitis B vaccine can produce good immunity and are widely used. However, it does not seem feasible in China. Many adults would not be able to finish the full vaccination schedule on time, due to the need to seek work in different places, resulting in negative anti-HBs without protection and susceptibility to HBV infection. To reduce the number of new cases of hepatitis B in adults, more studies on the adult immunization schedule have been conducted across China.
Chen Shizhu.31 believed that protection from hepatitis B vaccine could not be achieved according to the standard schedule (0-1-6 months) among migrants and people who often perform special tasks and require emergency or rapid immunization. In those cases, it is more suitable to use a 0-1-7-21-day or 0-7-14-day schedule, as these yeild higher anti-HBs conversion rates and protect individuals who do not obtain protective antibodies according to the 0-1-6-month schedule. Therefore, the rapid immunization schedule should be best used for high-risk groups and populations who need it. Compared with the standard immunization program, the rapid schedule had no significant differences regarding anti-HBs level at 1 year after full immunization. Saltoglu et al.32 compared the effects of the rapid and standard immunization programs among healthy adolescents and adults. They observed no differences in anti-HBs seroconversion rate between the 2 groups at 6 months after immunization. Furthermore, they found that the serum peak concentration of anti-HBs in the rapid immunization group was lower than in the standard group, suggesting that the protection time of rapid schedule might be shorter than that of the standard one. In consequence, another dose of vaccine at 12 months after rapid immunization program is recommended to improve the durability of antibody protection. However, Halperin SA et al33 observed that a 0-2-month schedule of an investigational hepatitis B vaccine consisting of HBsAg combined with an immunostimulatory phosphorothioate oligodeoxyribonucleotide adjuvant (HBV-ISS) could achieve protective levels more quickly than a 0-2-6-month schedule with Engerix-B. Six years later, they34 found that a 0-1-month and 0-2-month regimen of HBV-ISS induce similar antibody response in healthy young adults. There are no reports of the durability of the response induced by HBV-ISS. Apart from rapid immunization, Yao Jun et al.35 concluded that hepatitis B vaccination with both 0-1-6 and 0-1-12-month schedules in adults result in better level of immune responses.
Immune failure and booster immunization
Although hepatitis B vaccine could induce protective levels of anti-HBs in most healthy adults following a standard vaccination protocol, many studies have demonstrated that 1–10% of healthy ones did not produce protective levels of anti-HBs. What is the possible reason for this unresponsiveness? Soroosh P et al.36 showed that defective HBsAg-specific T-cell repertoire may not be an explanation for humoral unresponsiveness to hepatitis B vaccine. Amirzargar AA et al.37 confirmed in an Iranian population that lack of antibody response to hepatitis B is associated with increased frequencies of HLA DQB10201 allele. Whether the DQB10201 allele is increased in Chinese non-responders needs further investigation.
Although the mechanism is not fully understood, the non-responders should be protected as soon as possible. The questions are how to revaccinate them and what is the best vaccination protocol. Chaoshuang Lin et al.38 revaccinated adults with low or undetectable anti-HBs titers after hepatitis B vaccination using 3 different doses according to a 0-1-6-month schedule, and found that higher dose of hepatitis B vaccine could produce higher seroconversion rate and geometric mean titers for anti-HBs. This implies that for adults without response to hepatitis B immunization, anti-HBs seroconversion rates could be improved through increased dose. A clinical trial conducted by Pan HX et al.39 confirmed that booster vaccination with primary or higher dose of hepatitis B vaccine could provide greater immunogenicity in unresponsive adults. Except higher dose, vaccinated younger adults could produce higher immunogenicity because non-response reaction is known to increase with age. Vermeiren et al.40 demonstrated in a retrospective cohort study that immunosenescence starts at young age, especially among male adults. In addition, vaccination with multiple covalent combined vaccines, such as hepatitis A and B vaccines, produced better immunogenicity than hepatitis B vaccine in adults. They observed that repeatedly vaccinated adults without response to hepatitis B vaccine could achieve a higher anti-HBs seroconversion rate after revaccination with combined hepatitis A and B vaccine. For healthy non-responders, Lin C et al.41 found that co-administration of granulocyte-macrophage colony stimulating factor (GM-CSF) and the standard vaccine dose (20 μg) was effective, and augmentation of the vaccine dose produced better protective levels of anti-HBs than injecting the vaccine in combination with GM-CSF. Zhu JQ et al.42 also identified the important factors affecting the immunoreactions levels of revaccination, which are age, vaccine and dose.
Apart from adults with immune failure, who should be revaccinated; adults with successful vaccination should also receive booster vaccination. Wang Hongwu et al.43 observed that5 years after hepatitis B vaccination in adults, the anti-HBs positive rate was reduced to half. Therefore, booster vaccination against hepatitis B should be recommended among adults at 5 years after primary vaccination. What is the possible etiology? West et al.44 pointed out that healthy adult could have typical manifestations of an immune reaction, that is, substantial immunological memory and antibody growth. Immunological memory is related to memory B lymphocytes, which rapidly produce antibody when they encounter the same antigen for the second time. Adults with previous vaccination show significantly increased anti-HBs at several days after booster vaccination.
Cost-benefit study
In 1991, the World Health Organization called for wider use of hepatitis B vaccine. Hepatitis B vaccination is the most economic measure to prevent HBV infection. According to Professor Duan Zhongping, Deputy Director of the Chinese Foundation for Hepatitis Prevention and Control, who spoke at annual academic meeting of the China Association for Science and Technology in 2005, treatment takes ∼338 times more than prevention. Judging from the prevalence of hepatitis B around the world, China is a high-endemic areas at present, as the HBsAg carrier rate is still >8%. As adults are the major creators of social wealth, control of hepatitis Bin this group is important. However, the main recipients of hepatitis B vaccine are newborns, preschool children and high-risk groups in China. Therefore, to allow China to become a low-endemic country, we should extend the ranges of high-risk populations, and healthy adults also need to be protected. In the past, underinsurance restrained vaccination among children. To date, the same situation has occurred in adults. Lu et al.45 found that underinsurance is a barrier to vaccination among adults aged ≧18 years. Hence expanding health insurance coverage and implementing other effective strategies are needed to help improve hepatitis B vaccination among adults.
Conclusion
It is reported that the active hepatitis B vaccination among adults is low, with a rate of only 13.78% in China.46 Given the feasibility of adult hepatitis B immunization and the economic and social benefits that could be achieved, we should adhere to the strategy of neonatal hepatitis B immune, actively implement the EPI, and explore suitable hepatitis B immunization strategies for healthy adults. As there is no unified hepatitis B vaccination strategies for adults in China, we recommend 10 or 20 μg with a 0-1-6-month or 0-1-12-month schedule for migrant adults. For immunity failure, increasing the dose or covalent vaccine is suggested to provide protective antibodies. To enhance immunity among adults, hepatitis B vaccine should be included in health insurance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Project supported by the National Scientific and Technological Major Project of China (No. 2011ZX10004-901,2013ZX10004-904, No. 2014ZX10004008).
References
- [1].Bureau of disease prevention and control of Ministry of health, Chinese Center for disease control and prevention Sero-epidemiological survey of hepatitis B among national population. Beijing: People's Medical Press; 2011 [Google Scholar]
- [2].Cheng Li, Shu-qin Duan, Shaohong Yan. Epidemiology of hepatitis B in Inner Mongolia autonomous region, 1991–2010. Disease Surveillance 2012; 27(1):20-24. [Google Scholar]
- [3].Jingmin Zhang. Hepatitis B in Leshan, 2007–2011. J Preventive Med Information 2013; 29(1):43-6. [Google Scholar]
- [4].Hua Ke, Ling Chai. Epidemiological analysis on viral hepatitis B in Yunxian County of Hubei from 2004–2011. Occupation Health 2013; 29(6):742-3. [Google Scholar]
- [5].Zhou B, Cao DQ, Zhang YH, et al.. Epide-miological characteristics of viral hepatitis in Mentou district of Beijing. Capital J Public Health 2011; 5:14-7. [Google Scholar]
- [6].Jiaye Liu, Bingyu Yan, Zhang Li, et al.. Comparison on the Antibody Response and Influenced Factors of Hepatitis B Vacine Made by Recombinant Dexyribonucleic Acid Techniques among Adults. Chinese J Vaccines Immunization 2013; 19(2):142-6. [Google Scholar]
- [7].Li J, Yao J, Shan H, Chen Y, Jiang ZG, Ren JJ, Xu KJ, Ruan B, Yang SG, Wang B, et al.. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother 2015; 11(5):1108-13.; PMID:25607773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bin Lin, Kefen Zeng, Liang Xue, et al.. Survey on Adult HBV Infection Rate and Influence Factor in Karamay City. Practical Preventive Medicine 2006; 13(5): 1195-7. [Google Scholar]
- [9].Zhang W, Lin CY, Han Lili, Li LQ, Gao P, Lin H, Gong XH, Huang F, Tang YQ, Ma JX, et al.. Study on the immuno-effects and influencing factors of Chinese Hamster Ovary (CHO) cell hepatitis B vaccine among adults, under different dosages. Chinese J Epidemiol 2010; 31(7):767-70.; PMID:21162840 [PubMed] [Google Scholar]
- [10].Li Li, Xiaofeng Liang. The present status and countermeasures of controlling Chinese hepatitis A and B. Disease Surveillance 2009; 24(5):307-12. [Google Scholar]
- [11].Zhicheng Dai, Guoming Qi. Sero-epidemiological survey of viral hepatitis in China (1992–1995) (Vol. I). Beijing: Science Press; 1997 [Google Scholar]
- [12].Bureau of disease prevention and control , National Health and Family Commission of People's Republic of China. http://www.nhfpc.gov.cn/jkj/s3582/201307/518216575e544109b2caca07fca3b430.shtml. Published July 26, 2013 [Google Scholar]
- [13].Yanyang Zhang, Jun Li, Ying Ye, et al.. Epidemic analysis of hepatitis B in Henan province in 2013. Chronic Pathematol J 2014; 15(5): 352-6. [Google Scholar]
- [14].Shu Zhang, Jing Sun. Epidemiology of hepatitis B in Liaoning province. Chinese J Public Health Management 2014; 30(3):392-3. [Google Scholar]
- [15].Fujie Shen, Min Shu, Shu Wang, et al.. Analysis on epidemiological characteristics of acute viral hepatitis in Huangpu District of Shanghai from 2007–2012. Occupation Health 2014; 30(14):1925-7. [Google Scholar]
- [16].Xuejian Liu, Xin Liu, Peilin Luo, et al.. Analysis of the Epidemiological Characteristics of Hepatitis B in Xuyi from 2009 to 2013. Chinese Primary Health Care 2014; 28(7):114-5. [Google Scholar]
- [17].Jianfei Gao, Hongxing Zhang, Aihong Zhang. Analysis of the Epidemiological Characteristics of Hepatitis B in Rudong from 2005 to 2011. Shanghai J Preventive Med 2014; 24(7):373-4. [Google Scholar]
- [18].Shan Qi, Yi Zhi, Xingya Lin, et al.. Analysis of the Epidemiological Characteristics of Hepatitis B in XuZhou from 2007–2010. Jiangsu J Preventive Medicine 2012; 23(1):43-4. [Google Scholar]
- [19].Haihong Feng. Analysis of the Epidemiological Characteristics of Hepatitis B in Guangyun from 2005–2012. Jiangsu Health Care 2013; 15(4):11-2. [Google Scholar]
- [20].Huanzhang Yuan, Xie Liu, Jinjun Wen. Analysis of the Epidemiological Characteristics of Hepatitis B in Dongwan from 2007–2011. South China J Preventive Medicine 2013; 39(5):44-6. [Google Scholar]
- [21].Siyu Wang. Analysis of the Epidemiological Characteristics of Hepatitis B in Dongliao county from 2008–2012. China Practical Medicine 2013; 8(26):258 [Google Scholar]
- [22].Qimin Huang. Epidemiological analysis on viral hepatitis B in Nanjing City from 2008–2012. Occupation Health 2014; 30(7):940-1. [Google Scholar]
- [23].Liusha Xue. Analysis on epidemiological characteristics of virus hepatitis in Mengshan County of Guangxi from 2006–2011. Occupation Health 2013; 29(14):1782-4. [Google Scholar]
- [24].Jian Zhou, Yanyan Jiang, Lin Sun. Analysis on the epidemiological trend of hepatitis B from 1990 to 2011 in Weifang city. Progress Microbiol Immunol 2013; 41(6):51-4. [Google Scholar]
- [25].Jinsheng Wen, Qining Cao. The Safety and efficacy after immunization with recombinant hepatitis B vaccine in infants. Jiangxi Medical J 2009; 44 (6):608-9. [Google Scholar]
- [26].Zhongtai Cui, Shuxia Lin, yan Yan. An Analysis of the Effect of Domestic Recombinant Yeast Heptatitis B Vaccine of Different Doses. Health Med Res Practice 2011; 8 (1):46-4749. [Google Scholar]
- [27].Ren JJ, Dai XW, Jiang ZG, Shen LZ, Chen YD, Li Q, Ren W, Liu Y, Yao J, Li LJ. Immunological effects of a 10-μg dose of domestic hepatitis B vaccine in adults. J Zhejiang Univ Sci B 2012; 13(11):948-54.; PMID:23125088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yinzhong Chen, Renjie Jiang, Jinjin Shen, et al.. Observation on the Anti-HBs of Different Genetic Engineering Hepatitis B Vaccine in Adults. Chinese J Vaccines Immunization 2007; 13 (4): 320-4. [Google Scholar]
- [29].Shuyi An, Hui Jia, Yue F, et al.. Meta-analysis on immunological effect of recombinant yeast hepatitis B vaccine with different doses. Chinese J Health Statistics 2009; 26 (4):398-9. [Google Scholar]
- [30].Li J, Yao J, Shan H, Chen Y, Jiang ZG, Ren JJ, Xu KJ, Ruan B, Yang SG, Wang B, et al.. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother 2015; 11(5):1108-13.; PMID:25607773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shizhu Chen. The present status and problems of hepatitis B vaccine immune. World Chinese J Digestol 2006; 14 (27):2661-7. [Google Scholar]
- [32].Saltoglu N, Inal AS, Tasova Y, Kandemir O. Comparison of the accelerated and classic vaccination schedules against hepatitis B: three-week hepatitis B vaccination schedule provides immediate and protective immunity. Ann Clin Microbiol Antimicrob 2003; 2:1201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 2006; 24(1):20-6.; PMID:16198027 [DOI] [PubMed] [Google Scholar]
- [34].Halperin SA, McNeil S, Langley JM, Smith B, MacKinnon-Cameron D, McCall-Sani R, Heyward WL, Martin JT. Safety and immunogenicity of different two-dose regimens of an investigational hepatitis B vaccine (hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide) in healthy young adults. Vaccine 2012; 30(36):5445-8.; PMID:22704926; http://dx.doi.org/ 10.1016/j.vaccine.2012.05.074 [DOI] [PubMed] [Google Scholar]
- [35].Yao J, Li J, Chen Y, Shan H, Dai XW, Yang LN, Jiang ZG, Ren JJ, Xu KJ, Ruan B, et al.. The response of hepatitis B vaccination on seronegative adults with different vaccination schedules. Hum Vaccin Immunother 2015; 11(5):1102-7.; PMID:25621975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Soroosh P, Shokri F, Azizi M, Jeddi-Tehrani M. Analysis of T-cell receptor β chain variable gene segment usage in healthy adult responders and nonresponders to recombinant hepatitis B vaccine. Scand J Immunol 2003; 57(5):423-31.; PMID:12753498; http://dx.doi.org/ 10.1046/j.1365-3083.2003.01256.x [DOI] [PubMed] [Google Scholar]
- [37].Amirzargar AA, Mohseni N, Shokrgozar MA, Arjang Z, Ahmadi N, Yousefi Behzadi M, Amanzadeh A, Shokri F. HLA-DRB1, DQA1 and DQB1 alleles and haplotypes frequencies in Iranian healthy adult responders and non-responders to recombinant hepatitis B vaccine. Iran J Immunol 2008; 5(2):92-9.; PMID:18523354 [PubMed] [Google Scholar]
- [38].Chaoshuang Lin, Shibin Xie, Jing Liu, et al.. Effect of revaccination using different schemes among adults with low or undetectable anti-HBs titers after hepatitis B virus vaccination. Clin Vaccine Immunol 2010; 17(10):1548-51.; http://dx.doi.org/ 10.1128/CVI.00064-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pan HX, Zeng Y, Song XF, Zhang YJ, Xu K, Liang ZL, Zhu FC. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine 2014; 32(29):3706-12.; PMID:24681228; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.094 [DOI] [PubMed] [Google Scholar]
- [40].Vermeiren AP, Hoebe CJ, Dukers-Muijrers NH. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J Clin Virol 2013; 58(1):262-4.; PMID:23895931; http://dx.doi.org/ 10.1016/j.jcv.2013.07.003 [DOI] [PubMed] [Google Scholar]
- [41].Lin C, Zhu J, Zheng Y, Chen Y, Wu Z, Chong Y, Gao Z. Effect of GM-CSF in combination with hepatitis B vaccine on revacination of healthy adult non-responders. J Infect 2010; 60(4):264-70..; PMID:20138189; http://dx.doi.org/ 10.1016/j.jinf.2010.01.011 [DOI] [PubMed] [Google Scholar]
- [42].Zhu JQ, Huang ZY, Mao DB. Immunoreaction levels of revaccination in healthy population with hepatitis B vaccine. Zhongguo Yi Miao He Mian Yi 2009; 15(2):152-8.; PMID:20077662 [PubMed] [Google Scholar]
- [43].Hongwu Wang, Benxiao Luan, Lu Jia. The immune effect of hepatitis B vaccination in adults of: 9 year observation. J Preventive Medicine Anhui 2004; 10 (2): 84-85. [Google Scholar]
- [44].West DJ. Vaccine-induced HBsAg immune memories: its relationship with booster immunization strategy. Foreign Medicine Section Biol Products Prophylaxis Diagnosis Therapy 1997; 20(3): 84-85. [Google Scholar]
- [45].Lu PJ, O'Halloran A, Williams WW. Impact of Health Insurance Status on Vaccination Coverage among Adult Populations. Am J Prev Med 2015; 48(6):647-61.; PMID:25890684; http://dx.doi.org/ 10.1016/j.amepre.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xiaoqiu Qi, Wang Yu, Jingjin Yu, et al.. National population hepatitis B Sero-epidemiological survey report. Beijing: People's Medical Press; 2011. [Google Scholar]
- [47].Xu F, He F, Zhou BQ, Zhao QY, Yan R. Observation on immunization effect of hepatitis B vaccine in adults by different dosages and schedules. Disease Surveillance 2013; 28(1):38-41. [Google Scholar]
- [48].Chen SY, Wang XC, Dong XL, Xu HT, Wang FD, Tang ZF, Wang XL, Fan JL. Effects of two immunization schedules of recombinant yeast-derived hepatitis B vaccines for adults. Chin Prev Med 2013; 14(2):96-8. [Google Scholar]
- [49].Yuan YB, Wang ZQ, Ma XP, Zhang GH. Comparison of the immunogenicity of different dosages of recombinant yeast-derived hepatitis B vaccines in adults. J Prev Med Chin PLA 2003; 21(3):176-9. [Google Scholar]
- [50].Qi Y, Yang J, Ge GH. Different doses of recombinant hepatitis B vaccine on the immune effects of adult. Guide China Med 2011; 9(23):25-6. [Google Scholar]


