ABSTRACT
Presently the dose of rabies immunoglobulin (RIG) which is an integral part of rabies post exposure prophylaxis (PEP) is calculated based on body weight though the recommendation is to infiltrate the wound(s). This practice demands large quantities of RIG which may be unaffordable to many patients. In this background, we conducted this study to know if the quantity and cost of RIG can be reduced by restricting passive immunization to local infiltration alone and avoiding systemic intramuscular administration based on the available scientific evidence. Two hundred and sixty nine category III patients bitten by suspect or confirmed rabid dogs/animals were infiltrated with equine rabies immunoglobulin (ERIGs) in and around the wound. The quantity of ERIG used was proportionate to the size and number of wounds irrespective of their body weight. They were followed with a regular course of rabies vaccination by intra-dermal route. As against 363 vials of RIGs required for all these cases as per current recommendation based on body weight, they required only 42 vials of 5ml RIG. Minimum dose of RIGs given was 0.25 ml and maximum dose given was 8 ml. On an average 1.26 ml of RIGs was required per patient that costs Rs. 150 ($3). All the patients were followed for 9 months and they were healthy and normal at the end of observation period. With local infiltration, that required small quantities of RIG, the RIGs could be made available to all patients in times of short supply in the market. A total of 30 (11%) serum samples of patients were tested for rabies virus neutralizing antibodies by the rapid fluorescent focus inhibition test (RFFIT) and all showed antibody titers >0.5 IU/mL by day 14. In no case the dose was higher than that required based on body weight and no immunosuppression resulted. To conclude, this pilot study shows that local infiltration of RIG need to be considered in times of non-availability in the market or unaffordability by poor patients. This preliminary study needs to be done on larger scale in other centers with long term follow up to substantiate the results of our study.
Keywords: animal bites, passive immunization, rabies, rabies immunoglobulin
Introduction
Rabies is a fatal disease and an estimated 55,000 people die of rabies every year in the world1 and 20,000 deaths are reported from India alone.2 The main reason for high death rate in India is high cost of treatment and lack of awareness regarding first aid, vaccination and failure to use rabies immunoglobulins (RIGs) in animal bite cases. Vaccination induces >0.5 IU/mL levels of rabies virus neutralizing antibody (RVNA) only after 10-14 days.3 This window period may be crucial in cases with short incubation period. Use of RIGs is particularly required in all category III exposures where virus load may be more and incubation period short. Rabies immunoglobulins are not affordable because of the cost factor. (A cost of around Rs 1500 ($20) for equine RIGs to 30,000 Rupees ($500) for human RIGs for an average patient. Further there is shortage of RIGs in India mainly due to limited production. Presently the dose of RIG is calculated based on body weight of the patient, though the recommendation is to infiltrate locally in order to neutralize the virus at wound site as early as possible. If the dose of RIG is calculated based on body weight, most often the dose exceeds the quantity required for local infiltration, and this excess amount is injected intramuscularly in the gluteal region. It has been shown previously that systemic administration of RIG will not produce >0.5 IU/mL levels of RVNA in blood and hence may not be useful in neutralizing the virus at local wound site.4 On the other hand, these products have proved their efficiency when administered in the site of virus entry (Wound) in association with rabies vaccine. Dean and Baer had shown in a classic study in 1963 that intramuscular injection of Anti Rabies serum (ARS) will not provide a titer of >0.5 IU/mL at systemic level and that local injection of rabies virus-contaminated wounds is essential for survival in cases of severe exposure.5 Madhusudana et al, (2013) reiterate there is no basis for calculating the dose of immunoglobulin based on body weight.6 There is a case report of rabies occurring in a child where a single puncture wound on the finger was not infiltrated with RIGs but the entire calculated dose had been given intramuscularly in the gluteal region.7 As there was acute and severe shortage of RIGs in the study area coupled with un-affordability of majority of category III exposure patients, this study was conducted to know the efficacy of local infiltration of RIG alone depending on the wound size and numbers, without calculating the dose required based on body weight and thus avoiding systemic intramuscular (IM) administration.
Results
A total of 269 category III patients were included in the study for local infiltration of RIGs into the wound without any systemic administration. The details of patients, biting animals, average number of wounds, their location and average quantity of ERIG required for local infiltration in comparison to actual quantity required based on body weight is given in Tables 2 and 3. It is evident from the table that adult males and children were mainly involved and most of these had peripheral bites except in few cases where the bites were on face. While patient DDU/14 had 2cm long 3 scratches by wild monkey on the forehead DDU/69 had deep lacerated (4 × 4 × 4 cm) wound below knee joint, both had antibody titres 7.8 and 30 IU/Ml respectably. In an attack by wild pig in a rural village a lady (DDU/133) was mauled and had badly fractured leg with multiple lacerated wound all over the body and was brought on stretcher from the IG Medical College Shimla for RIG, she was given 8 ml of RIG diluted with 20 ml of normal saline and all the wounds were infiltrated sufficiently to cover whole of the surface area of the wounds. Serum samples tested on day 14 had titers 8.5 IU/ml. Another lady, DDU/202, had a scratch on her Rt. breast by a stray dog kept pet and survived with local 0.25 ml of local RIGs. The blood sample could not be tested. Some of the patients who were bitten by suspected rabid dogs like DDU/6 and DDU/321, where dogs were killed by villagers, the patients survived and the serum titers done for DDU/6 were 6.5 IU/ml. Interestingly 40% of the bites were inflicted by monkeys and this geographical region in India is known for dense population of monkeys. Rabies in monkeys has been documented by the authors by sending a freshly dead monkey’s brain to Central Research Institute (CRI), Kasauli that was found to be positive for rabies virus
Table 2.
Demographic data of the patients included in the study (n = 269) in relation to amount of ERIG administered locally and percentage reduction in dose of ERIG.
| Parameter | Number of patients | Average quantity of ERIG consumed | % reduction in dose of ERIG |
|---|---|---|---|
| Age of the patient | |||
| <5 yrs | 25 | 1.5 ML | 80% |
| 5-10 yrs | 40 | 2.0 mL | 60% |
| 10-15 yrs | 30 | 3.5 mL | 75% |
| >15 yrs | 174 | 3.5 mL | 80% |
| Biting Animal | |||
| Dogs | 140 | 2.5 mL | 80% |
| Monkeys | 116 | 2.0 mL | 75% |
| Cats | 8 | 2.5 mL | 80% |
| Mongoose | 2 | 2.0 mL | 80% |
| Pigs | 3 | 2.5 mL | 80% |
| Bite on face, head and neck with severe risk | 25 | 3.5 mL | 70% |
| Bites on upper limbs | 35 | 2.5 mL | 70% |
| Bites on lower limbs | 55 | 2.0 Ml | 60% |
| Bites on chest | 15 | 2.0 ML | 70% |
| Bites on abdomen | 10 | 1.5 mL | 80% |
Table 3.
Location of wound site and associated risk among those exposed to suspected rabid animal bites. The results clearly show that there was no immunosuppression with local RIGs and we did not exceed the total RIGs volume as prescribed by WHO.
| Pt. ID | Delay in seeking treatment | Wound site | Animal | RIGs given locally in the wound | Day of blood sample | RFFIT titers (IU/ml) | Animal follow-up | Pt follow up at 9 months or more |
|---|---|---|---|---|---|---|---|---|
| DDU 3 | 1 DAY | Rt. Calf | Dog | 4 ml | D 15 | 7.5 | Healthy | Healthy |
| DDU 6 | 1 Day | Leg | Suspected Rabid Dog | 2 ml | D 8 | 6.5 | Rabid Killed by People | Still Healthy after 1 year |
| DDU 7 | 0 | Arm | Monkey bite | 1 ml | D 12 | 5.6 | Could not be followed | Healthy |
| DDU 8 | 2 Days | Arm | Dog | 0.25 ml | D 14 | 7.5 | Healthy | Healthy |
| DDU 14 | 3 Days | Rt. Elbow | Monkey | 2 ml | D 12 | 7.8 | Could not be followed | Healthy |
| DDU 27 | 3 Days | Fingers Dot wound | Monkey | 0.25 ml | D 14 | 14.5 | Could not be followed | Healthy |
| DDU 28 | 1 Day | Lt. Gluteus | Stray Dog | 0.5 ml | D 14 | 6.9 | Could not be followed | Healthy |
| DDU 30 | 3 Days | Hands | Stray Dog | 0.5 ml | D 14 | 7.9 | Could not be followed | Healthy |
| DDU 46 | 1 Day | Dot wound on Rt Calf | Stray pup | 0.25 ml | D 14 | 10.5 | Could not be followed | Healthy |
| DDU 50 | 0 | Scratch in the back | Monkey | 0.5 ml | D 14 | 4.5 | Could not be followed | Healthy |
| DDU 54 | 3 Days | Dot wound fingers | Stray Dog | 1 ml | D 14 | 7.5 | Could not be followed | Healthy |
| DDU 69 | 0 | Below Rt knee | Stray Dog | 2 ml | D 14 | 30 | Could not be followed | Healthy |
| DDU 75 | 2 Days | Below Rt knee | Stray Dog | 1 ml | D 14 | 14.5 | Could not be followed | Healthy |
| DDU 77 | 3 days | Lt leg | Dog | 0.5 ml | D 14 | 15.6 | Healthy | Healthy |
| DDU 78 | 0 | Lt. gluteus | Stray Dog | 1 ml | D 14 | 7.5 | Could not be followed | Healthy |
| DDU 79 | 1 Day | Rt. Knee | Dog | 1 ml | D 14 | 15.6 | Healthy | Healthy |
| DDU 81 | 2 Days | Hands | Monkey | 0.5 ml | D 14 | 7.5 | Could not be followed | Healthy |
| DDU 119 | 0 | Rt Foot | Dog | 0.25 ml | D 14 | 7.5 | Healthy | Healthy |
| DDU 133 | 0 | Lt. Thigh and Lt. Arm 6x3x3 | Wild Pig | 8 ml diluted in 20 ml as pt. wt was 60 kg | D 10 | 8.5 | Could not be followed | Healthy |
| DDU 152 | 1 Day | Hand | Stray Dog | 0.25 ml | D 14 | 13.5 | Could not be followed | Healthy |
| DDU 154 | 2 Days | Rt thigh | dog | 0.25 ml | D 14 | 4.5 | Healthy | Healthy |
| DDU 160 | 2 Days | Rt Arm | Monkey | 3 ml | D 14 | 14.5 | Could not be followed | Healthy |
| DDU 161 | 2 Days | Lt Arm | Monkey | 0.25 ml | D 14 | 7.5 | Could not be followed | Healthy |
| DDU 172 | 2 Days | Rt. Arm | dog | 0.5 ml | D 14 | 12.8 | Healthy | Healthy |
| DDU 180 | 0 | On back | Stray Dog | 0.5 ml | D 14 | 12.5 | Could not be followed | Healthy |
| DDU 211 | 0 | Lt. leg | Monkey | 0.1 ml | D 14 | 13.4 | Could not be followed | Healthy |
| DDU 248 | 2 Days | Lt. Hand | Dog | 0.3 ml | D 14 | 12.5 | Healthy | Healthy |
| DDU 319 | 0 | Leg | Stray Dog | 0.25 ml | D 14 | 12.5 | Could not be followed | Healthy |
| DDU 384 | 73 Days | Rt. Hand Dorsum | Rabid pup of 2 months old | 2 ml given in the scar due to pup bite | D 8 | 12.5 | Pup rabid and died soon after | Healthy |
| DDU 324 | 0 | Leg | Monkey | 7 ml | D 14 | 14.5 | Could not be followed | Healthy |
As against 363 vials of RIGs required for all these cases as per WHO recommendation based on body weight the study could be done with only 42 vials thus saving significant quantities of ERIG and cost. Minimum dose of RIGs given was 0.25 ml with insulin syringe and maximum dose given was 8 ml. On an average 1.26 ml of RIGs was required per patient that costs Rs. 150 ($3). Till date no death has been reported. All suspected rabid dog bite victims have been followed up for more than 9 months now and overall follow-up rate is 82% and all were alive. With local infiltration of RIG the RIG could be made available to all patients in times of non-availability in the market.
Till May, 2015 in one year of local infiltration of RIG study, we have managed more than 2000 patients with local RIGs alone and out of that 64 were bitten by suspected rabid animals (Fig. 1) and 7 patients were bitten by Lab Confirmed Rabid dog (FAT test from CRI Kasauli Lab). Maximum incubation period for rabies documented in our state of Himachal Pradesh is 102 Days 8 and all the 7 patients bitten by confirmed rabid dog were followed for more than 102 days and were alive with no deaths reported till date. We are continuing this mode of treatment as ERIGs are still not available in the market.
Figure 1.
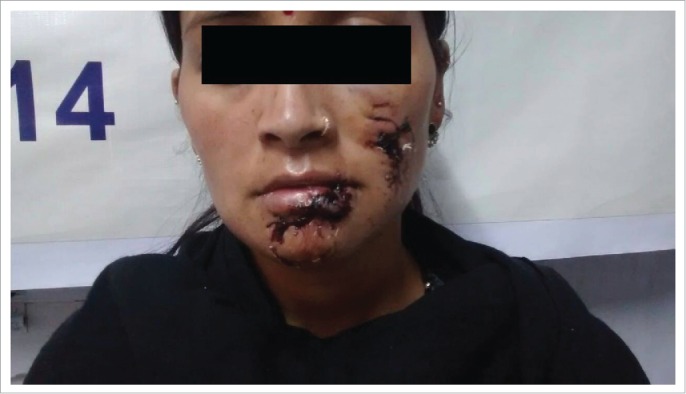
The woman and her daughter were bitten by a clinically confirmed rabid stray dog on face in Kullu district in April 2015. Both were given Local RIGs with IDRV and survived. But a similar patient from another area Rampur (2014), who was bitten on lower lip by a suspected rabid stray dog and given IM rabies vaccine without local RIGs, died of Rabies within 2 weeks of the bite. This demonstrates the life saving value of local RIGs with IDRV.
Adverse events
As we did not administer ERIG by systemic route, we did not encounter any major adverse event apart from local pain in 60% of cases and some redness around the wound(s) in 40% of cases. These adverse events subsided without any medication.
Discussion
Rabies is still considered 100% fatal despite reports of occasional survivors from time to time.9 The virus is highly neurotropic and resides in peripheral and central nervous system throughout its course of incubation period and clinical illness. There is no viremia any time during the infection and there is hardly any systemic immune response unless the patient is vaccinated. One way of preventing this dreaded disease is to neutralize the virus locally in the wound using rabies immunoglobulins. The utility of passive immunization in prevention of rabies was first demonstrated by Babes in 1940 and subsequent studies by Habel and Koprowsky firmly established the value of passive immunization in prevention of rabies.10,11
The administration of passive immunization was first advocated by the WHO in 1973.12 At that time only un-purified antirabies serum (ARS) of equine origin was available and because it was a heterologous protein the dose was calculated depending on the weight of the patient giving due consideration to body surface and biological half-life of the protein. As evidence mounted that systemically administered immunoglobulins may not reach adequate concentrations in the blood and body fluids, WHO in 1982 advocated that half the calculated dose of immunoglobulin to be infiltrated locally in to and around the wound and rest to be administered by intramuscular route13 In 1992, WHO revised its recommendation and advocated that as much as possible of RIG should be infiltrated locally and rest, if any, should be administered systemically.14 This recommendation has been retained in the latest expert report.15 Further, it was recommended that considering the unusual and prolonged incubation period of the disease, local wound infiltration should be considered even in cases who report weeks or months after the exposure even though the local wound had healed. This clearly indicates that local infiltration of RIG is crucial for effective neutralization of the virus to prevent its access to CNS.
It is difficult to establish the exact amount of RIG that is required to neutralize the virus at the local site. However, in a previous study conducted in experimental mice, it has been clearly shown that there is no basis for calculating the dose based on body weight.6 In this study there was 100% survival of peripherally challenged mice, which were infiltrated with 1/100 amount of immunoglobulin required calculated based on body weight. In these experiments mice had been challenged with 104 LD 50 of CVS. Even doses of ERIG as low as 0.01 IU could neutralize this amount of virus and prevent rabies where as based on body weight the amount of RIG to be used was 10 IU. This study clearly indicates that there is no basis for calculating dosage of RIG based on body weight. Further, these animals were not administered post-exposure vaccination but still there was 100% survival with local infiltration of RIGs alone emphasizing the importance of local infiltration of RIG in prevention of rabies. It is evident from Table 2 that though average consumption of ERIG calculated as per body weight would range from 7-10 Ml; we have used an average of 1.26 ml of RIG for local infiltration thus reducing the cost as much as 88%. Thus there is a significant reduction in usage of RIG eventually making the treatment affordable and simultaneously allowing usage of RIG in larger number of category III exposures. This affordability has enabled hospital administration to purchase ERIG to all patients for free for the first time.
The usage of RIG in developing countries particularly India is very limited for several reasons. Some reasons for this include perceived risk of anaphylaxis with ERIG, pain and practical difficulties associated with local infiltration particularly if there are large and multiple wounds, restricted supply of ERIG and the cost involved. This study was conducted keeping in view the severe shortage of ERIG in many parts of the country including our own province , un affordability of large quantities of ERIG in category III exposures after bites from suspect or confirmed rabid animals and a need to do some intervention in the interest of exposed people to save their lives. The wounds were thoroughly infiltrated with required volumes of ERIG which varied depending on the severity of exposure. In all, we administered 0.25 to 8 ml RIG locally in to around the wound. This was followed by complete course of rabies vaccination as per the up-dated TRC regimen. As detailed above, 8ml RIG dose diluted with 20 ml normal saline was given to the lady who was mauled by a wild pig so as to not to exceed the dose as prescribed by WHO as this may cause immunosupression.16
We estimated the levels of RVNA titers in 30 category III exposure patients that were bitten by animals with strong suspicion of rabies. All subjects had adequate amounts of RVNA in their blood that ranged from 4.5 IU/ml- 30 IU/ml on day 14 following vaccination (Table 3).
We have followed these patients now for over 9 months. All patients are alive and healthy. Considering the fact that the average incubation period in humans bitten by confirmed rabid dogs is generally 1-3 months, we can surmise that these patients are safe and unlikely to develop rabies encephalitis in future. The practice of giving local RIGs continues in our clinic at DDU Hospital Shimla as RIGs are still not available in the market.
To conclude, in this prospective study an attempt has been made to know the feasibility of reducing the dose of RIG in category III exposures by avoiding the calculation of dosage of RIG based on body weight and restricting administration of RIGs to local infiltration alone. The observations from our study are encouraging and this should encourage more studies to follow in other parts of developing countries where rabies due to dog bites are still a major economic and social burden and ERIG are either not available or not affordable by the patients.
Materials and methods
Subjects and post-exposure management
A total number of 269 patients who had category III exposures to suspect rabid animal bites who came to our center at DDU Hospital Shimla were included. The inclusion and exclusion criteria for patients is given in Table 1. These patients expressed their inability to purchase the full calculated dose of immunoglobulins but agreed to purchase required quantity of equine rabies immunoglobulin (ERIG) to infiltrate the wound. A thorough wound wash and antiseptic treatment with Betadine was done and wound was allowed to dry before RIGs administration. All patients were administered with the same batch of ERIG/ ARS. Anti-Rabies serum (ARS) from CRI Kasauli (Batch RS 1402 L-7) having a titer of 550 IU/ml was used throughout the study period. Also the patients were given same batch of purified chick embryo cell rabies vaccine (1ml), Vaxirab-N (Pitman-Moore strain, batch CM-132 having a potency of 7.18 IU/ 1ml vial). The schedule of vaccination followed was modified Thai Red Cross (TRC) Schedule of intradermal antirabies vaccination (IDRV), 2-2-2-0-2. Ethical clearance was taken from the Institutional Ethics Committee of Jaypee University of Information Technology in a meeting held on 23rd May, 2014 and was assigned the code as IEC/ Project no. 11-2014. The patient’s written consent was taken. Generally the patients reported to us 24 to 72 hours after the bite except in one case where reporting time was 2 months after the bite by a clinically confirmed rabid pup and the 2 ml ERIGs was given to 8 yrs old girl in the scar caused by the pup. The serum antibody titers were 12.5 IU/ml.
Table 1.
Inclusion and Exclusion criteria for the subjects recruited in the study.
| Inclusion Criteria | Exclusion criteria |
|---|---|
| All category III exposures | Immunompromised |
| Seeking PEP with in 72 hours after bite ( with one exception) | Severe malnourishment |
| Willing to give consent | Any associated disease |
| Available for follow up | Follow up not possible |
| Willing to provide blood sample if required |
The wounds of the patients were carefully examined and enough quantities of ERIG infiltrated in and around the wound without dilution. While administering ERIG following parameters were considered. The quantity of ERIG was calculated roughly based on the size of the wound(s). We did not consider anatomical location of the wound and wounds on face, head and neck were also injected with quantities sufficient to infiltrate the area until overflowing has occurred. The minimum quantity required was 0.25 ml and maximum required was 8 mL depending on the number and size of the wounds. We did not perform prior skin test as per the latest recommendation of WHO but all the facilities required to treat anaphylaxis (if required) were in place. The quantity of RIG administered was just sufficient to infiltrate wound(s) and there was no correlation with body weight. Irrespective of body weight, the patients were administered sufficient RIG to infiltrate single or multiple wounds. 20 of the severely bitten patients had serology done and in no case the dose was higher than that required based on body weight and no immunosuppression resulted (Table 3).
During the study period, 6 stray and wild animals that were reported dead in the surrounding Shimla Municipal Area, were sent for confirmation of rabies to Central Research Institute, Kasauli. All were positive for rabies by fluorescent antibody technique (FAT),17,18 thus confirming the endemicity of rabies in the region and value of local infiltration of ERIGs. The patients were followed both by house to house visit wherever possible and other patients were followed by regular telephonic contact. As most of the biting animals were stray and not traceable, we could not follow these animals.
Estimation of rabies virus neutralizing antibody titers (RVNA)
This was done at the department of Neurovirology at NIMHANS, a WHO collaborating center for reference and research on rabies.The blood samples were collected from patients with severe category III exposures due to bite from unprovoked dogs who had also bitten 3-4 people simultaneously. The procedure used was a microneutralization test as described in our earlier works.19 Briefly increasing dilutions of patient’s serum in 96 well tissue culture plate were mixed with 100FFD 50 of challenge virus standard (CVS), incubated for 1 hour in humid conditions. BHK 21 cells (ATCC CCL 10) were trypsinized, resuspended and 100 microliters of cell suspension was added to each well. The plate was incubated overnight at 37C in CO2 incubator after which cell monolayer was fixed in cold acetone and stained by FAT. The plate was read using a inverted fluorescence microscope (Nikon Eclipse). The highest dilution of the serum inhibiting 50% fluorescent foci in the wells was taken as end point titer. The titers were expressed in international units in comparison to a inhouse reference serum calibrated against 2nd WHO reference serum (obtained from National Institute of Bilogicals, UK).
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
References
- [1].World health Organization WHO expert consultation on rabies 2nd report WHO Tech Rep Ser 982, Geneva, WHO, 2013 [Google Scholar]
- [2].Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS Ashwathnarayana DH, Abdul Rahman S, Meslin FX, Lobo D, Ravikumr K, Gangaboriah . Assessing the burden of rabies in India. Results of a national multi-centre epidemiological survey. Int J Infect Dis, 2007; 11:29-35 [DOI] [PubMed] [Google Scholar]
- [3].Taylor LH, Costa P, Briggs DJ. Public health management of humans at risk. In: Jackson AC. (ed) Rabies 3d ed Oxford, Academic Press; 543-573 [Google Scholar]
- [4].Saesow N, Chaiwatanarat T, Mitmoonpitak C, Wilde H. Diffusion and fate of intramuscularly injected human rabies immune globulin. Acta Trop. 2000; 76(3):289-92; PMID:10974171; http://dx.doi.org/ 10.1016/S0001-706X(00)00107-8 [DOI] [PubMed] [Google Scholar]
- [5].Dean DJ, Bear GM, Thompson WR. Studies on the local treatment of rabies infected wounds. Bull World Hlth Org 1963; 28:477-84 [PMC free article] [PubMed] [Google Scholar]
- [6].Madhusudana SN, Ashwin BY, Sudarsahan S. Feasibility of reducing rabies immunoglobulin dosage for passive immunization against rabies; results of in vitro and in vivo studies. Hum Vaccine Immunother 2013; 9:1914-7; http://dx.doi.org/ 10.4161/hv.25431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine 2007; 25:7605-9; PMID:17905484; http://dx.doi.org/ 10.1016/j.vaccine.2007.08.054 [DOI] [PubMed] [Google Scholar]
- [8].Omesh K Bharti, Rajesh K Sood, Vidya Ramachandran, Archana Phull. A scratch with hind toes by rabid dog causes rabies- some case studies. Indian Journal of Applied Research. 2015; 5(1):576-577. [Google Scholar]
- [9].de Souza A, Madhusudana SN. Survival from rabies encephalitis. J Neurol Sci 2014; 339:8-14; http://dx.doi.org/ 10.1016/j.jns.2014.02.013 [DOI] [PubMed] [Google Scholar]
- [10].Habel K. Sero-prophylaxis in experimental rabies. Pub Health Rep 1945; 60:545-8; http://dx.doi.org/ 10.2307/458525719316026 [DOI] [Google Scholar]
- [11].Koprowsky H, Van der Sheen J, Blancau J. Use of hyper immune antirabies serum Concentrates in experimental rabies. Am J Med 1950; 8: 412-4; PMID:15410721; http://dx.doi.org/ 10.1016/0002-9343(50)90224-5 [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization Expert committee on rabies. 6th report Tech Rep Ser 523 WHO; Geneva, 1973 [PubMed] [Google Scholar]
- [13].World Health Organization Expert committee on rabies 7th report Tech rep Ser 709 WHO; Geneva, 1982 [PubMed] [Google Scholar]
- [14].World Health Organization Expert committee on rabies 9th report Tech rep Ser 824 WHO, Geneva, 1992 [PubMed] [Google Scholar]
- [15].World Health Organization WHO expert consultation on rabies 2nd report Tech rep Ser 982, WHO; Geneva, 2013 [Google Scholar]
- [16].Atanasiu P, Dean DJ et.al; Rabies Neutralizing Antibodies Response to Different Schedules of Serum & Vaccine inoculations in non–exposed persons: Part 4; Bull WHO; 1967; 36:361-365; PMID:5299668 [PMC free article] [PubMed] [Google Scholar]
- [17].Fekadu M, Shaddock JU, Baer GM. Excretion of rabies virus in saliva of rabid dogs. J Infect Dis 1982; 145:715-9 [DOI] [PubMed] [Google Scholar]
- [18].Dean DJ, Ableseth MK, Atanasiue P. The fluorescent antibody technique : Meslin FX, Kaplan MM, Koprowsky H (eds) laboratory techniques in rabies 4t ed. Geneva, WHO; 1996, 88-96 [Google Scholar]
- [19].Smith JS, Yager PA, Bear GM. A rapid fluorescent focus inhibition test for determining Rabies virus neutralizing antibody : Meslin FX, Kaplan MM, Koprowsky H (eds) Laboratory techniques in rabies 4th ed. Geneva, WHO; 1996, 181-192 [Google Scholar]


